Abstract
Background
We describe the organization of a prospective, randomized, multicenter trial comparing the effectiveness of open popliteal artery aneurysm repair (OPAR) and endovascular popliteal artery aneurysm repair (EPAR) of asymptomatic popliteal artery aneurysms (PAAs) as an example for how to use the Vascular Quality Initiative (VQI) framework. Given that many centers participate in the VQI, this model can be used to perform multicenters’ prospective trials on very modest budget.
Methods
VQI prospectively collects data on many vascular procedures. These data include many important perioperative, intraoperative, and postoperative details regarding both patients and their procedures. We describe a study where minimal changes to the collected data by participating centers can provide level-1 evidence regarding a significant clinical question. Data will be collected using modified VQI forms within the existing VQI data reporting structure. We plan to enroll 148 patients with asymptomatic PAAs into the open and endovascular surgery cohorts. Patients from participating VQI centers will be randomized 1:1 to either OPAR or EPAR and will be followed for an average of 2.5 years. Our primary hypothesis is that major adverse limb event–free survival is lower in the EPAR cohort and that EPAR is associated with more secondary interventions, improved quality of life, and decreased length of stay. The budget for this trial is fixed at $10,000/year for the course of the study, and the trial is judged to be feasible because of the functionality of the VQI platform.
Conclusions
Using the existing VQI infrastructure, Open versus Endovascular Repair of Popliteal Artery Aneurysm will provide level 1 data for PAA treatment on a modest budget. The proposed trial has an adequately powered comparative design that will use objective performance goals to describe limb-related morbidity and procedural reintervention rates.
INTRODUCTION
The Society of Vascular Surgery Vascular Quality Initiative (VQI) was launched in 20111 based on the success of the Vascular Group of New England (VSGNE). The VQI currently includes 290 centers and is composed of 16 collaborative regional centers.2 The purpose of the VQI is to “provide benchmark reports” to the participating centers and surgeons to improve quality of vascular care.1 Cronenwett et al.1 proposed many advantages of participating in this initiative including the opportunity to participate in clinical outcome projects where information about many index procedures is being stored. As the data on these procedures are prospectively collected, minor adjustments to the data being collected can be used to answer a clinical question or conduct prospective randomized trial. Here we describe the first multicenter, prospectively collected, randomized trial where the machinery of VQI will be used to collect level-1 data on a very modest budget. In this manner, this article describes another way to use the VQI database.
Popliteal artery aneurysms (PAAs) are the most common peripheral arterial aneurysms and are associated with significant morbidity.3 In patients with asymptomatic PAA, elective repair has been recommended for PAA > 2 cm and for smaller aneurysms with associated mural thrombus.3 Current treatment options include open popliteal artery aneurysm repair (OPAR) using surgical bypass with aneurysm exclusion and endovascular popliteal artery aneurysm repair (EPAR) using a stent graft.
Few studies have directly compared outcomes after OPAR with EPAR and with different methodologies4–9and there is only one published, prospective, randomized trial that evaluated outcomes after OPAR and EPAR that included only 30 patients.5
Published comparative studies of current PAA management suggest that clinical equipoise exists in the choice of treatment for patients with PAA.5,10,11 The decision to undertake OPAR or EPAR varies widely among practitioners, and is based on a range of factors including disease pattern, availability of autogenous conduit, surgical and endovascular skill sets, access to an appropriate procedural environment, and practitioner bias. The paucity of strong comparative data further clouds the decision process. An adequately powered, prospective, comparative study contrasting OPAR with EPAR would guide vascular specialists in their clinical decision making for treatment of PAA. Such a multicenter randomized trial requires significant resources. In parallel, fiscal issues facing the US government have hampered the ability to obtain federal funding for clinical research and trials. These obstacles led us to attempt to organize a randomized clinical trial within the VQI consortium on a modest budget.
The Open versus Endovascular Repair of Popliteal Artery Aneurysm (OVERPAR) trial, using objective performance guidelines (OPG)–approved limb specific end points12 will take advantage of the currently functioning data collection structure of the VQI with minimal alterations in the already existing and operational input fields. As such, this trial will be conducted at a substantially lower cost than would be required were it to be funded by an entity such as the National Institutes of Health (NIH). Hereby, we describe the organization of a multicenter randomized trial using VQI infrastructure as a proof of concept in our current era of cost containment.
METHODS
Study Plan
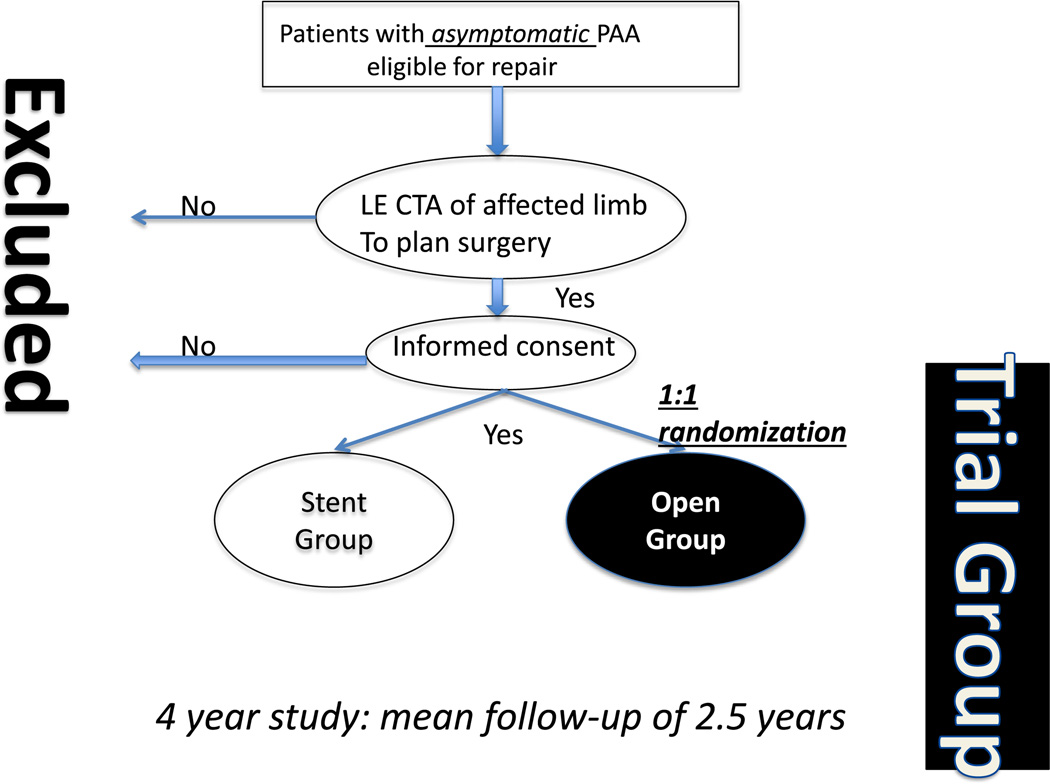
The OVERPAR trial is a prospective, randomized, open-label (2-arm), multicenter, superiority trial comparing the effectiveness of OPAR and EPAR in patients with asymptomatic clinically significant (>2.0 cm) PAA. Patients with asymptomatic PAA, referred to participating centers, are screened and standard-of-care lower extremity computed tomography angiography performed to assess for anatomic eligibility (Fig. 1). Patients are consented and randomized, 1:1, to either OPAR or EPAR. The objective of this trial was to compare the primary end point event rates of patients with PAA randomized to OPAR or EPAR. As this is an intention-to-treat study, every patient who consented to participate in the study will be followed in the surgical arm they were ultimately treated.
Fig. 1.
OVERPAR trial schematic. Patients with asymptomatic PAA are recruited and then randomized 1:1 to either OPAR or EPAR. LE CTA, lower extremity computed tomography angiography.
Primary and Secondary End points
Primary end point
The primary end points were chosen based on the primary hypothesis that major adverse limb event (MALE)–free survival in the EPAR group will be lower than that in the OPAR group. MALE was defined as any major limb amputation (above-ankle amputation of the index limb) or reintervention (new bypass graft, jump and/or interposition graft revision, angioplasty/stent/stent graft, or thrombectomy and/or thrombolysis). The MALE end points were adapted from the published OPG guidelines to include minor and major interventions, and the VQI database has been minimally adjusted to track these interventions.12
Secondary end points
Secondary end points were chosen to evaluate the secondary hypotheses of this trial; EPAR will be associated with more secondary interventions, increased independent living status, increased ambulatory status, decreased length of stay, and a comparatively better quality of life. These secondary end points are divided into 3 categories and are depicted in Table I.
Table I.
The secondary end points to be measured in OVERPAR trial
| Clinical categories |
|
| Functional and quality of life measure | Vascular quality of life |
| Resource utilization | Length of stay |
MACE, major adverse composite events.
Patient Population
One hundred forty-eight subjects (74 patients in each group) aged 35 or older with asymptomatic PAA undergoing OPAR or EPAR are being recruited from VQI sites. Patients are being randomized 1:1 to undergo either OPAR or EPAR (Fig. 1). At the time of article preparation, there were 21 VQI centers participating in this trial. Fourteen of these centers were members of VSGNE whereas the remainder were participants of other VQI centers, outside of New England (Table II). Before enrollment, institutional review board (IRB) was obtained at each center. Every center used the study protocol and informed consent form created by the core team (MHE, GD, MM, PPG, and AF) to apply for their IRB. In most institutions, fees were waived as this is an investigator-initiated trial.
Table II.
Participating Vascular Quality Initiative Centers at the time of the article preparation
| Center’s name | City, state |
|---|---|
| Albany Vascular Group | Albany, NY |
| Bay State Medical Center* | Springfield, MA |
| BI-Deaconess Hospital* | Boston, MA |
| Boston Medical Center* | Boston, MA |
| Rhode Island University Medical Center* | Providence, RI |
| Brigham and Women’s Hospital* | Boston, MA |
| Cardiothoracic Surgical Associates* | Manchester, NH |
| Charlton Memorial Hospital* | Falls River, MA |
| Danbury Medical Center* | Danbury, CT |
| Dartmouth Medical Center* | London, NH |
| Hartford Hospital* | Hartford, CT |
| Henry Ford Hospitals | Detroit, MI |
| Louisiana State University | Shreveport, LA |
| Maine Medical Center* | Portland, ME |
| Massachusetts General Hospital* | Boston, MA |
| St. Elizabeth’s Hospital* | Brighton, MA |
| Tufts Medical Center* | Boston, MA |
| University of Indiana | Indianapolis, IN |
| University of Vermont* | Burlington, VT |
VSGNE centers.
Inclusion and Exclusion Criteria
Patients with asymptomatic >2-cm PAA who are candidates for both OPAR and EPAR based on clinical and anatomic criteria are being enrolled. Inclusion and exclusion criteria are detailed in Table III. All the inclusion and none of the exclusion criteria must be met to qualify for enrollment. The most important exclusion criterion is that of PAA presenting with symptoms. Symptomatic patients will be treated with standard of care—open surgery or thrombolysis as decided by usual clinical judgment.
Table III.
Inclusion and exclusion criteria for OVERPAR trial
| Inclusion criteria (all must be present for inclusion): 1. Age ≥ 35 years | |
| 2. | Popliteal artery aneurysm ≥2 cm in diameter with or without the presence of mural thrombus |
| 3. | Candidates for both OPEN and EPAR as judged by the enrolling investigator. |
| 4. | Greater than 2-cm length of normal superficial femoral artery distal to the deep femoral artery takeoff and >2-cm length of normal popliteal artery proximal to the first patent tibial artery. |
| 5. | Patient signs consent to participate in the trial. |
| Exclusion criteria (none of these can be met for inclusion): 1. Popliteal artery thrombosis | |
| 2. | Popliteal artery aneurysm causing symptomatic thromboembolic disease or compressive symptoms. |
| 3. | Superficial femoral artery occlusion |
| 4. | Less than 2-cm length of normal artery to accommodate stent-graft seal |
| 5. | Life expectancy of less than 2 years. |
| 6. | Deemed excessive risk for surgical bypass (defined as prohibitive operative risk by formal preprocedural cardiac risk assessment undertaken by a cardiologist or internist according to established AHA guideline criteria). |
| 7. | A documented hypercoagulable state (defined as a known blood disorder associated with venous or arterial thrombosis). |
| 8. | Any infrainguinal revascularization procedure on index leg within 12 weeks before treatment initiation. |
| 9. | Current immunosuppressive medication, chemotherapy, or radiation therapy. |
| 10. | Absolute contraindication to iodinated contrast because of prior near-fatal anaphylactoid reaction (laryngospasm, bronchospasm, cardiorespiratory collapse, or equivalent), and which would preclude patient from participating in angiographic procedures. |
| 11. | Allergy to stainless steel or nitinol. |
| 12. | Pregnancy or lactation. |
| 13. | Inability or refusal to provide informed consent. |
AHA, American Heart Association.
Trial Therapies
OPAR includes standard-of-care infrainguinal bypass with PAA exclusion. Details of the procedure such as the location of the proximal and distal anastomoses, surgical approach, surgical technique, and the choice of conduit is left up to the surgeon. Both autogenous vein and prosthetic conduit are being allowed. Endovascular repair includes the deployment of a self-expanding stent graft. Vascular access location, details of stent-graft deployment, the number of stent grafts placed, and type of self-expanding stent graft used will be left up to the operator. Percutaneous or femoral cutdown-based vascular access is being allowed. After deployment, stent graft angioplasty is required. The choice, degree, reversal of anticoagulation, and choice of postoperative antiplatelet medication is left up to the operator. Our pragmatic approach that allows for inclusion of a range of surgical and endovascular techniques is designed to be reflective of the current clinical practice. In addition, all the details of the operation and/or intervention will be collected for patients enrolled in the OVERPAR trial. This will require modification of the current VQI forms. The modifications to the forms are minor but allow for collection of the specific details of each operation. This will allow for further subgroup and detailed analyses.
Randomization
Within each center, subjects are being randomized in a 1:1 ratio to 1 of the 2 treatment arms using a permuted block randomization plan with block size of 6 stratified according to clinical center. Electronically randomized sheets are generated for each center by biostatisticians at Boston Medical Center. Once a patient is consented at a trial site, the trial site coordinator calls the central study coordinator at Boston Medical Center, Boston, MA who accesses this electronic spread sheet and relays the result of randomization (OPAR or EPAR) to the requesting center.
Subjects in whom a randomly assigned procedure (OPAR or EPAR) is attempted, even if not completed, are considered appropriately treated as intended, irrespective of the corrective surgery or future procedures attempted in that subject. Crossover is defined as completion of open surgery when randomized to EPAR or vice versa without first attempting the randomly assigned procedure.
Study Procedures and Follow-Up
Intraoperative success is assessed by the surgeon and based on the surgeon’s clinical judgment. This may include postprocedural angiography, duplex, intraoperative Doppler, or distal pulse palpation.
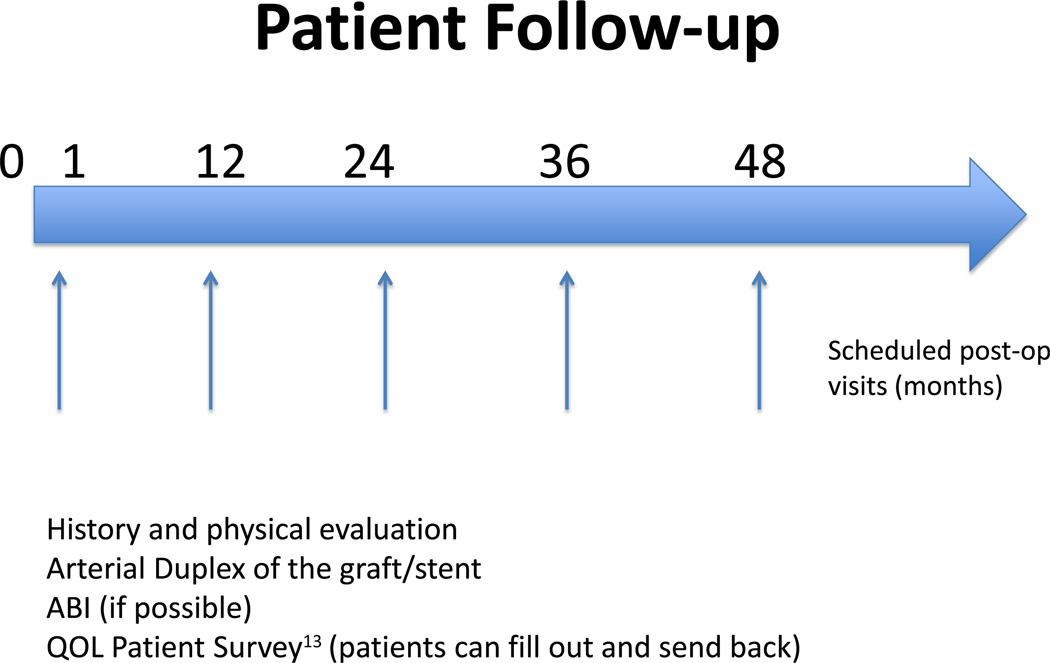
Follow-up is at 1 month, 1 year, 2 years, 3 years, and 4 years (Fig. 2). In that regard, the follow-up is more robust than the standard VQI follow-up period. This is another modification of VQI created to accommodate this trial. During follow-up, the patients will undergo a standard-of-care history and physical examination, graft duplex, and ankle-brachial indices. Clinical and laboratory data are collected using modified VQI forms created to accommodate this trial and in accordance with the practice guidelines of the participating centers. Dates of reintervention, amputation, or death are recorded. Graft and/or stent revision follow the current practice guidelines of each participating centers and enrolling clinicians. This study does not detail any specific guidelines for when such revision is required, and the revisions are at the discretion of the enrolling physicians. The specific indication for this revision will be included in the ultimate analysis and as part of the analysis of our secondary end points. VascuQOL13 (Vascular Quality of Life) surveys are being completed during the initial visit, at 1 month and annually as noted in Figure 2. Deidentified QOL surveys are being stored at Boston Medical Center for adjudication and final analysis.
Fig. 2.
Patients recruited into OVERPAR are followed at 1 month, and then annually. At each visit the data noted are to be collected by M2S.
Vascular Quality Initiative
VQI is a multicenter collaborative of regional vascular quality groups where data are prospectively collected on a number of open and endovascular procedures.14 The VQI and data collection are thoroughly detailed on the link previously mentioned.2 For this trial, data are collected using a computerized VQI interface currently in use by participating VQI sites. Data entry is performed at the sites by data coordinators, physician extenders, and physicians. Each hospital within VQI pays a fee to participate and employs an individual to locally oversee data entry. The database is managed by M2S (West Lebanon, NH). Deidentified data are provided to member institutions on a regular basis for benchmarking at biannual meetings of local VQI groups. The OVERPAR trial does not significantly tax the VQI endeavor as only minor changes to the VQI forms have been required for this trial. Yearly follow-up beyond 1 year is required as an average patient follow-up will be 2.5 years. These changes from typical VQI collection protocols are being supported without further charge to the centers by M2S.
Statistical Methods
The first phase of analysis will include a description of study variables. This step includes generating summary statistics for study characteristics. Summary statistics will be generated and assessed for the total sample size. The quartiles and median time-to-event outcomes (e.g., MALE-free survival, or MALE-postoperative day) will be estimated using the Kaplan–Meier method and will be reported with 2-sided 95% confidence intervals for each intervention arm.
For the time to the primary event, Kaplan–Meier product–limit estimates of the event-free survival time distributions will be computed for each group, and treatment groups will be compared using the nonparametric log-rank test. The hazard ratio and its 95% confidence interval will be estimated by a Cox regression model in which time to MALE + all deaths is the dependent variable and intervention is the independent variable.
The primary analysis will be conducted on an intention-to-treat basis (i.e., based on their randomization status), which includes all subjects randomized, after the last subject has been followed for 1 year or is off study. Other time-to-event outcomes will be analyzed similarly as the primary analysis. The previously mentioned analyses will also be run on the per-protocol basis (i.e., a comparison of treatment groups that includes only those patients who completed originally allocated).
Study Organization and Funding
The trial is being supported by the New England Society for Vascular Surgery which, in conjunction with VSGNE, has advertised compelling support for this endeavor through e-mail notification of members and presentation of the trial concept at its annual meetings.
A $10,000/year grant has been awarded to the principal investigators with contingent funding for a period of 3 years for the conduct of this trial. Although the trial will undoubtedly continue for a total of 4 years, no patient recruitment will occur beyond 3 years and thereby data will be generated as per VQI protocols irrespective of additional funding.
DISCUSSION
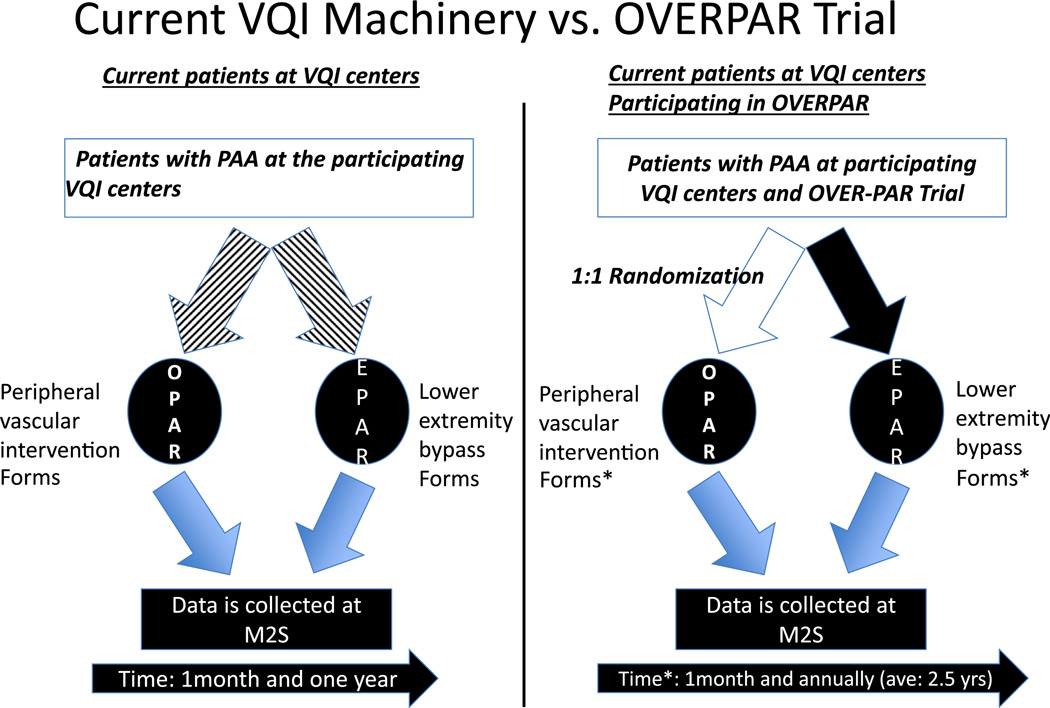
Organization of a multicenter, prospective, randomized trial is a costly endeavor. In that regard, any such trial, in addition to a core of interesting and relevant clinical questions will require significant organization for site and patient recruitment, data collection, and data analysis. VQI machinery easily provides patient and site recruitment and data collection pieces of this. To accommodate data collection, VQI forms need to be modified to answer the relevant clinical question and collection of the data needs to be extended to allow for a more meaningful analysis. In that regard, the OVERPAR trial clearly shows a viable example of another use of VQI machinery. The OVERPAR trial aims to be the largest trial ever performed in patients with asymptomatic PAA. This multicenter, prospective, randomized trial is designed to be adequately powered and will use objective performance goals to describe limb-related morbidity and procedural reintervention rates. The trial will be conducted using the currently existing VQI data collection software. On its completion, this comparative effectiveness study will provide level-1 evidence that will guide care of patients with PAA. This study will be a model for future multicenter trial using national quality improvement programs for clinical trial infrastructure. Minor modifications to the current data collection practices of centers and surgeons already participating in VQI to accommodate OVERPAR trial are schematically drawn in Figure 3. These changes are minor and as most centers regularly follow patients beyond 1 year postoperatively, this trial closely mirrors current practice patterns.
Fig. 3.
Comparison of the current practices for treatment of PAA at VQI centers and the minimal requirements that are needed to perform this study.
One of the limitations of this trial is the rate of postoperative follow-up in VQI. VQI requires high compliance with at least 80% follow-up but in practice these goals are not met. Given that these patients are registered as OVERPAR patients, we anticipate that the follow-up will be more rigorous. In addition, in our study-design, our power calculation accounted for 20% lost to follow-up. We, therefore, believe that these measures will improve the compliance with follow-up.
Another concern is the cost that each participating center incurs by participating in these trials. The cost to the center is minimal and includes time spent by the data collector in collecting the additional information unique to the OVERPAR trial. Participating center’s IRB costs in all the centers have been waived, given that each center acts as their own IRB and by noting that this study is an “investigator-initiated trial”. We provide each center with a copy of protocol, informed consent, QOL questionnaire, and provide assistance with the preparation of IRB forms. In addition, the budget has small allowance for these extra expenses.
The proposed budget for this study is $10,000/year for 3 years. It is important to compare this budgetary requirement to that of an externally funded multicenter trial. Although it is exceedingly difficult to make a direct comparison to similar NIH-funded trials, in 2012 the average cost of research grants was for $450,000/year.15 Using this value as a reference, a similar NIH-funded trial would require at least $1,000,000. Because of the existing VQI infrastructure, we aim to perform this trial on a much more limited budget. In that regard, this is a trial of concept that can potentially act as a model for future multicenter cooperative trials to answer key clinical questions. Clearly, similar clinical trials can be designed and using VQI platform be performed on a very modest budget. In this regard, VQI presents another clearly important role in the delivery of quality vascular surgery care.
We are actively seeking the participation of other VQI centers by presenting this study at different scientific forums and thereby expect to increase the enrollment of this trial to achieve our goal in a shorter time. We are confident that with cooperation of our colleagues these numbers are attainable.
CONCLUSIONS
This low budget trial will be the largest multicenter trial of patients with asymptomatic PAA that will provide level-1 data using VQI machinery. This study will be a model for future multicenter trials using VQI infrastructure and cooperative VQI surgeons.
REFERENCES
- 1.Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–1537. doi: 10.1016/j.jvs.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 2. http://www.vascularqualityinitiative.org.
- 3.Galland RB. History of the management of popliteal artery aneurysms. Eur J Vasc Endovasc Surg. 2008;35:466–472. doi: 10.1016/j.ejvs.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Tielliu IF, Verhoeven EL, Zeebregts CJ, et al. Endovascular treatment of popliteal artery aneurysms: results of a prospective cohort study. J Vasc Surg. 2005;41:561–567. doi: 10.1016/j.jvs.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 5.Antonello M, Frigati P, Battocchio P, et al. Open repair versus endovascular treatment for asymptomatic popliteal artery aneurysm: results of a prospective randomized study. J Vasc Surg. 2005;42:185–193. doi: 10.1016/j.jvs.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 6.Midy D, Berard X, Ferdani M, et al. A retrospective multicenter study of endovascular treatment of popliteal artery aneurysm. J Vasc Surg. 2010;51:850–856. doi: 10.1016/j.jvs.2009.10.107. [DOI] [PubMed] [Google Scholar]
- 7.Lovegrove RE, Javid M, Magee TR, et al. Endovascular and open approaches to non-thrombosed popliteal aneurysm repair: a meta-analysis. Eur J Vasc Endovasc Surg. 2008;36:96–100. doi: 10.1016/j.ejvs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Pulli R, Dorigo W, Fargion A, et al. Comparison of early and midterm results of open and endovascular treatment of popliteal artery aneurysms. Ann Vasc Surg. 2012;26:809–818. doi: 10.1016/j.avsg.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Pulli R, Dorigo W, Castelli P, et al. A multicentric experience with open surgical repair and endovascular exclusion of popliteal artery aneurysms. Eur J Vasc Endovasc Surg. 2012;45:357–363. doi: 10.1016/j.ejvs.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Tsilimparis N, Dayama A, Ricotta JJ., 2nd Open and endovascular repair of popliteal artery aneurysms: tabular review of the literature. Ann Vasc Surg. 2013;27:259–265. doi: 10.1016/j.avsg.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg. 2013;57:1306–1310. doi: 10.1016/j.jvs.2012.10.122. [DOI] [PubMed] [Google Scholar]
- 12.Goodney PP, Schanzer A, Demartino RR, et al. Validation of the Society for Vascular Surgery’s objective performance goals for critical limb ischemia in everyday vascular surgery practice. J Vasc Surg. 2011;54:100, 108.e4. doi: 10.1016/j.jvs.2010.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan MB, Crayford T, Murrin B, et al. Developing the Vascular Quality of Life Questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg. 2001;33:679–687. doi: 10.1067/mva.2001.112326. [DOI] [PubMed] [Google Scholar]
- 14.Hirshberg A, Wall MJ, Johnston RH, Jr, et al. Transcervical gunshot injuries. Am J Surg. 1994;167:309–312. doi: 10.1016/0002-9610(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 15.Rose SL, Mooe EF. Trauma angiography: the use of clinical findings to improve patient selection and case presentation. J Trauma. 1988;28:240. [PubMed] [Google Scholar]