Abstract
Viruses use various mechanisms to evade clearance by the host. Investigating how a few changes in the genome of a non-lethal virus can lead to altered disease, from survivable to immunosuppression/death, would provide valuable information into viral pathogenesis. The Daniels strain of Theiler’s murine encephalomyelitis virus causes an asymptomatic infection or acute encephalitis followed by viral clearance. A mutant, H101, carries several alterations in the viral genome. H101 infection causes profound immunosuppression and death. Thus, a virus that is normally cleared by its natural host can become lethal due to just a few changes in the viral genome.
Keywords: Picornavirus, Viral pathogenesis, Immunosuppression, T cell
1. Introduction
Infections with picornaviruses are often asymptomatic. Mild illnesses (gastrointestinal infection, colds) can be caused by some picornaviruses and, on occasion, more severe diseases, such as encephalitis (inflammation in the brain), can result. Picornaviridae family members include the polioviruses, Saffold virus (SAFV), Foot-and-mouth disease virus (FMDV) and the hepatitis A virus (Jones, et al. 2007; Nielsen, et al. 2012; Tapparel, et al. 2013). Recently, the picornaviruses: cosavirus, salivirus and SAFV have been detected in patients presenting with a wide range of symptoms, including gastroenteritis (Blinkova, et al. 2009; Chiu, et al. 2010; Chiu, et al. 2008).
The newly emerged SAFV currently has 11 genotypes worldwide, SAFV-1 to −11, all of which were recently isolated from fecal specimens collected in 2009 from 943 patients with acute flaccid paralysis in Pakistan and Afghanistan (Naeem, et al. 2014). Multiple SAFV strains represent all but one of the genotypes, SAFV-10, which is currently represented by only one strain. The genome diversity of SAFV was examined by comparing the full genome sequence of a representative strain from each SAFV genotype. High genetic diversity and extensive recombination were demonstrated among different SAFV genotypes (Naeem, et al. 2014). SAFV is the first virus of the Cardiovirus genus found to infect humans, and it is closely related to the mouse pathogen Theiler’s murine encephalomyelitis virus (TMEV) (Chiu, et al. 2008).
TMEV belongs to the Picornaviridae family and Cardiovirus genus. The genome of TMEV is a non-enveloped, single-stranded, positive-sense RNA of approximately 8100 nucleotides which is enclosed in a highly structured capsid. The RNA genome consists of a viral protein (VPg) covalently linked to the 5' untranslated region (UTR), an open reading frame that encodes a polyprotein, from which viral proteases post-translationally cleave 10–12 proteins, including 4 capsid proteins, followed by the 3'UTR and a poly A tail (Rueckert 1996). TMEV infection of mice through the natural route of infection (enteric) is mostly benign. However, rarely TMEV is able to enter into the central nervous system (CNS) and infection can lead to acute encephalitis. Mice of specific genetic backgrounds demonstrate varying susceptibilities to disease induced by TMEV (Lipton and Dal Canto 1979). More specifically, C57BL/6 (B6) mice infected intraperitoneally (i.p.) with the Daniels (DA) strain of TMEV do not show any overt signs of disease, but an abundant virus-specific CD8+ T cell response develops early following infection (Dethlefs, et al. 1997a, 1997b). B6 mice infected with the DA strain of TMEV via the intracerebral (i.c.) route develop acute encephalitis; mice survive the acute disease and clear the virus by approximately day 21 post infection (p.i.), and the mice are immune to subsequent DA virus infections (Fiette, et al. 1993). In comparison, SJL/J mice i.c. infected with the DA strain of TMEV are unable to clear the virus and subsequently present with chronic demyelinating disease, similar to multiple sclerosis (MS).
A mutant of the DA strain of TMEV, called H101, was inadvertently created as a result of transcription error(s) by the T7 polymerase while using a modified full-length infectious cDNA clone of the DA strain of TMEV as template (Zurbriggen, et al. 1991). The H101 mutant virus encodes a point mutation (Thr101Ile) in viral protein (VP) 1, which is a capsid protein. In addition, in sequencing the H101 viral genome, there were also several nucleotide substitutions in the 5’ UTR as well as additional amino acid substitutions in the capsid protein coding region, suggesting that there are a number of perturbations in the viral genome (Tsunoda, et al. 1997). B6 mice infected with the H101 mutant virus experienced profound immunosuppression (i.p. and i.c. routes of infection). Following i.c. infection of B6 mice, H101 viral antigen was not detected in the CNS, but infection led to greater than 90% mortality by day 7 p.i., suggesting that the H101 mutant virus led to a markedly different pathogenesis when compared to the parental DA strain of TMEV.
In this study, we provide a model of a murine virus (DA strain of TMEV) which, in the B6 mouse, can result in an asymptomatic infection and viral clearance (i.p. infection) or acute encephalitis and viral clearance (i.c. infection), but with just a few changes to the viral genome (H101 mutant virus) can result in immunosuppression (i.p. infection) and immunosuppression and death (i.c. infection). There are very few nucleotide differences between the DA strain of TMEV and the H101 mutant virus, therefore, this study provides experimental evidence that a virus that is normally cleared by its natural host can become lethal due to just a few changes to the viral genome.
2. Materials and Methods
2.1. Animals
Male B6 mice and female SJL/J mice at 4- to 5-weeks of age were obtained from the Jackson Laboratory (Bar Harbor, ME). All animal experiments were conducted in accordance with the guidelines prepared by the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council.
2.2. Viral infection
B6 mice were anesthetized with isofluorane by inhalation and infected i.c. or i.p. with the indicated plaque forming units (pfu) of the DA strain of TMEV, the H101 (H for hydrocephalus) strain of TMEV (Tsunoda, et al. 1997) or mock infected with phosphate-buffered saline (PBS) at a final volume of 20 µl per mouse. The DA and H101 strains of TMEV were propagated as previously described (Tsunoda, et al. 1997).
Anesthetized B6 mice were i.c. infected with 50 pfu of lymphocytic choriomeningitis virus (LCMV), Armstrong (Arm) strain (obtained from Dr. Buchmeier, UC Irvine). Viral stocks were prepared by passaging the virus in BHK-21 cells. Viral titer was determined by plaque assay using Vero cells.
2.3. Mortality
B6 mice were infected with increasing viral doses (3 × 103, 3 × 104, 3 × 105 pfu) of H101 by i.c. inoculation. Mice were weighed and assessed for mortality through day 7 p.i.
B6 mice were infected with 3 × 105 pfu of H101 by i.p. inoculation, followed by 50 pfu of LCMV (Arm) by i.c. inoculation on day 7 post-H101-infection. Mice were assessed for mortality through day 7 post-LCMV-infection (day 14 post-H101-infection).
2.4. Spleen weights
B6 mice were infected with 3 × 105 pfu of DA or H101 virus, or mock infect, by i.c. inoculation. Spleens were harvested from infected mice on days 3 and 5 p.i. and weighed.
2.5. Immunohistochemistry
B6 mice, i.c. infected with 3 × 105 pfu of DA or H101 virus, or mock infect, were euthanized on day 3 p.i. Animals were perfused with PBS followed by 4% paraformaldehyde. Brains were harvested, divided into five coronal slabs and embedded in paraffin. Four-µm-thick tissue sections were cut and mounted onto slides. Viral antigen-positive cells were detected on paraffin sections using TMEV hyperimmune rabbit serum, as previous described (Tsunoda, et al. 2001; Zurbriggen and Fujinami 1989). Reactivity of the TMEV hyperimmune rabbit serum for H101 viral antigens was demonstrated previously (Tsunoda, et al. 1997). Viral antigen-positive cells were enumerated and summed, in a blinded fashion, using one slide per brain and evaluating the hippocampal region of the tissue sections.
B6 mice, i.p. infected with 3 × 105 pfu of H101 followed by i.c. infection with 50 pfu of LCMV (Arm) on day 7 post-H101-infection, or mono-infected with either H101 or LCMV, were euthanized on day 7 p.i. Animals and tissues were processed as described above. CD8 antigen-positive cells were detected on paraffin sections using anti-mouse CD8-β antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The tissues were visualized using the avidin-biotin peroxidase complex technique with 3,3'-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO) in 0.01% hydrogen peroxide (Sigma) in PBS. The tissues were then counterstained with Harris’ hematoxylin (Electron Microscopy Sciences, Hatfield, PA).
SJL/J mice sensitized with myelin proteolipid protein (PLP) 139–151 peptide (see section 2.9. below) which were subsequently i.p. infected with 3 × 105 pfu of H101 on day 19 post sensitization were euthanized following the first relapse. Animals were perfused with PBS followed by 4% paraformaldehyde. Spinal cords were harvested, divided into 12 transverse portions and embedded in paraffin. Four-µm-thick tissue sections were cut and mounted onto slides. CD3 antigen-positive cells were detected on paraffin sections using anti-mouse CD3ε antibody (BD Bioscience, San Jose, CA) following antigen retrieval. The tissues were visualized and counterstained as described above. Each spinal cord cross section was visually divided into quarters: top, bottom, right and left. CD3 antigen-positive cells were enumerated within each available quarter, all available quarters were summed and the sum was divided by the total number of available quarters to obtain a pathologic score for each mouse.
2.6. Flow cytometry
Flow cytometry was performed as previously described (Cusick, et al. 2013). Briefly, B6 mice, i.c. infected with 3 × 105 pfu of DA or H101 virus, or mock infected, were euthanized and perfused with PBS on day 3 p.i. Subsequently, cells were mechanically isolated from the spleens and suspended in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 1% L-glutamine (Mediatech), 1% antibiotics (Mediatech), 50 µM 2-mercaptoethanol (Sigma), and 10% Cosmic Calf Serum (CCS; Hyclone, Logan, UT). Cells were further purified with Histopaque-1083 (Sigma). Cells were treated with Fc block (BD Bioscience), stained with the indicated anti-mouse antibodies for 30 minutes at 4°C [anti-CD45–v500 and anti-CD3ε–allophycocyanin (APC)-Cy7, (obtained from BD Bioscience); anti-CD19–v450 and anti-CD11b–APC (obtained from eBioscience, San Diego,CA)], and analyzed by flow cytometry on a BD FACSCanto II (BD Biosciences). Brain-derived cells were stained and analyzed individually for each mouse. Gating was determined based on fluorescence-minus-one (FMO) with isotype matched immunoglobulin (Ig) controls. More specifically, FMO controls contained each antibody conjugate used in the experiment except one, with the addition of the appropriate isotype control for the excluded fluorochrome. This was performed for each fluorochrome. Flow cytometry data analysis was performed using FlowJo software (Tree Star, Inc., Ashland, OR).
2.7. Mitogen-induced proliferation assays
Spleens were harvested, on day 7 p.i., from B6 mice i.p. infected with increasing viral doses of H101 (1 × 101, 1 × 102, 1 × 103, 1 × 104, 1 × 105 pfu). Mononuclear cells were isolated with Histopaque-1083. Cells were resuspended at 1 × 106 cells/ml in complete media (RPMI-1640 media supplemented with 1% L-glutamine, 1% antibiotics, 50 µM 2-mercaptoethanol and 10% CCS). Next, 100 µl of cells were added to each well of a 96-well round-bottomed plate (Corning, Corning, NY). Increasing concentrations (0.1, 1, 3, 10 µg/ml) of concanavalin A (Con A) in 100 µl of complete media were added into culture. Cells were incubated at 37°C, 5% CO2 for 72 hours in the presence of the indicated Con A doses. Sixteen-eighteen hours prior to harvesting cultures, the cells were pulsed with 1 µCi/well of 3H-thymidine (PerkinElmer, Boston, MA). The cells were harvested onto glass fiber filters (PerkinElmer) for measurement of radiolabel incorporation using a liquid scintillation counter (PerkinElmer).
2.8. Enzyme-linked immunosorbent assay (ELISA)
Sera were collected at day 7 p.i. from B6 mice infected i.c. with 3 × 105 pfu of DA or H101 virus. Sera were stored at −80°C until tested. Flat-bottomed 96-well plates (Corning) were coated with TMEV-antigen (DA or H101 antigen, 10 µg/ml in PBS) overnight at 4°C. DA and H101 virus antigens were prepared by infecting BHK-21 cells with virus at a multiplicity of infection of 0.1 pfu/cell as described (Kurtz, et al. 1995b). Plates were washed, incubated with sera in serial dilutions starting at 1:27 and diluted to 1:214, washed and incubated with secondary antibody, goat anti-mouse IgG-horse radish peroxidase (Invitrogen, San Diego, CA). Subsequently, substrate solution [citrate buffer (Fisher Scientific, Denver, CO), 10× o-phenylenediamine, hydrogen peroxide] was added for 30 minutes at room temperature in the dark. The reaction was stopped by adding hydrochloric acid. Fluorescence was measured using a Wallac Victor 2 Multi-label Counter (PerkinElmer).
2.9. Experimental autoimmune encephalomyelitis (EAE) induction
SJL/J mice were injected subcutaneously in the flanks with 200 ml of 1 mM PLP139–151 peptide. The emulsion solution was reconstituted complete Freund’s adjuvant (CFA), composed of Freund’s incomplete adjuvant (Pierce Biotechnology, Rockford, IL) containing Mycobacterium tuberculosis H37 Ra (2 mg/ml) (Difco Laboratories, Detroit, MI), and PLP139– 151. Mice were intravenously injected with 0.2 mg of Bordetella pertussis toxin (List Biological Laboratories, Campbell, CA), in a 100 ml final volume, on days 0 and 2 following sensitization. Mice developed a relapsing-remitting clinical course (RR-EAE). Mice were weighed and scored daily for clinical signs. Clinical scoring was as follows: 0, no clinical disease; 1, loss of tail tonicity; 2, presents with mild hind leg paralysis with no obvious gait disturbance; 3, mild leg aralysis with gait disturbance and paralysis; 4, hind limbs are paralyzed; and 5, moribund or dead. If the mice were paralyzed to the point where they could not feed or groom themselves (moribund), or they lost 20% of their body weight, the mice are euthanized via inhaled anesthetic.
2.10. Statistical analysis
The program SigmaPlot (Systat Software, Inc., Chicago, IL) was used for all statistical analyses performed. The Student’s t test was performed for pairwise comparison. The Chi-Square test was utilized for nominal data (survival: yes or no).
3. Results
3.1. Decreasing viral doses of H101 led to lower mortality
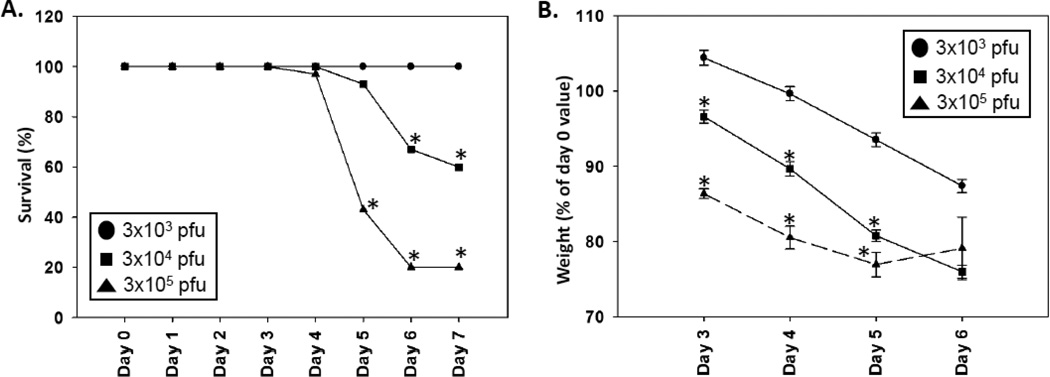
To determine the severity of the disease induced by i.c. infection of B6 mice with the H101 mutant virus, mice were infected with 3 × 103, 3 × 104 and 3 × 105 pfu of H101 and monitored daily for mortality and weight loss. No B6 mice succumbed to infection when H101 was administered via the i.p. route of infection (data not shown). For i.c. infection, we found that 100% of the mice infected with 3 × 103 pfu of the H101 mutant of TMEV survived through day 7 p.i. (Fig. 1A), while still experiencing steady weight loss for days 3–6 p.i. (Fig. 1B). The mortality rate significantly increased (p<0.05, Chi-Square test) on days 5–7 p.i. when mice were infected with higher viral doses of H101 (Fig. 1A), and this was accompanied by a significantly greater weight loss (p<0.05, Student’s t test) for days 3–5 p.i. (Fig. 1B). These results show that disease was more severe with higher doses of H101. In addition, although the mortality rate dropped to zero for the mice infected with 3 × 103 pfu of H101, there was still disease present as evidenced by the weight loss seen in these animals.
Figure 1.
Decreasing viral doses of H101 led to lower mortality. B6 mice were infected with decreasing viral doses of the H101 strain of TMEV by i.c. inoculation. (A) Percent of mice that survived. *p<0.05, Chi-Square test. (B) Weight of mice after infection. Results represent the mean ± standard error of the mean (SEM) for groups of 30 mice per group for 3 × 105 pfu and 20 mice per group for 3 × 104 and 3 × 103 pfu. *p<0.05, Student’s t test.
3.2. H101 viral antigens were not found within the hippocampal region of the brain
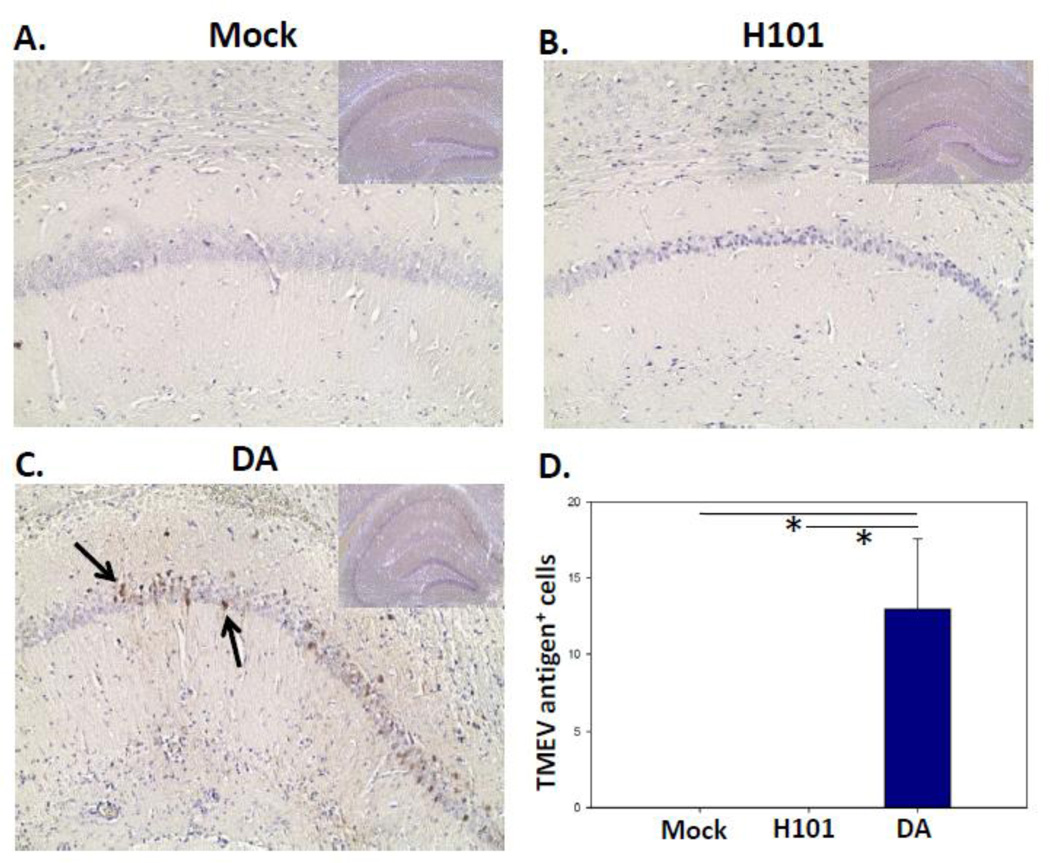
Although general infection of the brain parenchyma was not seen previously with H101 infection of SJL/J mice (Tsunoda, et al. 1997), the DA strain of TMEV is known to infect the pyramidal neurons of the CA1 region of the hippocampus within the brain of B6 mice (Kirkman, et al. 2010). Therefore, immunohistochemistry was performed using TMEV hyperimmune rabbit serum to determine whether the H101 strain of TMEV was also capable of infecting pyramidal neurons of the hippocampus of B6 mice. Pyramidal neurons of the hippocampus were not found to contain H101 viral antigens (Fig. 2). The hippocampal region of mock infected mice (Fig. 2A) and mice i.c. infected with 3 × 105 pfu of H101 (Fig. 2B), at day 3 p.i., had fewer viral antigen-positive cells compared to mice i.c. infected with 3 × 105 pfu of DA (Fig. 2C). These differences were significant (p<0.05, Student’s t test) (Fig. 2D). Therefore, the H101 strain of TMEV is unable to infect the pyramidal neurons of the hippocampus of B6 mice. The viral dose of 3 × 105 pfu will be used hereafter if not otherwise indicated.
Figure 2.
H101 viral antigens were not found within the hippocampal region of the brain. B6 mice were i.c. infected with 3 × 105 pfu of DA or H101, or mock infected. Representative tissue sections of the CA1 region of the hippocampus (inset: hippocampus) stained for TMEV antigen (arrow) from mock infected (A), H101 infected (B) and DA infected (C) mice at day 3 p.i. are shown. (D) Quantification of viral antigen-positive cells within the hippocampal region of the brain. Results represent the mean + SEM for groups of 4 mice per group. *p<0.05, Student’s t test.
3.3. H101 strain of TMEV ablates splenic T cells
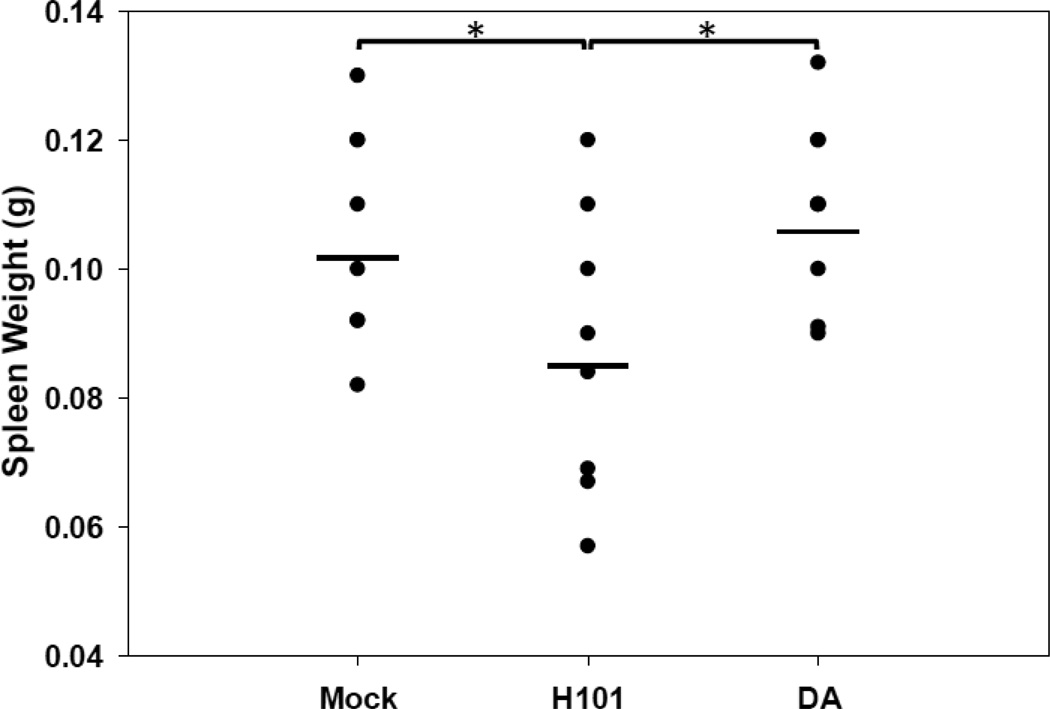
The profound immunosuppression induced by H101 was demonstrated in several ways. First, B6 mice i.c. infected with the H101 mutant virus had significantly lower (p<0.05, Student’s t test) spleen weights (0.085 ± 0.005g), in comparison to mock infected mice (0.106 ± 0.006g) and mice infected with DA (0.109 ± 0.005g) (Fig. 3), evaluated at days 3 and 5 p.i., suggesting that H101 was causing leukopenia. Spleen weights were also found to be reduced, albeit to a lesser extent, when H101 was administered via the i.p. route of infection (data not shown).
Figure 3.
H101 infection resulted in reduced spleen weight. B6 mice were i.c. infected with 3 × 105 pfu of DA or H101, or mock infected. Mouse spleens were collected on days 3 and 5 p.i. and weighed. Data shows groups with 8 mice per mock group and 9 mice per H101 and DA groups. *p<0.05, Student’s t test.
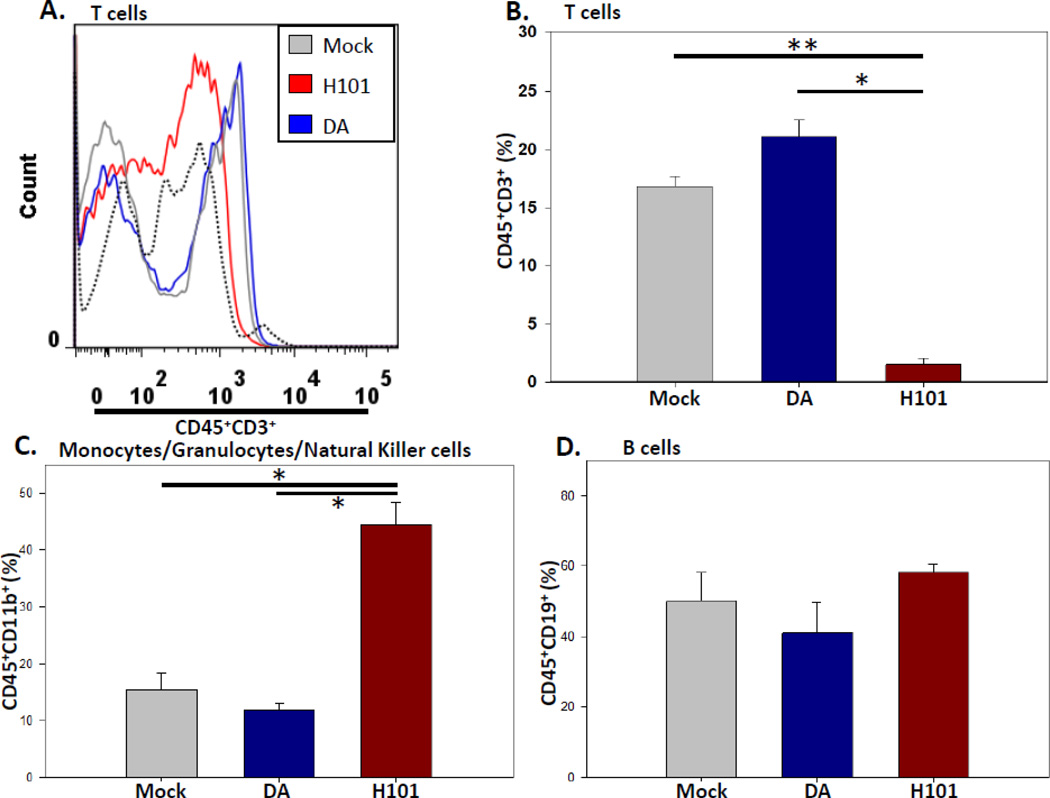
To determine what cell-types H101 could be targeting for ablation, spleen cells from mock infected B6 mice and mice i.c. infected with DA or H101 were immunophenotyped on day 3 p.i. using flow cytometry (Fig. 4). CD45+CD3+ T cells were significantly less in mice infected with H101, compared to mice infected with DA (p<0.05, Student’s t test) and mock infected mice (p<0.005, Student’s t test) (Fig. 4A,B). However, monocytes/granulocytes/natural killer cells (CD45+CD11b+) were significantly higher (p<0.05, Student’s t test) in spleens from mice infected with H101 (44.4 ± 4), in comparison to mock infected mouse spleens (15.5 ± 2.9) and spleens from mice infected with DA (11.9 ± 1.3) (Figs. 4C). Similarly, B cells (CD45+CD19+) in spleens from mice infected with H101 (58.2 ± 2.4) were higher, in comparison to mock infected mouse spleens (50.1 ± 8.2) and spleens from mice infected with DA (41 ± 8.6), although these differences did not reach significance (Fig 4D). The increase in both B cells and monocytes/granulocytes/natural killer cells in the spleens from mice infected with H101 can be accounted for by the low frequency of T cells. Therefore, spleens isolated on day 3 p.i. from B6 mice infected via the i.c. route with the H101 mutant virus had no CD45+CD3+ T cells, compared to the spleens of mock infected mice and mice infected with the DA strain of TMEV. Splenic T cells were also reduced when H101 was administered via the i.p. route of infection, although to a lesser extent than that seen following i.c. infection (data not shown).
Figure 4.
H101 infection resulted in reduce T cells in the spleen. B6 mice were i.c. infected with 3 × 105 pfu of DA or H101, or mock infected. Mouse spleens were collected on day 3 p.i. and analyzed by flow cytometry. (A) Representative flow cytometric histogram. Fluorescence minus one plus isotype antibody was used as a control (black dotted line). (B) Quantification of CD45+CD3+ T cells in the spleens. (C) Quantification of CD45+CD11b+
monocytes/granulocytes/natural killer cells in the spleens. (D) Quantification of CD45+CD19+ B cells in the spleens. Results represent the mean + SEM for groups of 4 mice per mock and DA groups and 5 mice per H101 group. *p<0.05, **p<0.005, Student’s t test.
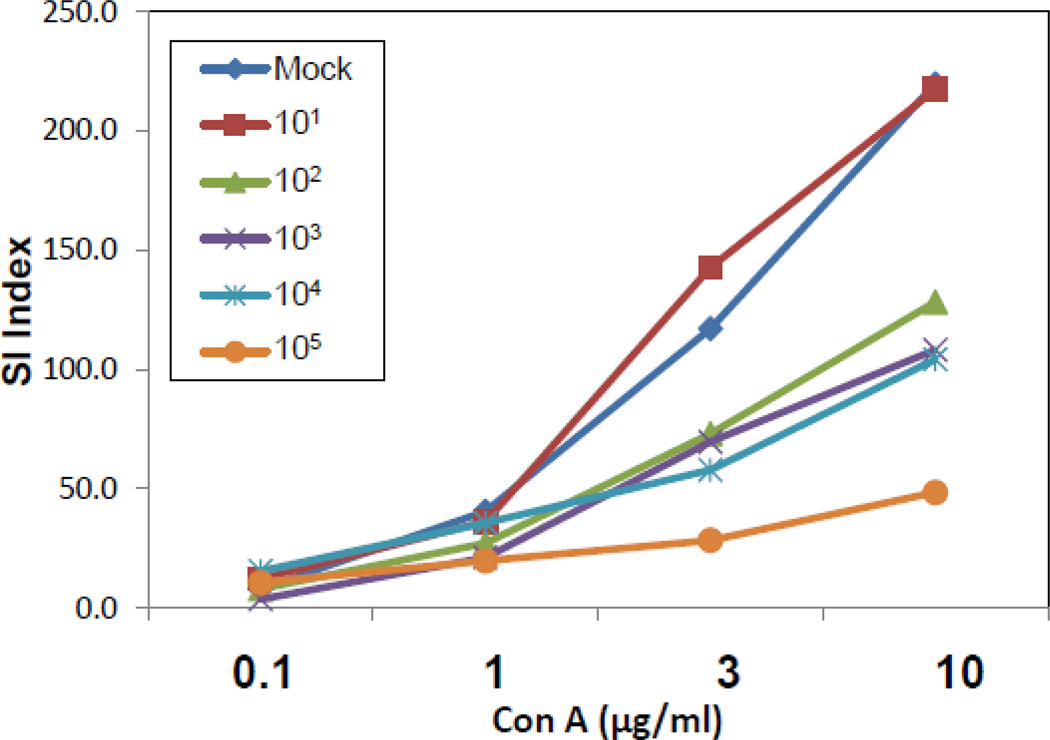
To test whether H101 had an effect on the functionality of T cells, B6 mice were mock infected or i.p. infected with increasing viral doses (1 × 101, 1 × 102, 1 × 103, 1 × 104, 1 × 105 pfu) of the H101 strain of TMEV. Spleens were collected on day 7 p.i. and cells were isolated and used to measure the proliferative response via 3H-thymidine incorporation assays. The same number of spleen cells were added to each well and cultured with increasing concentrations (0.1, 1, 3, 10 µg/ml) of Con A. Increasing viral doses of H101 resulted in decreasing lymphocyte proliferation in response to Con A (Fig. 5). Taken together, these results demonstrate that H101 infection leads to depletion of T cells in the spleen. We are currently investigating why T cell depletion is greater following i.c. infection compared to i.p. infection.
Figure 5.
Increasing viral doses of H101 resulted in decreasing lymphocyte proliferation to Con A. B6 mice were infected with decreasing viral doses (1 × 101, 1 × 102, 1 × 103, 1 × 104, 1 × 105 pfu) of H101 by i.p. inoculation. Spleens were harvested on day 7 p.i., lymphocytes were isolated and stimulated in vitro with increasing concentrations of Con A (0.1, 1, 3, 10 µg/ml). The proliferative response was measured by 3H-thymidine incorporation for the last 16–18 hours of a 72 hour assay. The stimulation index (SI) was used for proliferation analysis, in which the mean of the experimental data was divided by the mean of the background for each culture condition. Mouse spleen cells were pooled for proliferation assays, therefore statistics were not performed.
3.4. H101 strain of TMEV induced lower levels of virus-specific serum antibody
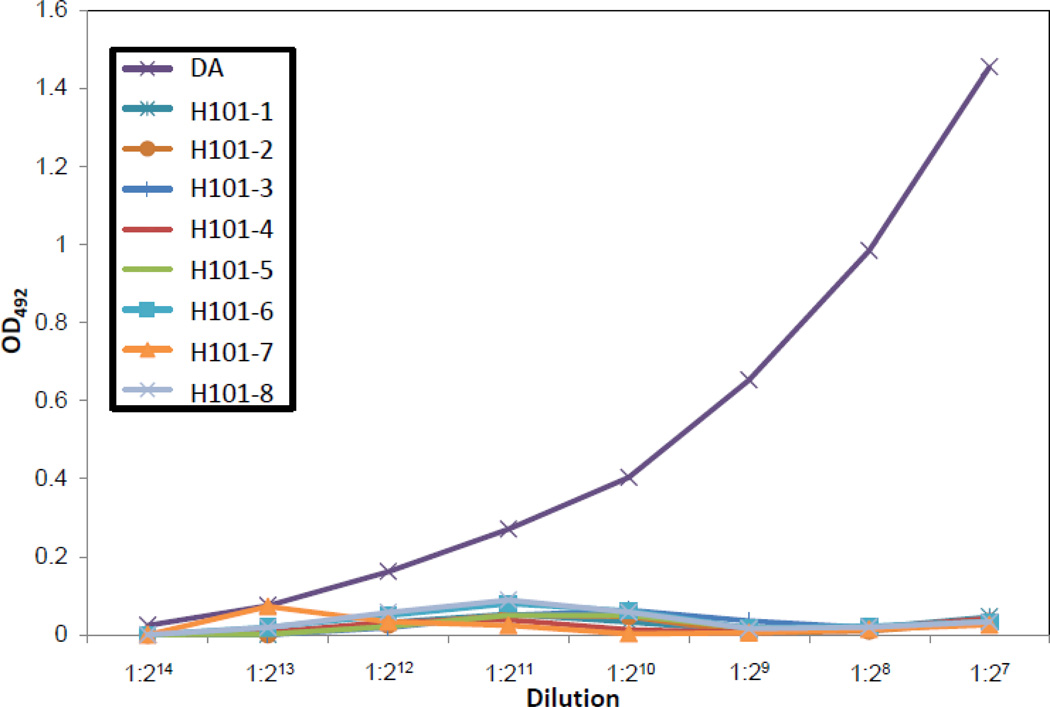
CD4+ T cells are required for protection from TMEV infection in SJL/J mice due to the “help” that CD4+ T cells provide for the development of an antiviral antibody response in the CNS (Kurtz, et al. 1995a, 1995b). Although there was no effect on the number of splenic B cells in B6 mice infected with the H101mutant strain of TMEV in comparison to B6 mice infected with the DA strain of TMEV (Fig. 4D), we quantified, by ELISA, anti-TMEV antibody levels in serum from B6 mice that were i.c. inoculated with either DA or H101 (Fig. 6). Quantification of virus-specific serum antibody on day 7 p.i. demonstrated a markedly lower virus-specific serum antibody level in mice infected with the H101 mutant than in mice infected with DA (Fig. 6). Quantification of total serum IgG, IgG1, IgG2a, IgG2b and IgG3 by ELISA showed no differences between mice infected with H101 or DA (data not shown). Therefore, H101 infection leads to depletion of virus-specific antibody in the serum, likely through a reduction in CD4+ T helper cells.
Figure 6.
H101 infection resulted in reduced virus-specific serum antibody levels. B6 mice were i.c. infected with 3 × 105 pfu of DA or H101. Serum was collected on day 7 p.i. and an ELISA for TMEV-specific antibody was performed. Each individual curve was generated from a single mouse.
3.5. H101 strain of TMEV protected against subsequent LCMV infection
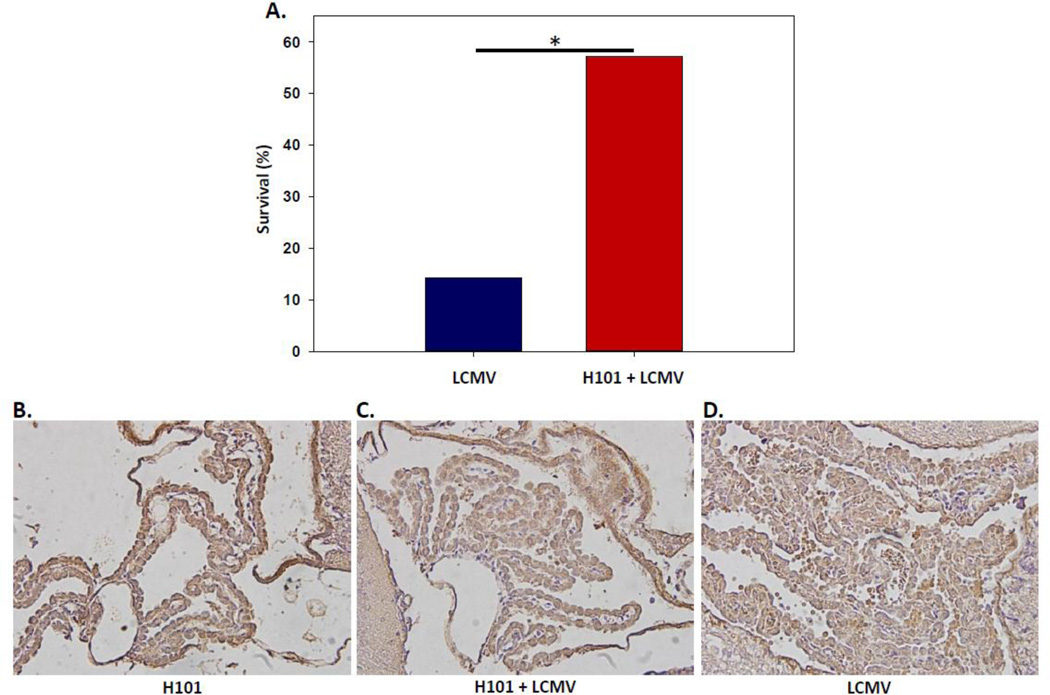
Fatal meningitis results by approximately day 6 p.i. from i.c. inoculation of B6 mice with LCMV; the meningitis is due to infiltrating CD8+ T cells and monocytes (Kim, et al. 2009). To test the functional ability of the H101 mutant strain of TMEV to kill T cells in vivo, B6 mice were i.p. infected with the H101 strain of TMEV 7 days prior to i.c. infection with LCMV (Arm, 50 pfu). As controls, a group of B6 mice were i.c. inoculated with LCMV alone, and a group of B6 mice were i.p. infected with H101 alone. H101 mono-infection had no biological effect on the mice (N=4) (data not shown). LCMV mono-infection led to significantly greater (p<0.05, Chi-Square test) mortality at day 7 post-LCMV-infection, in comparison to H101 plus LCMV dual-infection at day 7 post-LCMV-infection (day 14 post-H101-infection) (Fig. 7A). Examination of the choroid plexus of the brain from H101 mono-infected (Fig. 7B), H101 plus LCMV dual-infected (Fig. 7C) and LCMV mono-infected (Fig. 7D) mice for CD8+ T cells showed a much larger amount of cellular infiltration in the LCMV mono-infected mice (Fig. 7D) in comparison to either the H101 mono-infected mice or the H101 plus LCMV dual-infected mice. A portion of the cellular infiltrate was CD8+ T cells. These results demonstrate that the H101 strain of TMEV provides protection against T cell-mediated meningitis, thereby providing in vivo evidence that H101 targets T cells.
Figure 7.
H101 infection protected against subsequent LCMV infection and choriomeningitis. B6 mice were i.p. infected with 3 × 105 pfu of H101, followed on day 7 p.i. with 50 pfu LCMV (Arm) by the i.c. route. (A) Mice were assessed for mortality through day 7 post-LCMV-infection (day 14 post-H101-infection) (H101 + LCMV). In tandem, B6 littermates were mono-infected with LCMV by the i.c. route and assessed for mortality through day 7 post-LCMV-infection (LCMV). Results present groups of 14 mice per group. *p<0.05, Chi-Square test. Representative tissue sections of the choroid plexus of the brain stained for CD8 antigen from H101 mono-infected (B), H101 + LCMV dual-infected (C) and LCMV mono-infected (D) mice at day 7 p.i. are shown.
3.6. H101 strain of TMEV ameliorated RR-EAE
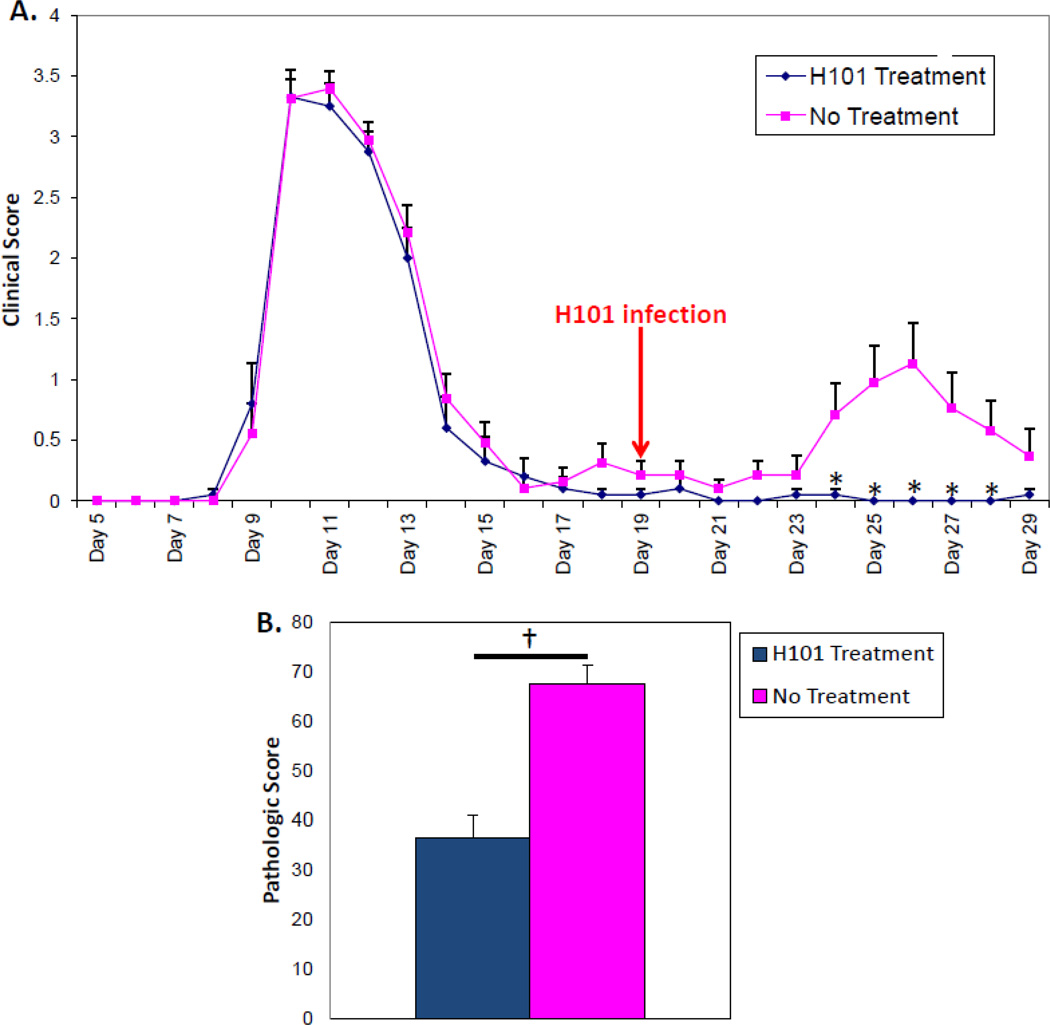
Previously, using the PLP139–151-induced EAE model in SJL/J mice, infection with the H101 strain of TMEV was shown to significantly reduce the clinical signs of EAE at the peak of the acute phase if it was administered i.p. on day 5 post-sensitization, 4 days prior to the first observable signs of disease (Cusick, et al. 2014). In comparison, administration of the DA strain of TMEV i.p. on day 5 post-sensitization also significantly reduce the clinical signs of EAE at the peak of the acute phase, but not to the same extent as the H101 strain of TMEV (data not shown). As the SJL/J mice in the PLP139–151-induced EAE model develop a RR-EAE clinical course, this model was used to determine if H101 could prevent, dampen and/or alter a T cell-mediated disease that was well underway. SJL/J mice were sensitized with PLP139–151 peptide in CFA. SJL/J mice experienced an acute attack between days 9–15 and recovered (Fig. 8A). One group of EAE mice was i.p. infected with the H101 strain of TMEV on day 19 post-sensitization, which was in the middle of the remission between the acute attack and the first relapse (started on day 24). A control group of EAE mice was left untreated (No Treatment). The group of EAE mice that was treated with H101 had significantly lower clinical scores (p<0.05, Student’s t test) on days 24–28 post-sensitization, compared to the untreated group (Fig. 8A). More specifically, the average clinical score, the mean clinical maximum and the cumulative disease index, an average score of the clinical score over the course of the first relapse (days 23–29), were all significantly lower (p<0.01, Student’s t test) in the EAE mice that were treated with H101, compared to the untreated group (Table 1). A further comparison showed that i.p. infection of EAE mice with the DA strain of TMEV during the remission between the acute attack and the first relapse did not significantly lower the clinical scores over the course of the first relapse in comparison to no treatment; in fact the clinical scores were increased over the no treatment group (data not shown). Upon examination of spinal cord sections from EAE mice treated with H101 during the remission or left untreated for the presence of CD3 antigen-positive cells, we found that the mice treated with H101 had significantly fewer CD3+ T cells infiltrating the spinal cords, compared to the no treatment mice (p<0.0001, Student’s t test) (Fig. 8B). Likewise, the mice treated with H101 had significantly fewer CD3+ T cells infiltrating the spinal cords, compared to mice treated with DA during the remission (p<0.001, Student’s t test) (data not shown). Mice treated with DA were not significantly different from untreated mice (data not shown). Therefore, H101, but not DA, could ameliorate a T cell-mediated disease that was well underway.
Figure 8.
H101 infection ameliorated RR-EAE. SJL/J mice were sensitized with PLP139-151 in CFA. (A) Sensitized mice were infected i.p. with 3 × 105 pfu of the H101 strain of TMEV at day 19 post-sensitization (arrow). PLP139–151-sensitized mice were used as a control (No treatment). Data are representative of the mean + SEM for groups of 20 mice for the H101 Treatment group and 19 mice for the No Treatment group. *p<0.05, Student’s t test. (B) Quantification of CD3 antigen-positive cells within the spinal cords. Pathologic score was calculated as described in the methods. Results represent the mean + SEM for groups of 20 mice for the H101 Treatment group and 18 mice for the No Treatment group. †p<0.0001, Student’s t test.
Table 1.
H101 Infection Ameliorates RR-EAE.
| N | Average Clinical Score |
Mean Clinical Maximum |
Cumulative Disease Index |
|
|---|---|---|---|---|
| No Treatment | 19 | 0.68 ± 0.10 | 1.5 ± 0.34 | 4.7 ± 1.51 |
| H101 Treatment | 20 | 0.02 ± 0.01* | 0.10 ± 0.07* | 0.15 ± 0.11* |
p< 0.01, Student’s t test
4. Discussion
There is an ongoing evolutionary battle between host cell immunity and viruses. As viruses develop new ways to evade the host immune response, the host, if it is to survive, must develop new ways to combat the viral infection. There are a multitude of viral immune evasion strategies that function through down regulation of both innate and adaptive components of the host immune response (Pratheek, et al. 2013). Some mechanisms of evasion lead to defective viral antigen processing and presentation and therefore no effector T cell response is induced. Some mechanisms of evasion lead to alteration in the host cellular microenvironment (cytokines and chemokines), thus altering the effector T cell response possibly through the induction of a regulatory T cell response. Yet other mechanisms of evasion involve the targeting of different immune cell types, such as natural killer cells, B cells or dendritic cells, or the regulation of the host complement system, which is a mechanism known to be used by picornavirus (Pratheek, et al. 2013). The H101 mutant strain of TMEV has acquired a new mechanism of evasion: the ability to target T cells that are critical to the host’s ability to combat the viral infection and clear the virus. As a result, the pathogenesis of the H101 strain of TMEV has been altered dramatically from that of the parental DA strain of TMEV. The H101 strain of TMEV no longer has the ability to infect neurons within the brain parenchyma. Instead, there is a profound immunosuppression, due to the ablation of splenic T cells which, in turn, causes a great reduction in virus-specific serum antibodies. Ultimately, a virus (DA strain) that was routinely cleared by the host, through just a few changes to its genome, has become a virus (H101 strain) that now has the ability to kill the host.
Picornaviruses have demonstrated the capability of evolving through recombination and mutation, thus imbuing them with the ability to evade immunity and adapt to new ecological niches (Benschop, et al. 2010; Boros, et al. 2012; Lau, et al. 2014; Yip, et al. 2013; Yip, et al. 2010). This new immune evasion strategy and altered pathogenesis for the H101 mutant strain of TMEV appears to be linked to just a few changes in the viral genome. Another picornavirus that appears to be evolving through a relative few alterations to its genome is FMDV (Aphthovirus genus), which is a highly infectious and contagious picornavirus that infects cloven hoofed livestock, and is endemic in India (Subramaniam, et al. 2013). There are currently six prevalent serotypes of FMDV worldwide. Within serotype O, there are 11 geographically restricted topotypes. Within the Middle East-South Asia topotype, the PanAsia lineage caused most of the outbreaks of foot-and-mouth disease in India between 1996–2008, while the Ind2001 lineage of FMDV caused sporadic cases of foot-and-mouth disease in India during 2003–2005, re-surged in 2008 and continued to be prevalent through 2011. An examination of the capsid coding region of 141 FMDV isolates collected during 2009–2012 demonstrated that a new genetic lineage, named Ind2011, had emerged with 9.8–14.8% and 9.7–12.8% nucleotide divergence from the Ind2001 and PanAsia lineages, respectively. The Ind2011 lineage appeared to share ancestry with the PanAsia lineage, not the Ind2001 lineage. The Ind2011 lineage had two amino acid substitutions at positions VP1-(L36F) and VP2-(Q133T) that appeared to be specific for the Ind2011 lineage (Subramaniam, et al. 2013).
In this study, two disease models, EAE and LCMV infection, were used to demonstrate the ability of the H101 mutant strain of TMEV to functionally target both CD4+ and CD8+ T cells in vivo. EAE is predominantly mediated by autoreactive CNS-specific CD4+ T cells (Libbey, et al. 2010), while the fatal meningitis induced by LCMV is mediated through CD8+ T cells (Kim, et al. 2009). Administration of the H101 strain of TMEV prior to infection with LCMV protected the mice from LCMV-induced meningitis and death (Fig. 7), thus demonstrating the ability of H101 to target the CD8+ effector T cells. Administration of the H101, but not DA, strain of TMEV to EAE mice during the remission, that followed the acute attack and preceded the first relapse, ameliorated the clinical signs of disease during the first relapse (Fig. 8), thus demonstrating the ability of H101 to target the CD4+ effector T cells. Previously, the ability of H101 to ameliorate the clinical signs of disease during the acute attack was demonstrated through administration of the H101 strain of TMEV to EAE mice prior to the acute attack (Cusick, et al. 2014). Therefore, administration of the H101 strain of TMEV was not only able to prevent a T cell-mediated disease from developing (LCMV) and ameliorate a newly developing T cell-mediated disease (EAE prior to acute attack), but administration of the H101 strain of TMEV was also able to ameliorate a T cell-mediated disease that was already well underway (EAE during remission). An important question that is currently being investigated is whether the elimination of T cells through infection with the H101 strain of TMEV is occurring through a direct or indirect mechanism.
Highlights.
Infection with the H101 mutant strain of TMEV resulted in high mortality.
H101 did not infect pyramidal neurons in the CA1 region of the hippocampus.
The H101 mutant strain of TMEV ablated splenic T cells.
H101 protected against CD8+ T cell-mediated death resulting from LCMV infection.
H101, but not DA, ameliorated the clinical signs of CD4+ T cell-mediated EAE.
Acknowledgments
We would like to thank F. Lynn Sonderegger, PhD, Jedediah Doane, BS, Jordan T. Sim, BA, and Mitchell A. Wilson for excellent technical assistance and Daniel J. Harper for the outstanding preparation of the manuscript. This work was supported by NIH T32AI055434 (M.F.C.), 1R01NS065714 (R.S.F.) and the Emma Mary Deland Foundation (R.S.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Benschop KS, de Vries M, Minnaar RP, Stanway G, van der Hoek L, Wolthers KC, Simmonds P. Comprehensive full-length sequence analyses of human parechoviruses: diversity and recombination. J.Gen.Virol. 2010;91:145–154. doi: 10.1099/vir.0.014670-0. [DOI] [PubMed] [Google Scholar]
- Blinkova O, Kapoor A, Victoria J, Jones M, Wolfe N, Naeem A, Shaukat S, Sharif S, Alam MM, Angez M, Zaidi S, Delwart EL. Cardioviruses are genetically diverse and cause common enteric infections in South Asian children. J.Virol. 2009;83:4631–4641. doi: 10.1128/JVI.02085-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros A, Pankovics P, Knowles NJ, Reuter G. Natural interspecies recombinant bovine/porcine enterovirus in sheep. J.Gen.Virol. 2012;93:1941–1951. doi: 10.1099/vir.0.041335-0. [DOI] [PubMed] [Google Scholar]
- Chiu CY, Greninger AL, Chen EC, Haggerty TD, Parsonnet J, Delwart E, DeRisi JL, Ganem D. Cultivation and serological characterization of a human Theiler’s-like cardiovirus associated with diarrheal disease. J.Virol. 2010;84:4407–4414. doi: 10.1128/JVI.02536-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CY, Greninger AL, Kanada K, Kwok T, Fischer KF, Runckel C, Louie JK, Glaser CA, Yagi S, Schnurr DP, Haggerty TD, Parsonnet J, Ganem D, DeRisi JL. Identification of cardioviruses related to Theiler’s murine encephalomyelitis virus in human infections. Proc.Natl.Acad.Sci.USA. 2008;105:14124–14129. doi: 10.1073/pnas.0805968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick MF, Libbey JE, Fujinami RS. Picornavirus infection leading to immunosuppression. Future Virol. 2014;9:475–482. doi: 10.2217/fvl.14.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. Infiltrating Macrophages are Key to the Development of Seizures Following Virus Infection. J.Virol. 2013;87:1849–1860. doi: 10.1128/JVI.02747-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefs S, Brahic M, Larsson-Sciard E-L. An early, abundant cytotoxic T-lymphocyte response against Theiler’s virus is critical for preventing viral persistence. J.Virol. 1997a;71:8875–8878. doi: 10.1128/jvi.71.11.8875-8878.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefs S, Escriou N, Brahic M, van der Werf S, Larsson-Sciard E-L. Theiler’s virus and Mengo virus induce cross-reactive cytotoxic T lymphocytes restricted to the same immunodominant VP2 epitope in B6 mice. J.Virol. 1997b;71:5361–5365. doi: 10.1128/jvi.71.7.5361-5365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiette L, Aubert C, Brahic M, Pena-Rossi C. Theiler’s virus infection of p2-microglobulin-deficient mice. J.Virol. 1993;67:589–592. doi: 10.1128/jvi.67.1.589-592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MS, Lukashov VV, Ganac RD, Schnurr DP. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J.Clin.Microbiol. 2007;45:2144–2150. doi: 10.1128/JCM.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz CIB, Sun XM, Fujinami RS. B-lymphocyte requirement for vaccine-mediated protection from Theiler’s murine encephalomyelitis virus-induced central nervous system disease. J.Virol. 1995a;69:5152–5155. doi: 10.1128/jvi.69.8.5152-5155.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz CIB, Sun XM, Fujinami RS. Protection of SJL/J mice from demyelinating disease mediated by Theiler’s murine encephalomyelitis virus. Microb.Pathog. 1995b;18:11–27. doi: 10.1016/s0882-4010(05)80009-9. [DOI] [PubMed] [Google Scholar]
- Lau SK, Woo PC, Yip CC, Li KS, Fan RY, Bai R, Huang Y, Chan KH, Yuen KY. Chickens host diverse picornaviruses originated from potential interspecies transmission with recombination. J.Gen.Virol. 2014 Jun 6; doi: 10.1099/vir.0.066597-0. Epub ahead of print 2014. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Tsunoda I, Fujinami RS. Studies in the modulation of experimental autoimmune encephalomyelitis. J.Neuroimmune Pharmacol. 2010;5:168–175. doi: 10.1007/s11481-010-9215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL, Dal Canto MC. Susceptibility of inbred mice to chronic central nervous system infection by Theiler’s murine encephalomyelitis virus. Infect.Immun. 1979;26:369–374. doi: 10.1128/iai.26.1.369-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem A, Hosomi T, Nishimura Y, Alam MM, Oka T, Zaidi SS, Shimizu H. Genetic Diversity of Circulating Saffold Viruses in Pakistan and Afghanistan. J.Gen.Virol. 2014 Jun 4; doi: 10.1099/vir.0.066498-0. Epub ahead of print 2014. [DOI] [PubMed] [Google Scholar]
- Nielsen ACY, Böttiger B, Banner J, Hoffmann T, Nielsen LP. Serious invasive Saffold virus infections in children, 2009. Emerg.Infect.Dis. 2012;18:7–12. doi: 10.3201/eid1801.110725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratheek BM, Saha S, Maiti PK, Chattopadhyay S, Chattopadhyay S. Immune regulation and evasion of Mammalian host cell immunity during viral infection. Indian J.Virol. 2013;24:1–15. doi: 10.1007/s13337-013-0130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert RR. Picornaviridae: The viruses and their replication. In: Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, Roizman B, Straus SE, editors. Fields Virology. Third Edition. Philadelphia: Lippincott-Raven; 1996. pp. 609–654. [Google Scholar]
- Subramaniam S, Sanyal A, Mohapatra JK, Sharma GK, Biswal JK, Ranjan R, Rout M, Das B, Bisht P, Mathapati BS, Dash BB, Pattnaik B. Emergence of a novel lineage genetically divergent from the predominant Ind2001 lineage of serotype O foot-and-mouth disease virus in India. Infect.Genet.Evol. 2013;18:1–7. doi: 10.1016/j.meegid.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Tapparel C, Siegrist F, Petty TJ, Kaiser L. Picornavirus and enterovirus diversity with associated human diseases. Infect.Genet.Evol. 2013;14C:282–293. doi: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, McCright IJ, Kuang L-Q, Zurbriggen A, Fujinami RS. Hydrocephalus in mice infected with a Theiler’s murine encephalomyelitis virus variant. J.Neuropathol.Exp.Neurol. 1997;56:1302–1313. doi: 10.1097/00005072-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Wada Y, Libbey JE, Cannon TS, Whitby FG, Fujinami RS. Prolonged gray matter disease without demyelination caused by Theiler’s murine encephalomyelitis virus with a mutation in VP2 puff B. J.Virol. 2001;75:7494–7505. doi: 10.1128/JVI.75.16.7494-7505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip CC, Lau SK, Lo JY, Chan KH, Woo PC, Yuen KY. Genetic characterization of EV71 isolates from 2004 to 2010 reveals predominance and persistent circulation of the newly proposed genotype D and recent emergence of a distinct lineage of subgenotype C2 in Hong Kong. Virol.J. 2013;10:222. doi: 10.1186/1743-422X-10-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip CC, Lau SK, Zhou B, Zhang MX, Tsoi HW, Chan KH, Chen XC, Woo PC, Yuen KY. Emergence of enterovirus 71 “double-recombinant” strains belonging to a novel genotype D originating from southern China: first evidence for combination of intratypic and intertypic recombination events in EV71. Arch.Virol. 2010;155:1413–1424. doi: 10.1007/s00705-010-0722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A, Fujinami RS. A neutralization-resistant Theiler’s virus variant produces an altered disease pattern in the mouse central nervous system. J.Virol. 1989;63:1505–1513. doi: 10.1128/jvi.63.4.1505-1513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A, Thomas C, Yamada M, Roos RP, Fujinami RS. Direct evidence of a role for amino acid 101 of VP-1 in central nervous system disease in Theiler’s murine encephalomyelitis virus infection. J.Virol. 1991;65:1929–1937. doi: 10.1128/jvi.65.4.1929-1937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]