Abstract
IMPORTANCE
Most out-of-hospital cardiac arrests receiving emergency medical services in the United States are treated by ambulance service providers trained in advanced life support (ALS), but supporting evidence for the use of ALS over basic life support (BLS) is limited.
OBJECTIVE
To compare the effects of BLS and ALS on outcomes after out-of-hospital cardiac arrest.
DESIGN, SETTING, AND PARTICIPANTS
Observational cohort study of a nationally representative sample of traditional Medicare beneficiaries from nonrural counties who experienced out-of-hospital cardiac arrest between January 1, 2009, and October 2, 2011, and for whom ALS or BLS ambulance services were billed to Medicare (31 292 ALS cases and 1643 BLS cases). Propensity score methods were used to compare the effects of ALS and BLS on patient survival, neurological performance, and medical spending after cardiac arrest.
MAIN OUTCOMES AND MEASURES
Survival to hospital discharge, to 30 days, and to 90 days; neurological performance; and incremental medical spending per additional survivor to 1 year.
RESULTS
Survival to hospital discharge was greater among patients receiving BLS (13.1% vs 9.2% for ALS; 4.0 [95% CI, 2.3–5.7] percentage point difference), as was survival to 90 days (8.0% vs 5.4% for ALS; 2.6 [95% CI, 1.2–4.0] percentage point difference). Basic life support was associated with better neurological functioning among hospitalized patients (21.8% vs 44.8% with poor neurological functioning for ALS; 23.0 [95% CI, 18.6–27.4] percentage point difference). Incremental medical spending per additional survivor to 1 year for BLS relative to ALS was $154 333.
CONCLUSIONS AND RELEVANCE
Patients with out-of-hospital cardiac arrest who received BLS had higher survival at hospital discharge and at 90 days compared with those who received ALS and were less likely to experience poor neurological functioning.
American emergency medical services (EMS) respond to an estimated 380 000 out-of-hospital cardiac arrests of primary cardiac etiology annually.1 Although 90% of these patients do not survive to hospital discharge, community training, rapid and appropriate delivery of prehospital care, and high-quality hospital cardiac care may substantially improve survival rates.2–7 In the United States and in other developed countries, an important strategy for responding to out-of-hospital cardiac arrest has been the delivery of advanced life support (ALS) by ambulance service providers.8
Advanced life support providers, or paramedics, are trained to use sophisticated, invasive interventions to treat cardiac arrest, including endotracheal intubation, intravenous fluid and drug delivery, and semiautomatic defibrillation.9 In contrast, basic life support (BLS) providers, or emergency medical technicians, use simple devices such as bag valve masks and automated external defibrillators. As a result, ALS providers tend to spend substantially more time at the location of the cardiac arrest than BLS providers.10 Reflecting ALS’s additional training and equipment, insurance reimbursement for it is higher.11
However, ALS has no established benefit over BLS for patients with cardiac arrest.10,12 Of the few high-quality comparisons that exist, the most robust is a before-after study10 from Ontario, Canada, which found that ALS did not improve survival to hospital discharge compared with a BLS system that optimized the time to defibrillation. Research from the United States is scant, but observational studies13,14 from urban areas of other high-income countries have also failed to find a benefit of prehospital ALS. Similarly, studies15,16 on the effectiveness of airway management favor BLS, and evidence of the benefits of intravenous drug delivery in the prehospital setting is limited.17–21 Understanding the comparative effects of ALS and BLS on health outcomes and medical spending after out-of-hospital cardiac arrest is important not only for countries such as the United States with developed ALS-based emergency response systems but also for developing countries in the process of designing cost-effective prehospital emergency response systems.
Methods
Study Population and Data Linkage
This research was approved by institutional review boards at Harvard University and the National Bureau of Economic Research. Informed consent was not required because the analysis is based on deidentified Medicare claims. We analyzed a 20% simple random sample of fee-for-service Medicare beneficiaries from nonrural counties who experienced out-of-hospital cardiac arrest between January 1, 2009, and October 2, 2011. We identified ground emergency ambulance rides by Health Care Financing Administration Common Procedural Coding System codes A0429 (BLS emergency), A0427 (ALS level 1 emergency), and A0433 (ALS level 2)11 with origin and destination codes RH (residence to hospital), SH (scene of accident or acute event to hospital), NH (skilled nursing facility [SNF] to hospital), or EH (residential, domiciliary, or custodial facility or nursing home other than SNF to hospital). We linked 95.7% of these rides to inpatient and outpatient claims by matching on beneficiary identification numbers and dates of service.
For 43 760 ambulance rides, an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code of 427.5 for cardiac arrest was present on an outpatient claim or an inpatient claim marked as “present on admission.” To focus on cardiac arrests arising from a nontraumatic etiology and to allow comparison with other studies,10 we removed observations with an injury ICD-9-CM diagnosis code (800–999 or E800–E900). We also removed cases (3.1%) from Connecticut, Delaware, Hawaii, and the District of Columbia, where billing practices make it difficult to determine whether ALS provided the service. Forexample,in Delaware, ALS is supported by local government funds and does not generally bill Medicare. We excluded observations (approximately 10% of the sample) from rural counties as defined by the US Bureau of the Census because they exhibited large differences on baseline characteristics. Finally, we removed cases from North Dakota, Vermont, and Wyoming because they had no BLS cases in nonrural areas. Our final sample size was 32 935 ambulance rides (Figure 1). We linked eachobservation to beneficiary data on demographics, death, and chronic conditions. Using claims for services during the 1 year before cardiac arrest, we constructed combined Charlson and Elixhauser comorbidity scores.22 We ascertained total Medicare spending from claims. We obtained demographic data from the 2009 Population Estimates for Zip Code Tabulation Areas,23 county-level demographic and health information for the most recent year available before 2011 for each variable from the Area Health Resources Files,24 and hospital process measures and mortality rates for 2009 to 2011 from the Hospital Compare data sets.25
Figure 1. Flowchart of Cardiac Arrest Sample Construction.
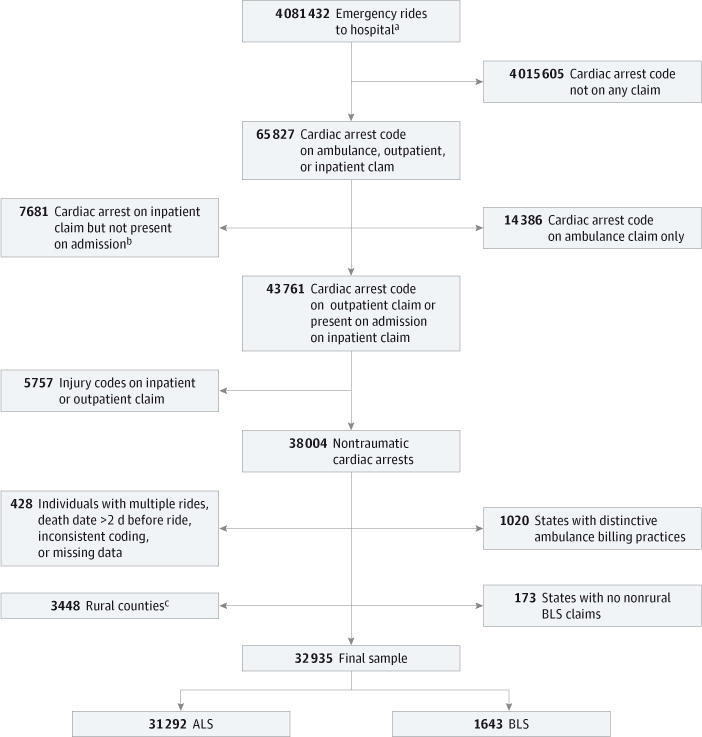
Codes refer to International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes. ALS indicates advanced life support; BLS, basic life support.
a Pickup locations included residence, scene of accident or acute event, skilled nursing facility, and non–skilled nursing facility residential, domiciliary, custodial, or nursing home facility.
b Present on admission status for cardiac arrest is either no or unknown.
c Rural areas are defined as counties that do not meet the metropolitan or micropolitan criteria as defined by the US Bureau of the Census. Metropolitan counties have at least 1 urbanized area of 50 000 or more population, and micropolitan counties have at least 1 urban cluster of at least 10 000 but less than 50 000 population. Both types have adjacent territory that has a high degree of social and economic integration with the core as measured by commuting ties.
Comparison Groups
We compared BLS and ALS transports defined by the service level billed on the Medicare ambulance claim, as indicated by the Health Care Financing Administration Common Procedural Coding System code. This code reflects the level of service that was deemed medically necessary. Crucially for our purposes, Medicare allows billing at the ALS level if assessment by ALS-trained providers was considered necessary at dispatch, even if ALS providers delivered only BLS interventions. Medicare pays a single amount for the service level that is inclusive of all items, and there is no itemized list of interventions in the claims. Therefore, although we cannot observe the specific combination of provider training, local protocols, or clinical interventions that a patient experienced, the ambulance crew level is an indicator for the set of interventions and scene and transport times that are characteristic of that level.
Guidelines and training for ALS providers direct them to provide ALS care for cardiac arrest or its antecedent conditions.8,20 Still, a potential concern may be that, after evaluating a patient, ALS-trained providers will deliver BLS interventions to patients who appear healthier and therefore bill at the BLS level. However, as noted above, ALS providers can still bill at the ALS level in these cases, and it is unlikely that they would not do so given the reimbursement differences. Therefore, it is unlikely that BLS cases in our sample were treated by providers trained in ALS.
A second potential concern with comparing outcomes for patients receiving ALS vs BLS is that, if more severe cases were to be triaged by dispatchers toward ALS, our analyses may be confounded by making ALS outcomes appear worse than they would be if patients were randomized to ALS. However, based on telephone interviews with EMS officials in 45 states, we established that existing dispatch protocols generally lead to BLS dispatch for cardiac arrest or any of its prodromal symptoms (eg, chest pain, breathing difficulty, or fainting) only if ALS is unavailable within a reasonable amount of time, either due to travel distance, attendance at another call, or a staffing shortage.
Outcome Measures
Our primary outcome measures were patient survival to hospital discharge, to 30 days, and to 90 days. Our secondary outcomes included neurological performance and medical spending. We inferred Cerebral Performance Categories Scale26 item 4 (coma or vegetative state) and item 5 (brain death) by the presence of ICD-9-CM diagnosis codes for anoxic brain injury (348.1), coma (700.01), persistent vegetative state (780.03), or brain dead (348.82). We combined these items to create an indicator for poor neurological functioning. For cardiac arrests that occurred in 2009 and 2010, we computed total medical spending up to 1 year after the cardiac arrest or until death.
Statistical Analysis
We first modeled the probability (P) that a beneficiary received ALS using logistic regression. The predicted propensity scores P were used to derive balancing weights.27 Because ALS cases outnumbered BLS cases, we chose weights to adjust the ALS distribution to the observed BLS distribution over the set of covariates. Therefore, each BLS observation received a weight of 1, and each ALS observation received a weight of (1 − P)/P. We chose this approach over propensity score–based matching or stratifying because it provided exact balance most efficiently. Furthermore, unlike using the propensity score as a covariate in a multivariable model, it allowed balance checking.
We tested the following individual-level variables in the propensity score regression: ambulance mileage, history of 27 chronic conditions, and a 6-category zip code–level indicator combining high (>$40 000) or low median household income and racial/ethnic composition (>80% black, >80% white, or integrated).28 To account for differences in the quality of hospital care that may be correlated with both outcomes and the propensity of a beneficiary to receive prehospital ALS, we also created zip code–level hospital quality measures, as described in the eAppendix in the Supplement.
Our final propensity score model adjusted for age (linear spline), sex, race/ethnicity, pickup location, and 3 chronic conditions at the individual level (the model coefficients are summarized in the eAppendix in the Supplement). At the zipcode level, we adjusted for race/ethnicity, the median household income, and hospital quality (eAppendix in the Supplement). We also adjusted for urbanicity, percentage older than 25 years with 4 or more years of college, percentage of primary care practitioners, and the presence of any medical school–affiliated hospital at the county level. We included binary variables for all states with 15 or more BLS observations (ie, state fixed effects) and created groups by region defined by the US Bureau of the Census for the remaining states. The Hosmer-Lemeshow test was not statistically significant for this model, suggesting that the link function was appropriate.
We used statistical software to construct (SAS version 9.3) and analyze (R version 3.1.0) the sample. All statistical tests were 2-sided at the 5% level. All differences were evaluated using t tests. Kaplan-Meier survival curves were prepared from the weighted observations, with end points defined by death or survival beyond the end of our data on December 31, 2011. Medical spending included Medicare and any non-Medicare primary insurer payments, as well as beneficiary payments, geographically adjusted using the Medicare Hospital Wage Index for an estimated 70% labor share of inputs. For medical spending and survival to 1 year, we used balancing weights estimated for observations in 2009 and 2010, and for survival to 2 years, we used only 2009 data.
Sensitivity Analyses
We conducted several sensitivity analyses, described in the eAppendix in the Supplement. First, to assess the extent to which unmeasured disease severity could confound our results,we estimated potential unmeasured confounding by introducing incremental changes to comorbidity scores. Second, we assessed the sensitivity of our results to alternative analytic methods by regressing survival on a binary indicator for ambulance type and other variables from our main analysis. Third, we assessed sensitivity to the inclusion of beneficiaries who appeared to have died en route to the hospital. We excluded this group in the main analysis because diagnosis is only available from ambulance claims and coding may be inaccurate. Fourth, we used other data sets to check the sensitivity of our results to the exclusion of individuals who may have died at the scene and therefore were not transported. Fifth, we estimated the effect of ALS, excluding patients from nursing homes who may have received different on-site care compared with other patients. Sixth, we assessed the sensitivity of our results to situations in which BLS called for ALS backup by calculating the number of BLS cases that would have to have been incorrectly attributed to ALS to reverse the direction of our findings. Seventh, we estimated the effect of ALS compared with BLS for patients with a primary cardiac etiology by excluding patients with acute respiratory failure codes. Eighth, we assessed the robustness of our results to a less sensitive but more specific definition of poor neurological functioning that included only patients with persistent vegetative state or brain death.
Results
Out-of-hospital cardiac arrest mortality rates were high (Table 1) and comparable to those of other studies10,29,30 that used primary data. Beneficiaries who received ALS were slightly younger, were more likely to be male, and were less likely to have most chronic conditions (Table 2). They were more often picked up at a residence, whereas patients receiving BLS were more often picked up at a skilled nursing facility. The distributions of household income and race/ethnicity, urbanicity, and the presence of medical school–affiliated hospitals differed (Table 3). Beneficiaries receiving ALS services were taken to hospitals that had somewhat better performance on process measures but had slightly worse 30-day mortality from acute myocardial infarction, heart failure, or pneumonia. After applying the propensity score–derived balancing weights to the ALS observations, there were no meaningful differences on any observed measure between the BLS and ALS groups.
Table 1.
Comparison of Medicare Claims–Based Sample and Primary Data–Based Samples on Mortality at Discharge for Individuals Brought to a Hospital
| Variable | Medicarea | CARES | ROC | OPALS Study |
|---|---|---|---|---|
| No. of patients who arrived at the hospital via EMS | 32 935 | 24 843 | 7486 | 4247 |
| Inpatients who died before discharge, % | 66 | 63 | NA | NA |
| Inpatients and outpatients who died before discharge, % | 90 | 88 | 87 | 95 |
Abbreviations: CARES, Cardiac Arrest Registry to Enhance Survival29; EMS, emergency medical services; NA, not available; OPALS, Ontario Prehospital Advanced Life Support10; ROC, Resuscitation Outcomes Consortium.30
Discharge status for Medicare outpatient claims was approximated using 2-day mortality because discharge status was poorly coded.
Table 2.
Differences in Patient Characteristics by Ambulance Service Levela
| Variable | BLS | Unweighted ALS | P Value | Weighted ALS |
|---|---|---|---|---|
| Age, mean, y | 77 | 75 | <.001 | 77 |
| Female sex, % | 52 | 46 | <.001 | 52 |
| Race/ethnicity, % | <.001b | |||
| White | 72 | 77 | 72 | |
| Black | 21 | 17 | 21 | |
| Hispanic | 3 | 2 | 3 | |
| Asian | 2 | 2 | 2 | |
| Other | 2 | 2 | 2 | |
| Ambulance mileage, mean, km | 8.7 | 9.5 | .002 | 8.5 |
| Pickup location, % | <.001b | |||
| Residence | 55 | 65 | 55 | |
| Skilled nursing facility | 27 | 14 | 27 | |
| Scene | 14 | 17 | 14 | |
| Non–skilled nursing facility nursing homec | 5 | 4 | 5 | |
| Comorbidity score, mean | 5.5 | 4.8 | <.001 | 5.5 |
| Chronic conditions, % | ||||
| Acute myocardial infarction | 13 | 14 | .17 | 14 |
| Alzheimer disease | 20 | 15 | <.001 | 20 |
| Alzheimer disease or dementiad | 42 | 31 | <.001 | 42 |
| Atrial fibrillation | 30 | 29 | .25 | 31 |
| Cataract | 66 | 62 | <.001 | 65 |
| Chronic kidney disease | 53 | 48 | <.001 | 52 |
| Chronic obstructive pulmonary disease | 49 | 49 | .69 | 49 |
| Heart failure | 66 | 62 | .001 | 67 |
| Diabetes mellitus | 58 | 53 | <.001 | 58 |
| Glaucoma | 27 | 22 | <.001 | 25 |
| Hip or pelvic fracture | 9 | 8 | .06 | 9 |
| Ischemic heart disease | 75 | 72 | <.001 | 76 |
| Depression | 43 | 40 | .005 | 43 |
| Osteoporosis | 24 | 20 | <.001 | 23 |
| Rheumatoid arthritis or osteoarthritis | 59 | 55 | <.001 | 58 |
| Stroke or transient ischemic attack | 32 | 27 | <.001 | 31 |
| Breast cancer | 5 | 4 | .14 | 5 |
| Colorectal cancer | 6 | 4 | .02 | 5 |
| Prostate cancer | 7 | 7 | .98 | 7 |
| Lung cancer | 5 | 4 | .87 | 4 |
| Endometrial cancer | 1 | 1 | .95 | 1 |
| Anemia | 80 | 72 | <.001 | 79 |
| Asthma | 19 | 20 | .31 | 19 |
| Hyperlipidemia | 76 | 75 | .43 | 77 |
| Benign prostatic hyperplasia | 23 | 22 | .68 | 21 |
| Hypertension | 91 | 90 | .04 | 92 |
| Acquired hypothyroidism | 25 | 22 | .004 | 24 |
Abbreviations: ALS, advanced life support; BLS, basic life support.
Differences between BLS and unweighted ALS observations were tested for statistical significance using t test or χ2 test, as appropriate. Because of missing data, some measures are based on less data than the full sample. Hospital-level measures are based on data from the Hospital Compare data sets.25
χ2 Test of independence was used for this categorical variable.
This includes non–skilled nursing facility residential, domiciliary, custodial, or nursing home facilities.
Alzheimer disease or dementia includes Alzheimer-related diseases and senile dementia.
Table 3.
Differences in Community and Hospital Characteristics by Ambulance Service Levela
| Variable | BLS | Unweighted ALS | P Value | Weighted ALS |
|---|---|---|---|---|
| Zip Code Level, % | ||||
| Household income/race/ethnicity groupb | <.001c | |||
| High/white | 37 | 43 | 38 | |
| Low/white | 7 | 8 | 7 | |
| High/black | 2 | 1 | 2 | |
| Low/black | 3 | 2 | 3 | |
| High/integrated | 35 | 30 | 34 | |
| Low/integrated | 16 | 16 | 16 | |
| Female sex | 51 | 51 | <.001 | 51 |
| Age ≥65 y | 14 | 14 | .30 | 14 |
| County Level, % | ||||
| Metropolitand | 87 | 85 | .01 | 87 |
| Persons with ≥4 y of college | 24 | 23 | <.001 | 24 |
| General practice physicians | 14 | 16 | <.001 | 14 |
| Any hospital with medical school affiliation | 70 | 63 | <.001 | 69 |
| Hospital Level, % | ||||
| Given aspirin at arrivale | 98 | 98 | .58 | 98 |
| Given aspirin at dischargee | 98 | 98 | .63 | 98 |
| Given β-blocker at dischargee | 97 | 98 | .003 | 98 |
| Given evaluation for LVSDf | 97 | 98 | <.001 | 98 |
| Given angiotensin-converting enzyme inhibitor or angiotensin receptor blocker for LVSDf | 94 | 95 | .05 | 95 |
| Initial blood culture performed before first dose of antibioticsg | 95 | 96 | <.001 | 96 |
| Given the most appropriate initial antibioticg | 93 | 93 | .01 | 93 |
| Heart failure 30-d mortality rate | 11 | 11 | <.001 | 11 |
| Myocardial infarction 30-d mortality rate | 15 | 16 | <.001 | 15 |
| Pneumonia 30 d mortality rate | 11 | 12 | <.001 | 11 |
Abbreviations: ALS, advanced life support; BLS, basic life support; LVSD, left ventricular systolic dysfunction.
Differences between BLS and unweighted ALS observations were tested for statistical significance using t test or χ2 test, as appropriate. Because of missing data, some measures are based on less data than the full sample. Hospital-level measures are based on data from the Hospital Compare data sets.25
This was high if the median household income exceeded $40 000 (otherwise low) and predominantly black if more than 80% black, predominantly white if more than 80% white, and otherwise integrated.
χ2 Test of independence was used for this categorical variable.
Metropolitan counties have at least 1 urbanized area of 50 000 or more population, and micropolitan counties have at least 1 urban cluster of at least 10 000 but less than 50 000 population. Both types have adjacent territory that has a high degree of social and economic integration with the core as measured by commuting ties.
The denominator for these measures is patients with myocardial infarction.
The denominator for these measures is patients with heart failure.
The denominator for these measures is patients with pneumonia.
Differences in Patient Survival
Unadjusted survival to hospital discharge was 3.5 (95% CI, 1.9–5.2) percentage points higher among patients receiving BLS (13.1% vs 9.6% for ALS) (Table 4). Unadjusted survival after BLS was also greater at 30 days (9.6% vs 6.5% for ALS; 3.1 [95% CI, 1.6–4.5] percentage point difference) and at 90 days (8.0% vs 5.8% for ALS; 2.2 [95% CI, 0.9–3.6] percentage point difference).
Table 4.
Health and Payment Outcomes by Ambulance Service Levela
| Variable | % (95% CI)
|
Ratio (95% CI) | ||
|---|---|---|---|---|
| BLS | ALS | Percentage Point Differenceb | ||
| Unadjusted Outcomes | ||||
|
| ||||
| Survival to hospital discharge | 13.1 (11.5–14.8) | 9.6 (9.3–9.9) | 3.5 (1.9–5.2) | 1.4 (1.2–1.5) |
|
| ||||
| Survival to 30 d | 9.6 (8.1–11.0) | 6.5 (6.2–6.8) | 3.1 (1.6–4.5) | 1.5 (1.2–1.7) |
|
| ||||
| Survival to 90 d | 8.0 (6.7–9.3) | 5.8 (5.5–6.1) | 2.2 (0.9–3.6) | 1.4 (1.2–1.6) |
|
| ||||
| Adjusted Outcomes | ||||
|
| ||||
| Survival | ||||
|
| ||||
| Survival to hospital discharge | 13.1 (11.5–14.8) | 9.2 (8.7–9.7) | 4.0 (2.3–5.7) | 1.4 (1.2–1.6) |
|
| ||||
| Survival to 30 d | 9.6 (8.1–11.0) | 6.2 (5.8–6.6) | 3.4 (1.9–4.8) | 1.5 (1.3–1.8) |
|
| ||||
| Survival to 90 d | 8.0 (6.7–9.3) | 5.4 (5.0–5.8) | 2.6 (1.2–4.0) | 1.5 (1.2–1.8) |
|
| ||||
| Survival to 1 y | 6.2 (4.9–7.6) | 4.4 (4.0–4.8) | 1.8 (0.4–3.3) | 1.4 (1.1–1.8) |
|
| ||||
| Survival to 2 y | 6.8 (4.8–8.9) | 3.9 (3.3–4.5) | 2.9 (0.8–5.0) | 1.7 (1.2–2.4) |
|
| ||||
| Other health measures | ||||
|
| ||||
| Poor neurological performance | 6.1 (5.0–7.3) | 9.7 (9.1–10.2) | 3.5 (2.2–4.8) | 0.6 (0.5–0.8) |
|
| ||||
| Admission to hospital | 25.4 (23.3–27.5) | 20.5 (19.8–21.2) | 4.9 (2.7–7.1) | 1.2 (1.1–1.4) |
|
| ||||
| Payments, mean, $ | ||||
|
| ||||
| 1-y Medical spending for all beneficiaries | 11 875 (9754–13 995) | 9097 (8527–9666) | 2778 (582–4973) | 1.3 (1.1–1.6) |
|
| ||||
| 1-y Medical spending per additional survivor to 1 y | 190 153 (150 041–230 265) | 206 775 (189 909–223 641) | NA | NA |
Abbreviations: ALS, advanced life support; BLS, basic life support; NA, not applicable.
Unless noted otherwise, estimates are adjusted by propensity score–based balancing weights. Estimates for survival to 1 year used only data from 2009 and 2010, and estimates for survival to 2 years used only data from 2009. Medical spending includes total payments to the provider by Medicare, the beneficiary, and a non-Medicare primary payer if one exists. Payments are geographically adjusted using the Medicare Hospital Wage Index for an estimated 70% labor share of inputs.
Discrepancies in differences are due to rounding.
After propensity score adjustment, survival to hospital discharge was 4.0 (95% CI, 2.3–5.7) percentage points, or 43%, higher among patients receiving BLS (13.1% vs 9.2% for ALS) (Table 4). Survival after BLS was also greater at 30 days (9.6% vs 6.2% for ALS; 3.4 [95% CI, 1.9–4.8] percentage point difference) and at 90 days (8.0% vs 5.4% for ALS; 2.6 [95% CI, 1.2–4.0] percentage point difference). Kaplan-Meier estimates show that much of the difference in survival between ALS and BLS is explained by higher mortality in the first few days after cardiac arrest for patients receiving ALS (Figure 2). After this period, the near constancy in the survival ratios to different time points suggests that patients receiving BLS survive at least as well as those receiving ALS. These findings were unaffected by various sensitivity analyses (eAppendix in the Supplement).
Figure 2. Kaplan-Meier Analysis of Survival After Cardiac Arrest by Ambulance Service Level.
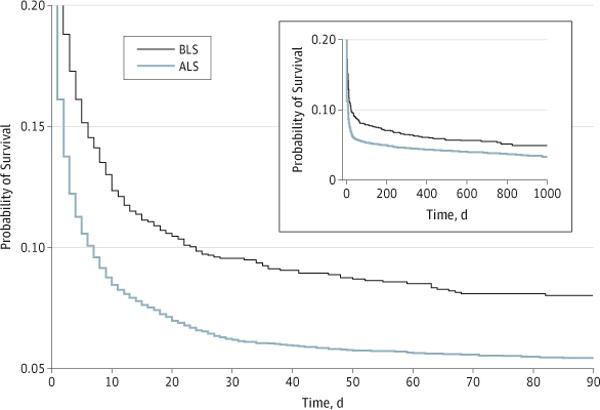
The main plot shows survival probability during the first 90 days, and the inset shows survival probability over the full observational period. Survival analysis was based on cardiac arrests that occurred between January 1, 2009, and October 2, 2011. Mortality was observed until December 31, 2011, when the data were censored; thus, there was follow-up to at least 90 days for each beneficiary. ALS indicates advanced life support; BLS, basic life support.
Differences in Neurological Performance
Among all individuals experiencing an out-of-hospital cardiac arrest, the percentage with poor neurological functioning after cardiac arrest was lower among those who received BLS vs ALS (6.1% vs 9.7%; 3.5 [95% CI, 2.2–4.8] percentage point difference). Among individuals who were admitted to the hospital, rates of poor neurological functioning were markedly lower for BLS compared with ALS (21.8% vs 44.8%; 23.0 [95% CI, 18.6–27.4] percentage point difference).
Differences in Medical Spending
The mean medical spending was higher among beneficiaries receiving BLS ($11 875 vs $9097 for ALS; $2778 [95% CI, $582–$4973] difference), in part because individuals who received BLS survived longer and had more opportunity to receive medical care (Table 4). Incremental medical spending per additional survivor to 1 year for BLS relative to ALS was $154 333 ([$11 875 – $9097]/[6.2% – 4.4%]), less than the mean medical spending per survivor to 1 year for ALS ($206 775).
Sensitivity Analyses
With one exception, our results were robust to all the sensitivity analyses described above and in the eAppendix in the Supplement. The exception is that, after restricting the definition of poor neurological functioning to only persistent vegetative state or brain death, there was no observed difference in neurological functioning between patients receiving ALS vs BLS.
Discussion
Using a nationally representative sample of traditional Medicare beneficiaries from nonrural counties who experienced out-of-hospital cardiac arrest between 2009 and 2011 and for whom EMS were billed to Medicare, we compared the effects of out-of-hospital BLS and ALS on survival, neurological performance, and medical spending. Ninety-day survival and neurological performance were substantially better among beneficiaries who received out-of-hospital BLS rather than ALS. Our estimates suggest that each year 1479 (95% CI, 683–2276) additional Medicare beneficiaries who experience out-of-hospital cardiac arrest would survive to 90 days if provided BLS instead of ALS. Furthermore, incremental medical spending per additional survivor to 1 year for BLS relative to ALS was $154 333, substantially less than the mean medical spending per survivor to 1 year for ALS ($206 775).
Prehospital care is complex, expensive, and critical to survival after out-of-hospital cardiac arrest, making it crucial to understand the combined effect on morbidity and mortality of the medical interventions, transport time, and training that characterize the 2 dominant models of prehospital care. Results of our study, to our knowledge the first large-scale systematic comparison of BLS and ALS in the United States, are consistent with those of international studies,10,13,14 which found that ALS does not improve survival to hospital discharge after cardiac arrest. In contrast, our results suggest that the use of ALS is associated with higher mortality than the use of BLS in patients with cardiac arrest. However, most out-of-hospital cardiac arrests treated by EMS in the United States are provided with ALS care.
Although ALS is often assumed to improve clinical outcomes by providing advanced airway management and intravenous drug therapy, other studies have described mechanisms by which ALS may lead to the worse outcomes that we found. First, prehospital endotracheal intubation entails risks, including unrecognized esophageal intubation, aspiration of gastric contents, aggravation of existing injuries such as cervical spine damage, and interference with chest compressions.31 Furthermore, successful intubation requires high levels of competency and regular practice, but in a Pennsylvania study32 paramedics performed a median of only one intubation per year. Therefore, bag valve mask ventilation may improve outcomes over endotracheal intubation in out-of-hospital cardiac arrest.15,16 Consistent with these risks of prehospital intubation, a large study15 of cardiac arrests in Japan found greater neurologically favorable survival with the use of bag valve masks compared with advanced airways. Similarly, an analysis of out-of-hospital cardiac arrests in Los Angeles, California, found that advanced airway methods were associated with decreased survival to hospital discharge compared with bag valve mask ventilation.16 Second, evidence on the benefits of intravenous drug delivery in out-of-hospital cardiac arrest is limited.17–21 Third, and perhaps most important, ALS may entail delays in hospital care10 that would otherwise offer definitive clinical management of the underlying disease (eg, percutaneous coronary intervention for acute myocardial infarction).
Because a randomized controlled trial of ALS vs BLS is unlikely to occur, we performed an observational analysis. Although our analysis is the largest to date in the United States to our knowledge, it has several limitations. Patients receiving ALS may be at higher risk of mortality irrespective of the intervention, which would confound our estimates. This would be most likely to occur if ALS was dispatched to patients with higher preexisting mortality risk based either on symptoms or preexisting conditions. However, telephone interviews with 45 state EMS agencies demonstrated that if ALS was available it would always be provided in cases of known cardiac arrest or for any typical prodromal symptoms (eg, chest pain, syncope, etc) that would be known to the dispatcher at the time of dispatch. In other words, BLS would only be dispatched when ALS is unavailable, leaving no clear remaining mechanisms to explain why less severely ill patients would be preferentially dispatched BLS. Moreover, beneficiaries who received BLS had on average more preexisting comorbidities than those who received ALS, suggesting that outcomes among patients receiving BLS would (if anything) be worse and not better. Finally, in analyses of sensitivity to unmeasured confounding, our findings that outcomes under BLS were better than under ALS would continue to hold unless an implausibly high difference in unobserved severity was postulated.
An additional source of confounding may be that individuals who can be more easily resuscitated at the scene (eg, those with ventricular fibrillation) might be overrepresented among BLS cases, while individuals who cannot be resuscitated by BLS wait to be treated by ALS rather than undergoing direct transport to the hospital. Advanced life support would then be spuriously associated with worse outcomes that should have been attributed to BLS. However, our sensitivity analysis of situations in which BLS waits for ALS backup found that this would have to occur in an implausibly high proportion of BLS cases to change the direction of our effect (eAppendix in the Supplement).
Additional factors that influence outcomes after cardiac arrest may potentially confound our analysis. For example, shorter ambulance response times to the scene33 and the presence of a shockable rhythm29 are associated with improved outcomes. However, no evidence exists that these factors differ between patients receiving ALS vs BLS. However, ALS providers on average spend significantly more time at the scene,10 which suggests how BLS may improve outcomes over ALS via rapid transport to the hospital. Other factors such as the quality of cardiopulmonary resuscitation (CPR) and the use of endotracheal intubation or intravenous drugs are similarly potential mediators of ALS and BLS treatment effects and, like scene and travel time, should not be viewed as confounders. Finally, although bystander-initiated CPR has been associated with improved outcomes,28 we could not directly control for bystander-initiated CPR and defibrillation. However, we adjusted for area-level race/ethnicity and household income, which have been shown to be important determinants of bystander-initiated treatment.28
An additional limitation is that we used administrative claims, which may be inaccurate and subject to coding errors in diagnoses and procedures. For example, our identification of ALS and BLS exposures may not accurately reflect the service level of the ambulance. However, Medicare policy allows billing at the ALS level if assessment by an ALS-trained crew was considered necessary at dispatch. Based on telephone interviews with state EMS officials, we found some instances of joint BLS and ALS response in which Medicare is billed for only BLS. However, states with distinctive billing practices such as this comprise about 3% of the sample, and our findings were unaffected by their exclusion. Nonetheless, services provided by EMS may differ across areas, which may not be reflected in the level of billing to Medicare. Because we could not identify specific interventions provided to each patient, our conclusions are limited to differences in outcomes associated with the overall practices of BLS and ALS providers.
Conclusions
Our study calls into question the widespread assumption that advanced prehospital care improves outcomes of out-of-hospital cardiac arrest relative to care following the principles of BLS, including rapid transport and basic interventions such as effective chest compressions, bag valve mask ventilation, and automated external defibrillation. It is crucial to evaluate BLS and ALS use in other diagnosis groups and settings and to investigate the clinical mechanisms behind our results to identify the most effective prehospital care strategies for saving lives and improving quality of life conditional on survival.
Supplementary Material
Acknowledgments
Funding/Support: Ms Sanghavi was supported by a National Science Foundation Graduate Research Fellowship and a Health Services Research Dissertation Award (R36) from the Agency for Healthcare Research and Quality. Dr Jena was supported by Early Independence Award 1DP5OD017897-01 from the National Institutes of Health.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Ms Sanghavi had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sanghavi, Jena, Zaslavsky.
Acquisition, analysis, or interpretation of data: Sanghavi, Jena, Newhouse.
Drafting of the manuscript: Sanghavi, Jena, Zaslavsky.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: All authors.
Obtained funding: Sanghavi.
Study supervision: Jena, Newhouse.
Conflict of Interest Disclosures: Dr Newhouse reported being a director of and holding equity in Aetna. No other disclosures were reported.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics: 2012 update: a report from the American Heart Association [published correction appears in Circulation. 2012;125(22):e1002] Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallstrom AP, Ornato JP, Weisfeldt M, et al. Public Access Defibrillation Trial Investigators Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351(7):637–646. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 3.Stiell IG, Wells GA, Field BJ, III, et al. Improved out-of-hospital cardiac arrest survival through the inexpensive optimization of an existing defibrillation program: OPALS study phase II: Ontario Prehospital Advanced Life Support. JAMA. 1999;281(13):1175–1181. doi: 10.1001/jama.281.13.1175. [DOI] [PubMed] [Google Scholar]
- 4.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 5.Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 6.Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation. 2007;74(2):227–234. doi: 10.1016/j.resuscitation.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Travers AH, Rea TD, Bobrow BJ, et al. Part 4: CPR overview: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 suppl 3):S676–S684. doi: 10.1161/CIRCULATIONAHA.110.970913. [DOI] [PubMed] [Google Scholar]
- 9.National Highway Traffic Safety Administration. National EMS scope of practice model. 2007 http://www.ems.gov/education/EMSScope.pdf. Accessed January 7, 2014.
- 10.Stiell IG, Wells GA, Field B, et al. Ontario Prehospital Advanced Life Support Study Group Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004;351(7):647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 11.Medicare Payment Advisory Committee (MedPAC) Ambulance services payment system: payment basics. 2013 Oct; http://www.medpac.gov/documents/payment-basics/ambulance-services-payment-system.pdf?sfvrsn=0. Accessed September 11, 2014.
- 12.Isenberg DL, Bissell R. Does advanced life support provide benefits to patients? a literature review. Prehosp Disaster Med. 2005;20(4):265–270. doi: 10.1017/s1049023x0000265x. [DOI] [PubMed] [Google Scholar]
- 13.Bur A, Kittler H, Sterz F, et al. Effects of bystander first aid, defibrillation and advanced life support on neurologic outcome and hospital costs in patients after ventricular fibrillation cardiac arrest. Intensive Care Med. 2001;27(9):1474–1480. doi: 10.1007/s001340101045. [DOI] [PubMed] [Google Scholar]
- 14.Ma MH, Chiang WC, Ko PC, et al. Outcomes from out-of-hospital cardiac arrest in metropolitan Taipei: does an advanced life support service make a difference? Resuscitation. 2007;74(3):461–469. doi: 10.1016/j.resuscitation.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa K, Hiraide A, Chang Y, Brown DF. Association of prehospital advanced airway management with neurologic outcome and survival in patients with out-of-hospital cardiac arrest. JAMA. 2013;309(3):257–266. doi: 10.1001/jama.2012.187612. [DOI] [PubMed] [Google Scholar]
- 16.Hanif MA, Kaji AH, Niemann JT. Advanced airway management does not improve outcome of out-of-hospital cardiac arrest. Acad Emerg Med. 2010;17(9):926–931. doi: 10.1111/j.1553-2712.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- 17.Gueugniaud PY, David JS, Chanzy E, et al. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2008;359(1):21–30. doi: 10.1056/NEJMoa0706873. [DOI] [PubMed] [Google Scholar]
- 18.Stiell IG, Wells GA, Hebert PC, Laupacis A, Weitzman BN. Association of drug therapy with survival in cardiac arrest: limited role of advanced cardiac life support drugs. Acad Emerg Med. 1995;2(4):264–273. doi: 10.1111/j.1553-2712.1995.tb03220.x. [DOI] [PubMed] [Google Scholar]
- 19.Nolan JP, De Latorre FJ, Steen PA, Chamberlain DA, Bossaert LL. Advanced life support drugs: do they really work? Curr Opin Crit Care. 2002;8(3):212–218. doi: 10.1097/00075198-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care [published corrections appear in Circulation 2013;128(25):e480 and 2011;123(6):e236] Circulation. 2010;122(18 suppl 3):S729–S767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 21.Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA. 2012;307(11):1161–1168. doi: 10.1001/jama.2012.294. [DOI] [PubMed] [Google Scholar]
- 22.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Center for Health Policy Research, The Dartmouth Institute for Health Policy and Clinical Research. Population estimates for Zip Code Tabulation Areas (ZCTAs) and Primary Care Service Areas (PCSAs) 2009 http://datawarehouse.hrsa.gov/DataDownload/PCSA/2009/Population%20Estimates%202009.pdf. Accessed September 10, 2014.
- 24.US Department of Health and Human Services, Health Resources and Services Administration. Area Health Resources Files (AHRF) http://arf.hrsa.gov. Accessed January 7, 2014.
- 25.Centers for Medicare and Medicaid Services. Hospital Compare. http://www.medicare.gov/hospitalcompare/Data/About.html. Accessed January 7, 2014.
- 26.Safar P. Resuscitation After Brain Ischemia: Brain Failure and Resuscitation. New York, NY: Churchill Livingstone; 1981. pp. 155–184. [Google Scholar]
- 27.Hirano K, Imbens GW. Estimation of causal effects using propensity score weighting: an application to data on right heart catheterization. Health Serv Outcomes Res Methodol. 2001;2(3–4):259–278. [Google Scholar]
- 28.Sasson C, Magid DJ, Chan P, et al. CARES Surveillance Group Association of neighborhood characteristics with bystander-initiated CPR. N Engl J Med. 2012;367(17):1607–1615. doi: 10.1056/NEJMoa1110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally B, Robb R, Mehta M, et al. Centers for Disease Control and Prevention Out-of-hospital cardiac arrest surveillance: Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005—December 31, 2010. MMWR Surveill Summ. 2011;60(8):1–19. [PubMed] [Google Scholar]
- 30.Nichol G, Thomas E, Callaway CW, et al. Resuscitation Outcomes Consortium Investigators Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300(12):1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HE, Mann NC, Mears G, Jacobson K, Yealy DM. Out-of-hospital airway management in the United States. Resuscitation. 2011;82(4):378–385. doi: 10.1016/j.resuscitation.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Wang HE, Yealy DM. Managing the airway during cardiac arrest. JAMA. 2013;309(3):285–286. doi: 10.1001/jama.2012.216998. [DOI] [PubMed] [Google Scholar]
- 33.Pell JP, Sirel JM, Marsden AK, Ford I, Cobbe SM. Effect of reducing ambulance response times on deaths from out of hospital cardiac arrest: cohort study. BMJ. 2001;322(7299):1385–1388. doi: 10.1136/bmj.322.7299.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


