Summary
We constructed a multiple myeloma (MM)-specific gene panel for targeted sequencing and investigated 72 untreated high-risk (del17p) MM patients. Mutations were identified in 78% of the patients. While the majority of studied genes were mutated at similar frequency to published literature, the prevalence of TP53 mutation was increased (28%) and no mutations were found in FAM46C. This study provides a comprehensive insight into the mutational landscape of del17p high-risk MM. Additionally, our work demonstrates the practical use of a customized sequencing panel, as an easy, cheap and fast approach to characterize the mutational profile of MM.
Keywords: myeloma, cancer genetics, DNA mutation, DRUG resistance, genetic analysis
In multiple myeloma (MM), risk assessment based on individual tumour cytogenetics, fluorescence in-situ hybridization (FISH) and gene expression profiling is already established, however these prognostic indices fail to capitalize on the technological revolution in speed and reduced cost of genomic next generation sequencing (NGS). Two recent large sequencing studies on 270 primary MM samples have essentially defined the mutation landscape for this disease (Bolli et al, 2014; Lohr et al, 2014). The existence of baseline clonal heterogeneity, linear and branching evolution and therapeutic selection resulting in clonal tides suggest an imminent need for rapid, accurate and comprehensive evaluation of patient genomic profiles to guide precision therapy. Discoveries on the role of CRBN, IKZF1 and IKZF3 for the efficacy of immunomodulatory drugs (IMiDs), XBP1s and IRE1 in proteasome inhibitor therapy and druggable targets, such as BRAF mutations in MM, have had a major impact on the understanding of the disease and emphasize the need for tailored treatment. With this in mind, we generated a 47-gene MM Mutation Panel (M3P) that requires small amounts of DNA and provides deep coverage results in clinically meaningful timeframes.
Material and methods
Tumour DNA from 72 newly diagnosed MM patients with del17p was collected by the German MM study group (DSMM), including 40 corresponding germline samples. Plasma cells were purified using anti-CD138+ immunomagnetic beads (median purity 95%). DNA was extracted from cell pellets stored at −80°C (AllPrep DNA/RNA Mini Kit, Qiagen, Venlo, The Netherlands). Baseline FISH confirmed del17p in all 72 cases. In a subset, additional abnormalities were screened with 35% (24/68) having gain of 1q21, 81% (58/72) del(13q), 29% (19/66) t(4;14), 40%(16/40) t(11;14) and 3%(1/36) t(14;16). To generate M3P we selected the top mutated genes (≥3%), (Lohr et al, 2014), expressed in public datasets (http://www.broad.mit.edu/mmgp), ending up with a list of 39 genes. Next we added eight genes targeted by commonly used therapies, associated with resistance to IMiDs (CRBN, CUL4A, CUL4B, DDB1 and IRF4), proteasome inhibitors (PSMG2, PSMB5) and glucocorticoid therapies (NR3C1). Overall, 2875 amplicons, covering 96% of the M3P exons (Tables SI, SII), were analysed per sample, multiplexed in two library preparations, using 20 ng of input DNA. Enriched templates were sequenced using semiconductor technology (PGM™, Life Technologies, Carlsbad, CA, USA) and analysed with Ion Reporter Software v1.6 (Life Technologies). A minimum of 20× depth coverage was required. Mutation calls were considered positive when called by ≥10% variant reads. In already characterized cancer-related mutations (COSMIC database, http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/), the threshold was reduced to 3%. Variants called in samples without corresponding germline available were excluded if listed in the Single Nucleotide Polymorphism Database (dbSNP, http://www.ncbi.nlm.nih.gov/SNP/), as well as single nucleotide variants (SNV) that were identified in multiple samples, unless described in COSMIC (Table I).
Table I.
Genes included in the Multiple Myeloma Mutation Panel (M3P) v1·0.
| M3P | ||||
|---|---|---|---|---|
| ADAMTS9 | DIS3 | IRF4 | NRAS | TP53 |
| ANK2 | DNAH5 | KRAS | PRDM1 | TRAF3 |
| ATM | EGFR | LRP1B | PSMB5 | TRIP12 |
| BRAF | EGR1 | LTN1 | PSMG2 | VCAN |
| CCND1 | FAM46C | LYST | PTPRD | XBP1 |
| CRBN | FAT1 | MECOM | RASA2 | ZFHX3 |
| CUL4A | FAT3 | KMT2C | RB1 | ZFHX4 |
| CUL4B | FAT4 | NBPF1 | SP140 | |
| CYLD | FRYL | NEB | SPEN | |
| DDB1 | HECW1 | NR3C1 | TIAM1 | |
Results and discussion
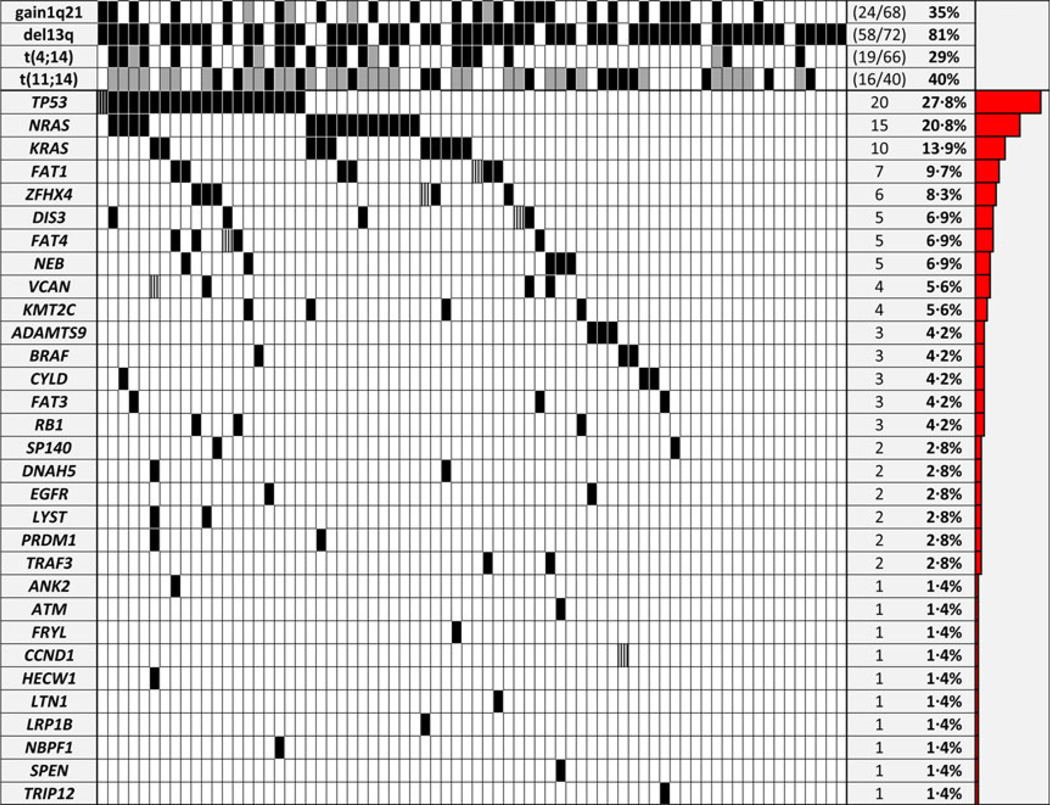
NGS has served as a catalyst in understanding the genomic landscape and subclonal complexity in haematological diseases. Individual genomic profiling prior to therapy and the tracking of clonal evolution during treatment will probably be the basis of individualized treatment decisions and the standard of care in the very near future. We capitalized on the availability of fast turnaround NGS technologies and early access to one of the recent large MM whole exome sequencing efforts to design a comprehensive, MM-specific mutation panel (M3P), containing the most frequently mutated genes and a selection of clinically relevant genes. A mean sequencing depth coverage of 298× (tumour) and 221× (germline) was achieved. In total, 123 nonsynonymous missense/nonsense SNVs and three indels were detected. 124 mutations were rated by Polyphene-2, SIFT and Provean, 108 were predicted damaging (87%) and 53 (43%) were listed in the COSMIC database (Fig 1). The average mutation prevalence was 1·75 (range:0–8). Mutations were found in 78% (56/72) of the patients and in 66% (31/47) of the genes. More than one mutation in one patient was present in seven genes including CCND (Pro18Ser, Cys47Gly), DIS3 (Tyr246Asp, Gln931*), FAT1 (Asp3799Gly, Arg549Trp), FAT4 (Leu784Met, Thr4281Ala), VCAN (Gln1009*, Ser2445Phe), ZFHX4 (Glu3379Asp, Glu3380*) and TP53 (Lys132Asn, Met237Ile, Val173Met, (6%/4%/5% VR). TP53 was the most frequently mutated gene (27·8%), followed by NRAS (20·8%) and KRAS (13·9%). Overall, the MAPK/ERK pathway was mutated in 38%, including three combined NRAS and KRAS mutations and three BRAF mutations, two of which were at the known targetable Val600E position. NRAS and KRAS is less frequently mutated in high-risk MM, including del17p (Chng et al, 2008), and more often in relapsed patients (Lohr et al, 2014), both explaining the lower prevalence in our untreated del17p cohort. TP53 is commonly assumed to be the major target of del17p MM (Munshi & Avet-Loiseau, 2011). Previous work suggested that TP53 mutations are correlated (Bolli et al, 2014) with or even be exclusively present in del17p (Lode et al, 2010) and, indeed, it was more prevalent than in published data from unselected MM patients (Bolli et al, 2014; Lohr et al, 2014). TP53 mutation has been associated with impaired event-free survival (Bolli et al, 2014) and overall survival (OS) (Chng et al, 2007) in non-del17p cohorts; however, as survival data was available in only 61 cases no significant difference in progression-free survival (n = 50) or OS (n = 61) by TP53 status could be observed (Figure S1), as the observation time was variable (follow up 60–2550 days, median 598 days) and numbers might still be too small to derive meaningful conclusions. DIS3 was mutated in five patients (Arg418Gly, Ser756Phe, His764Asp, Asp784Asn), including one case with two mutations (Tyr246Asp and Gln931*). DIS3 mutations have been reported to be exclusively present in t(4;14) and t (11;14) patients (Walker et al, 2012) and to be del13q14-dependent. In our del17p cohort all DIS3-mutated patients harboured a del13q, however only three of five patients had a t(4;14) or t(11;14). Truncating mutations of SP140, involved in the pathogenesis of acute leukaemia and viral infection, have been recently described in MM (Bolli et al, 2014), and two (Arg576*, Glu627*) were present in our cohort. FAT family genes showed a significant number of mutations, with FAT1 as the most frequently mutated (9%), followed by FAT4 (7%) and FAT3 (4%). Alterations of FAT cadherins (Bolli et al, 2014; Messina et al, 2014) and other large M3P genes, such as the dynein protein (DNAH5; Morgan et al, 2012) (3%), the low density lipoprotein receptorrelated protein 1B (LRPB1P; Leich et al, 2013) (1%) and the histone modifying enzyme KMT2C (MLL3; Chapman et al, 2011) (6%), have been reported in cancer, including MM. ZFHX4 (8%) has not yet been associated with MM. As a cautionary note, the frequent occurrence of mutations in implausible genes in sequencing studies has been identified as a confounding issue (Lawrence et al, 2013), thus functional investigation of these findings is needed to determine the relevance to MM. FAM46C is recurrently mutated in MM, however, understanding of the function of this gene in particular is very limited. An 9% overall mutation prevalence (Boyd et al, 2011; Bolli et al, 2014; Lohr et al, 2014) and impaired survival by abnormal FAM46C (Boyd et al, 2011) have been reported. Interestingly, no FAM46C mutation was seen in our cohort. The correlation of FAM46C mutation status and ploidy is controversial: Bolli et al (2014) reported a correlation of FAM46C mutations to hyperdiploid MM whereas Lohr et al (2014) did not find a significant correlation. Ploidy information in our cohort was incomplete, however, del17p is enriched in non-hyperdiploid MM (Van Wier et al, 2013), which might partly explain the lack of FAM46C mutations in our del17p-restricted cohort. Alternatively, TP53 and FAM46C might activate similar pathways, which would make mutations in both genes redundant. In published data FAM46C mutations seem to be extremely uncommon in del17p/mutant TP53 MM with a mean prevalence of 0·4% (Van Wier et al, 2013; Bolli et al, 2014; Lohr et al, 2014). Almost two-thirds of patients with FAM46C mutations were untreated in previous reports (Bolli et al, 2014; Lohr et al, 2014). We therefore suggest that FAM46C mutations might be a marker for lower risk disease rather than of progression, however further investigation is needed. Of note, no mutations in genes related to drug resistance were seen above our chosen cut-off point. However, mutations were identified in minor subclones below cut off threshold in CUL4B (Asp426Gly, 9% VR), DDB1 (Ala971Asp, 4% VR) and IRF4 (Gly43Ser, 6% VR), affecting the CRBN pathway, and in the steroid receptor NR3C1 (Lys772Asn, 8% VR). The CUL4B, IRF4 and NR3C1 mutations were predicted to be damaging by polyphene-2 or SIFT. Furthermore, according to the Universal Protein Resource database (www.uniprot. org) the identified DDB1 mutation potentially affects interaction with CUL4A, which is part of the ubiquitin ligase complex of CRBN, the IRF4 mutation is located in the DNA binding and the NR3C1 mutation in the steroid binding region, thus these findings provide a potential source of later IMiD and steroid drug resistance, respectively.
Fig 1.
Mutation prevalence in untreated patients with newly diagnosed and untreated del17p multiple myeloma and fluorescence in situ hybridization (FISH) results. All patients had a confirmed del 17p. Grey shading indicates lack of FISH data; black and striped shading indicates, multiple mutations in the same gene and timepoint. No mutation above the chosen threshold was found in CRBN, CUL4A/B, DDB1, EGR1, FAM46C, IRF4, MECOM, NR3C1, PSMB5, PSMG2, PTPRD, RASA2, TIAM1, XBP1 or ZFHX3.
In summary, we present the first MM-specific gene panel that characterizes the individual mutation status in a fast, accurate and cost-effective manner. Minimal DNA is required to run the assay and sensitivity down to minor clonal frequencies is attainable. We believe that a targeted panel of mutation detection, such as M3P, will become a diagnostic tool of choice to further improve MM classification, track clonal variability, more precisely predict prognosis and better guide treatment decisions in times of genetic-based risk assessment and personalized medicine.
Supplementary Material
Acknowledgements
This work is supported by grants R01 CA83724, CA167511 and CA183968, ECOG CA 21115T, Predolin Foundation, Mayo Clinic Cancer Center, the Mayo Foundation and the DFG (Ko 4604/1-1 to KMK, BU 1339/7-2 and BU 1339/3-1 to LBu, and LA 2414/2-1 to CL); EB has support by the Henry Predolin Foundation, the Marriott Specialized Workforce Development Awards in Individualized Medicine and the Fraternal Order of Eagles.
RF is a Clinical Investigator of the Damon Runyon Cancer Research Fund and received a patent for the prognostication of MM based on genetic categorization of the disease. He has received consulting fees from Medtronic, Otsuka, Celgene, Genzyme, BMS, Lilly, Onyx, Binding Site, Millennium and AMGEN. He also has sponsored research from Cylene and Onyx.
Footnotes
Authorship contributions
KMK designed and performed research, analysed data and wrote the paper; EB, RF, HE and AKS designed research, analysed and interpreted data and edited the paper; MDC analysed data; CL, LBu, MK, PL, LR and SK contributed sample and data; LBr, CXS, YXZ, JS, JO, JBE, PJ, JM, SVW and GA performed research and analysed data; All authors revised the paper and approved submission.
Disclosure of conflicts of interest
KMK, CL, EB, JM, LBr, JBE, PL, YXZ, CXS, PJ, JS, JO, LBu, MK, MDC, SVW, GA, LR, SK, HE, AKS and EB have nothing to disclose.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig S1. Survival del17p mutant versus wildtype.
Table SI. Variant table.
Table SII. Amplicon details of the M3P gene panel.
References
- Bolli N, Avet-Loiseau H, Wedge DC, Van Loo P, Alexandrov LB, Martincorena I, Dawson KJ, Iorio F, Nik-Zainal S, Bignell GR, Hinton JW, Li Y, Tubio JM, McLaren S, S OM, Butler AP, Teague JW, Mudie L, Anderson E, Rashid N, Tai YT, Shammas MA, Sperling AS, Fulciniti M, Richardson PG, Parmigiani G, Magrangeas F, Minvielle S, Moreau P, Attal M, Facon T, Futreal PA, Anderson KC, Campbell PJ, Munshi NC. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nature Communications. 2014;5:2997. doi: 10.1038/ncomms3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd KD, Ross FM, Walker BA, Wardell CP, Tapper WJ, Chiecchio L, Dagrada G, Konn ZJ, Gregory WM, Jackson GH, Child JA, Davies FE, Morgan GJ, Group NHOS. Mapping of chromosome 1p deletions in myeloma identifies FAM46C at 1p12 and CDKN2C at 1p32.3 as being genes in regions associated with adverse survival. Clinical Cancer Research. 2011;17:7776–7784. doi: 10.1158/1078-0432.CCR-11-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, Schinzel AC, Harview CL, Brunet JP, Ahmann GJ, Adli M, Anderson KC, Ardlie KG, Auclair D, Baker A, Bergsagel PL, Bernstein BE, Drier Y, Fonseca R, Gabriel SB, Hofmeister CC, Jagannath S, Jakubowiak AJ, Krishnan A, Levy J, Liefeld T, Lonial S, Mahan S, Mfuko B, Monti S, Perkins LM, Onofrio R, Pugh TJ, Rajkumar SV, Ramos AH, Siegel DS, Sivachenko A, Stewart AK, Trudel S, Vij R, Voet D, Winckler W, Zimmerman T, Carpten J, Trent J, Hahn WC, Garraway LA, Meyerson M, Lander ES, Getz G, Golub TR. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chng WJ, Price-Troska T, Gonzalez-Paz N, Van Wier S, Jacobus S, Blood E, Henderson K, Oken M, Van Ness B, Greipp P, Rajkumar SV, Fonseca R. Clinical significance of TP53 mutation in myeloma. Leukemia. 2007;21:582–584. doi: 10.1038/sj.leu.2404524. [DOI] [PubMed] [Google Scholar]
- Chng WJ, Gonzalez-Paz N, Price-Troska T, Jacobus S, Rajkumar SV, Oken MM, Kyle RA, Henderson KJ, Van Wier S, Greipp P, Van Ness B, Fonseca R. Clinical and biological significance of RAS mutations in multiple myeloma. Leukemia. 2008;22:2280–2284. doi: 10.1038/leu.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortes ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Lee RS, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CW, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leich E, Weissbach S, Klein HU, Grieb T, Pischimarov J, Stuhmer T, Chatterjee M, Steinbrunn T, Langer C, Eilers M, Knop S, Einsele H, Bargou R, Rosenwald A. Multiple myeloma is affected by multiple and heterogeneous somatic mutations in adhesionand receptor tyrosine kinase signaling molecules. Blood Cancer Journal. 2013;3:e102. doi: 10.1038/bcj.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode L, Eveillard M, Trichet V, Soussi T, Wuilleme S, Richebourg S, Magrangeas F, Ifrah N, Campion L, Traulle C, Guilhot F, Caillot D, Marit G, Mathiot C, Facon T, Attal M, Harousseau JL, Moreau P, Minvielle S, Avet-Loiseau H. Mutations in TP53 are exclusively associated with del(17p) in multiple myeloma. Haematologica. 2010;95:1973–1976. doi: 10.3324/haematol.2010.023697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JG, Stojanov P, Carter SL, Cruz-Gordillo P, Lawrence MS, Auclair D, Sougnez C, Knoechel B, Gould J, Saksena G, Cibulskis K, McKenna A, Chapman MA, Straussman R, Levy J, Perkins LM, Keats JJ, Schumacher SE, Rosenberg M, Multiple Myeloma Research C, Getz G, Golub TR. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell. 2014;25:91–101. doi: 10.1016/j.ccr.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina M, Del Giudice I, Khiabanian H, Rossi D, Chiaretti S, Rasi S, Spina V, Holmes AB, Marinelli M, Fabbri G, Piciocchi A, Mauro FR, Guarini A, Gaidano G, Dalla-Favera R, Pasqualucci L, Rabadan R, Foa R. Genetic lesions associated with chronic lymphocytic leukemia chemo-refractoriness. Blood. 2014;123:2378–2388. doi: 10.1182/blood-2013-10-534271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nature Reviews Cancer. 2012;12:335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- Munshi NC, Avet-Loiseau H. Genomics in multiple myeloma. Clinical Cancer Research. 2011;17:1234–1242. doi: 10.1158/1078-0432.CCR-10-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wier S, Braggio E, Baker A, Ahmann G, Levy J, Carpten JD, Fonseca R. Hypodiploid multiple myeloma is characterized by more aggressive molecular markers than non-hyperdiploid multiple myeloma. Haematologica. 2013;98:1586–1592. doi: 10.3324/haematol.2012.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BA, Wardell CP, Melchor L, Hulkki S, Potter NE, Johnson DC, Fenwick K, Kozarewa I, Gonzalez D, Lord CJ, Ashworth A, Davies FE, Morgan GJ. Intraclonal heterogeneity and distinct molecular mechanisms characterize the development of t(4;14) and t(11;14) myeloma. Blood. 2012;120:1077–1086. doi: 10.1182/blood-2012-03-412981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.