Abstract
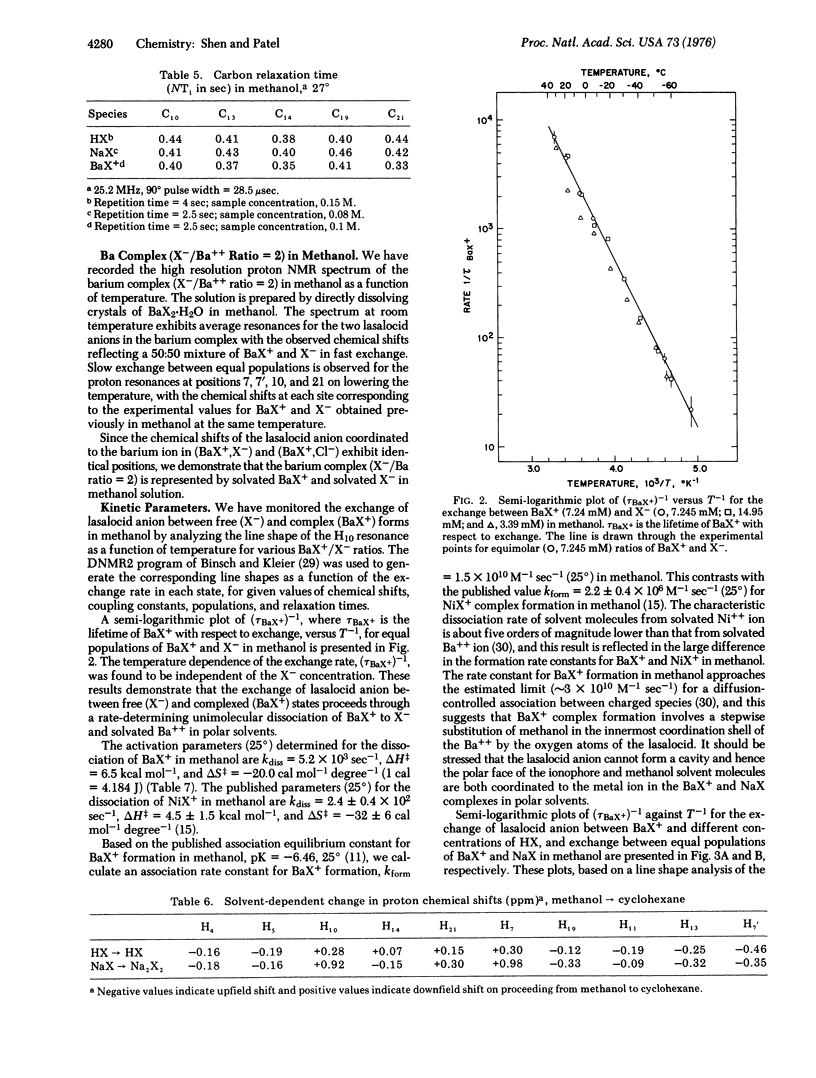
We report below on nuclear magnetic resonance investigations of the structure and exchange kinetics for the free acid, anion, sodium complex, and barium complex of the ionophore lasalocid A (X537A) in methanol solution. A comparison between the proton and carbon longitudinal relaxation times of lasalocid in nonpolar and polar solvents demonstrates that the free acid (HX) is a monomer in methanol solution. Parallel proton and carbon relaxation measurements demonstrate that the anion (X-), sodium complex (NaX), and barium complex (BaX+) are also monomeric in methanol solution. These results are in contrast to the Na2X2 dimer and the BaX2-H20 dimer observed in crystals and in nonpolar (cyclohexane and methylene chloride) solutions. Large downfield shifts on complex formation (X- to NaX and BaX+) are detected for protons located on the polar face of the ionophore with their C-H bonds directed towards and proximal to the metal ion. The exchange of lasalocid anion between free (X-) and complexed (BaX+) states in methanol can be monitored from the temperature-dependent line shapes of the proton resonances at superconducting fields. The exchange rates are independent of the reactant concentrations and are characteristic of a rate-determining dissociation of BaX+ in methanol solution with activation parameters delta H++ = 6.5 kcal mol-1 (25 degrees) and delta S++ = -20.0 cal mol-1 degree -1 (1 cal = 4.184 J). The rate constants for dissociation and formation of BaX+ complex in methanol, 25 degrees, are 5.2 X 10(3) sec-1 and 1.5 X 10(10) M-1 sec-1, respectively. These studies were extended to derive the activation parameters for the exchange of lasalocid anion between BaX+ and NaX and between BaX+ and HX in methanol, while the exchange among HX, X-, and NaX is too rapid to be monitored on the time scale of nuclear magnetic resonance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpha S. R., Brady A. H. Optical activity and conformation of the cation carrier X537A. J Am Chem Soc. 1973 Oct 17;95(21):7043–7049. doi: 10.1021/ja00802a027. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Pressman B. C. Kinetics of transport of divalent cations across sarcoplasmic reticulum vesicles induced by ionophores. Biochem Biophys Res Commun. 1972 Oct 6;49(1):292–298. doi: 10.1016/0006-291x(72)90043-5. [DOI] [PubMed] [Google Scholar]
- Cornelius G., Gärtner W., Haynes D. H. Cation complexation by valinomycin- and nigericin-type inophores registered by the fluorescence signal of Tl+. Biochemistry. 1974 Jul 16;13(15):3052–3057. doi: 10.1021/bi00712a009. [DOI] [PubMed] [Google Scholar]
- Deber C. M., Pfieffer D. R. Ionophore A23187. Solution conformations of the calcium complex and free acid deduced from proton and carbon-13 nuclear magnetic resonance studies. Biochemistry. 1976 Jan 13;15(1):132–141. doi: 10.1021/bi00646a020. [DOI] [PubMed] [Google Scholar]
- Degani H., Friedman H. L. Ion binding by X-537A. Formulas, formation constants, and spectra of complexes. Biochemistry. 1974 Nov 19;13(24):5022–5032. doi: 10.1021/bi00721a025. [DOI] [PubMed] [Google Scholar]
- Degani H., Friedman H. L. Ion binding by X-537A. Rates of complexation of Ni2+ and Mn2+ in methanol. Biochemistry. 1975 Aug 26;14(17):3755–3761. doi: 10.1021/bi00688a006. [DOI] [PubMed] [Google Scholar]
- Haynes D. H., Pressman B. C. X537A: a Ca2+ ionophore with a polarity-dependent and complexation-dependent fluorescence signal. J Membr Biol. 1974;16(2):195–205. doi: 10.1007/BF01872414. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Herrin J., Liu S. J., Paul I. C. The crystal and molecular structure of the barium salt of an antibiotic containing a high proportion of oxygen. J Am Chem Soc. 1970 Jul 15;92(14):4428–4435. doi: 10.1021/ja00717a047. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Shen C. Structural and kinetic studies of lasalocid A (X537A) and its silver, sodium, and barium salts in nonpolar solvents. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1786–1790. doi: 10.1073/pnas.73.6.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer D. R., Reed P. W., Lardy H. A. Ultraviolet and fluorescent spectral properties of the divalent cation ionophore A23187 and its metal ion complexes. Biochemistry. 1974 Sep 10;13(19):4007–4014. doi: 10.1021/bi00716a029. [DOI] [PubMed] [Google Scholar]
- Westley J. W. A proposed numbering system for polyether antibiotics. J Antibiot (Tokyo) 1976 May;29(5):584–586. doi: 10.7164/antibiotics.29.584. [DOI] [PubMed] [Google Scholar]


