Abstract
Consistent evidence indicates that exercise improves cognition and mood, with preliminary evidence suggesting that brain-derived neurotrophic factor (BDNF) may mediate these effects. The aim of the current meta-analysis was to provide an estimate of the strength of the association between exercise and increased BDNF levels in humans across multiple exercise paradigms. We conducted a meta-analysis of 29 studies (N = 1,111 participants) examining the effect of exercise on BDNF levels in three exercise paradigms: (1) a single session of exercise, (2) a session of exercise following a program of regular exercise, and (3) resting BDNF levels following a program of regular exercise. Moderators of this effect were also examined. Results demonstrated a moderate effect size for increases in BDNF following a single session of exercise (Hedges’ g = 0.46, p < 0.001). Further, regular exercise intensified the effect of a session of exercise on BDNF levels (Hedges’ g = 0.58, p = 0.02). Finally, results indicated a small effect of regular exercise on resting BDNF levels (Hedges’ g = 0.28, p = 0.005). When analyzing results across paradigms, sex significantly moderated the effect of exercise on BDNF levels, such that studies with more women showed less BDNF change resulting from exercise. Effect size analysis supports the role of exercise as a strategy for enhancing BDNF activity in humans, but indicates that the magnitude of these effects may be lower in females relative to males.
Keywords: exercise, physical activity, brain-derived neurotrophic factor, BDNF, meta-analysis
Introduction
There is a wealth of evidence that exercise improves both cognition (Roig et al., 2013; Smith et al., 2010) and mood (Josefsson et al., 2014; Rethorst and Trivedi, 2013; Stathopoulou et al., 2006), with evidence suggesting that brain-derived neurotrophic factor (BDNF) activity may mediate these effects (Erickson et al., 2012; Heyman et al., 2012; van Praag et al., 2005; Vaynman et al., 2004). BDNF is a protein found in high concentrations in the central nervous system, primarily in the brain regions of the hippocampus, cerebral cortex, hypothalamus, and cerebellum (Murer et al., 2001). Central BDNF can cross the blood-brain barrier and therefore be stored in other areas of the body; however, BDNF can also be produced by tissues in the periphery, making it difficult to identify in humans whether BDNF changes in serum levels result from changes in central or peripheral BDNF (Erickson et al., 2012; Murer et al., 2001). BDNF has been implicated in neural development and functioning, including neurogenesis, dendritic growth, and long-term potentiation of neurons (Altar, 1999; Gorski et al., 2003; Huang et al., 1999; Lu et al., 2005). Non-quantitative reviews from both the animal and human literature (Huang et al., 2014; Zoladz and Pilc, 2010) provide evidence that BDNF increases following exercise in rodents and acute and programmed aerobic exercise in humans. Animal models provide more consistent evidence for exercise-induced upregulation of BDNF, given the ability to measure BDNF centrally; however, studies indicate that peripheral (serum and plasma) and central BDNF levels are correlated in mouse models (Angelucci et al., 2011; Karege et al., 2002), with some evidence for similar associations in humans (Krabbe et al., 2007). There is also tentative evidence that peripheral levels of BDNF may have central effects (Schmidt and Duman, 2010). Given the evidence for BDNF increases, exercise can be viewed as a potential strategy for inducing BDNF activity for application to the enhancement of mood or cognition.
Indeed, individual studies have demonstrated the effect of higher BDNF levels on numerous cognitive processes. For example, higher BDNF levels have been associated with better spatial (Erickson et al., 2009; Rex et al., 2006), episodic (Egan et al., 2003), recognition (Komulainen et al., 2008; Whiteman et al., 2014), and verbal memory (Grassi-Oliveira et al., 2008) as well as better hippocampal functioning (Erickson et al., 2012). In addition, decreased levels of BDNF, particularly in older adults, have been associated with hippocampal atrophy and may contribute to memory impairment, which may be linked to cognitive challenges experienced in Alzheimer’s (Erickson et al., 2012; Murer et al., 2001).
Qualitative reviews (e.g., Huang et al., 2014; Zoladz and Pilc, 2010) present significant evidence that exercise enhances BDNF levels; however, they do not provide the magnitude or reliability of this effect. The goal of the present quantitative meta-analytic investigation was to document the level and reliability of the effect of exercise on changes in BDNF activity in humans. Three distinct paradigms have been used to study this effect: (1) changes in BDNF levels across a single session of acute exercise, (2) changes in BDNF levels across a session of exercise following a program of regular exercise (showing changes in BDNF release following repeated bouts of exercise), and (3) changes in resting BDNF levels following a program of regular exercise. Each of these was examined separately due to evidence that the effects of exercise on BDNF vary across paradigms (Huang et al., 2014). In the current meta-analysis, potential moderators (e.g., sex, age, assay type, diagnostic status, and exercise frequency) of the effect of exercise on BDNF were investigated.
Materials and Methods
Search Strategy
Studies published in English through February 2013 were identified using the search engines PubMed, PsycINFO, and Google Scholar. The following search terms were used in combination: brain-derived neurotrophic factor, BDNF, exercise, and physical activity. Reference sections of identified articles and relevant reviews were also examined to detect articles not captured by this search.
Study Selection and Data Abstraction
Identified studies were selected for inclusion in analyses based on the following criteria: (1) focus on a human population, (2) use of a precise measure of BDNF concentration levels (i.e., plasma or serum), and (3) administration of an exercise procedure or measure (i.e., acute exercise test, programmed regular exercise). If levels of BDNF were not able to be determined in the text of the article, such data were requested from the study authors. In the current report, data were abstracted from the articles by one of the first two authors and independently checked for accuracy. Any inconsistencies regarding inclusion or data extraction were resolved in a consensus meeting with the senior author.
Study Characteristics
Within each of the identified studies, several variables were evaluated to determine if they moderated the association between exercise and changes in BDNF levels from pre-exercise to post-exercise. These included sex (expressed as % female), age, assay type (i.e., serum or plasma), diagnostic status, and exercise frequency (defined as greater than or equal to American College of Sports Medicine guidelines, Garber et al., 2011).
Most studies of programmed exercise included for analysis involved a training program of aerobic exercise (n = 15); however, five studies included strength or resistance training, with two studies examining both strength and endurance (aerobic) training. Aerobic exercises varied among programs, with some programs combining multiple exercises, in the following frequencies: cycling (n = 8), running (n = 5), walking (n = 3), swimming (n = 1), rowing (n = 1), and unspecified/individualized (n = 4). Most programs required supervised training sessions (n = 16), whereas a few offered home-based interventions (n = 2). All but one of the programs that reported exercise intensity required moderate intensity exercise, with the other program involving high intensity exercise. Several 0 compared a training vs. sedentary control (or other active control, such as stretching) group (n = 9); eight studies utilized within-subject designs.
Data Synthesis
Random-effects analyses were used in this meta-analysis. Random-effects analyses are considered to be superior to fixed-effects methods, which assume homogeneous population effect sizes, due to minimization of type I error rates (Hunter and Schmidt, 2004; Lipsey and Wilson, 2000) and more realistic representations of heterogeneous effect sizes in the population (Field, 2001).
Effect sizes were calculated using Hedges’ g (Hedges and Olkin, 1985), a variation of Cohen’s d that corrects for biases due to sample sizes. To keep samples independent, one estimate of effect size was used per study; if a study included multiple effect sizes for a single construct, these effect sizes were averaged prior to data synthesis with other studies. Effect sizes of exercise at different intensities were averaged for overall study effect size; however, control groups (e.g., stretching) were not included in the overall effect size. We completed a separate moderator analysis of active (e.g., stretching) versus non-active (e.g., sedentary, waitlist) control groups, which revealed no moderation effects of control group type across paradigms (Q(1) = 0.06, p = 0.81) or within paradigm (all p > 0.07). Analyses were conducted combining both aerobic and strength/resistance training as well as with strength/resistance training examined separately, given the potential differential effect of these styles of interventions. Effect sizes were interpreted in the following manner: small effect (g = 0.2), medium effect (g = 0.5), and large effect (g = 0.8), based on Cohen’s (1992) standards.
For categorical moderators (e.g., assay type, diagnostic status, exercise frequency), effects were tested by computing Q tests to evaluate if effects varied systematically between groups. For continuous moderators (e.g., sex, age), we used bivariate correlational analyses to evaluate significance.
Finally, publication bias was assessed. Publication bias allows for protection against the “file drawer effect,” which posits that studies with null findings are less likely to be published and therefore unrepresented in the literature search and following meta-analysis. We evaluated publication bias using multiple methods. First, we examined the fail-safe N, which determines the number of additional studies with a null result required to reduce the overall effect size to non-significance (Rosenthal, 1991; Rosenthal and Rubin, 1988). If the fail-safe N is greater than 5 times the number of studies in the analysis, the results can be interpreted as robust (Rosenthal, 1991). Second, we visually inspected the funnel plot for symmetry relative to the mean effect size. Greater symmetry indicates lesser likelihood of publication bias. All data analyses were conducted using Comprehensive Meta-Analysis Software (Bornstein et al., 2005).
Results
Trial Flow
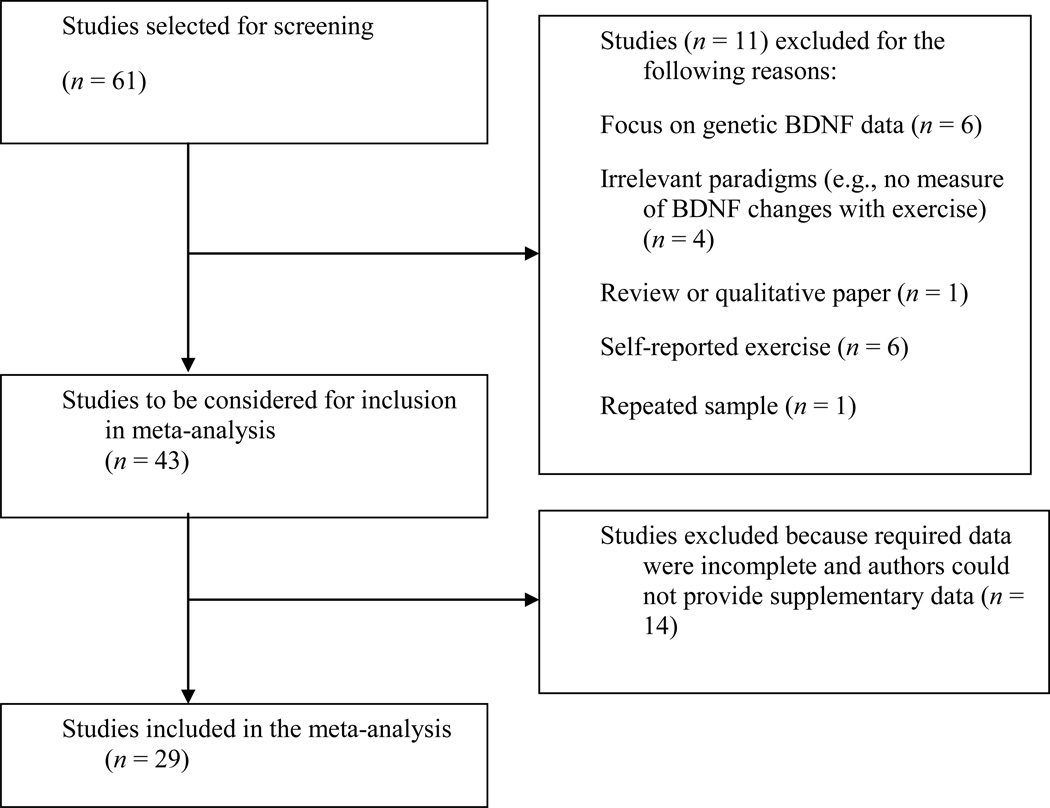
A total of 61 studies was initially identified as likely meeting inclusion criteria; evaluation of these studies resulted in a final sample for analysis that included 29 studies (see Figure 1). Studies were excluded when sufficient data were not available (following several contact attempts with authors), when outcomes were not based on BDNF levels following a bout of exercise, or when BDNF genotypes rather than levels were assessed.
Figure 1.
Study selection process
Table 1 displays the characteristics of the 29 studies, representing 1,111 participants (mean age = 42.1, % female = 46.6%), included in the analysis. Fourteen studies (48%) examined changes in BDNF levels after a single session of exercise, eight studies (27%) examined changes in BDNF levels immediately post-exercise in a design evaluating the effects of a program of regular exercise, and thirteen studies (45%) examined changes in resting BDNF levels following a program of regular exercise. Four studies included patients with a diagnosis of a mental disorder: three of individuals with major depressive disorder (Gustafsson et al., 2009; Laske et al., 2010; Toups et al., 2011) and one of individuals with panic disorder (Strohle et al., 2010). Of these studies, three examined the effect of acute exercise (Gustafsson et al., 2009; Laske et al., 2010; Strohle et al., 2010), whereas one (targeting depression) examined the effect of programmed exercise on resting BDNF levels (Toups et al., 2011).
Table 1.
Sample characteristics for studies of the association between exercise and BDNF levels
| Study | Population studied |
N | % female |
Mean Age |
BDNF measure |
Duration of exercise program |
Type of exercise |
Intensity of exercise |
Supervised/ Home-based |
Randomized/ control group |
Hedge’s g |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BDNF collected after acute exercise | |||||||||||
| Goekint et al., 2008 | Healthy | 11 | 0 | 22.9 | serum | n/a | 60 min ergometric cycling | Moderate (55–75% of VO2max) | n/a | Placebo vs. reboxetine | 1.72 |
| Tang et al., 2008 | Healthy | 16 | 50 | UN | serum | n/a | 15 min stepping | Not reported | n/a | n/a | 0.35 |
| Rasmussen et al., 2009 | Healthy | 8 | 0 | UN | plasma | n/a | 4h rowing | High (85–95% VO2max) | n/a | n/a | 0.74 |
| Cho et al., 2012 | Healthy | 18 | 0 | 19 | plasma & serum | n/a | Bruce maximal treadmill | Until volitional exhaustion | n/a | n/a | 0.89 |
| Correia et al., 2010 | Healthy | 16 | 0 | 24.6 | plasma | n/a | Strength (knee/elbow) | Maximal knee and elbow contractions | n/a | n/a | −0.08 |
| Seifert et al., 2010 | Healthy | 12 | 0 | 29.8 | plasma | n/a | Ergometer cycling | Until exhaustion | n/a | n/a | 0.65 |
| Bos et al., 2011 | Healthy | 38 | 26 | 43 | serum | n/a | cycling | Moderate (74% VO2max) | n/a | n/a | 0.25 |
| Griffin et al., 2011 | Healthy | 47 | 0 | 22 | serum | n/a | Ergometer graded exercise test | until exhaustion | n/a | Exercisers vs. sedentary control | 0.37 |
| Rojas Vega et al., 2011 | Healthy | 20 | 100 | 35.2 | serum | n/a | Graded exercise test | To 150bpm | n/a | n/a | 0.48 |
| Brunelli et al., 2012 | Healthy | 10 | 0 | 22 | serum | n/a | Cycling | Volitional exhaustion | n/a | n/a | 0.74 |
| Schmidt-Kassow et al., 2012 | Healthy | 40 | 50 | 23 | serum | n/a | 30 min cycle ergometer | n/a | Light vs. intense exercise | 0.28 | |
| Gustafsson et al., 2009 | MDD | 36 | 50 | UN | plasma | n/a | Computerized ergometer | Until exhaustion | n/a | Depressed vs. healthy controls | 0.77 |
| Laske et al., 2010 | MDD | 55 | 100 | 60.3 | serum | n/a | Incremental exercise test on treadmill | Until volitional exhaustion | n/a | Depressed vs. healthy | 0.18 |
| Strohle et al., 2010 | Panic | 12 | 75 | 31.9 | serum | n/a | 30 min treadmill test | Moderate (70%) VO2max) | n/a | Exercise vs. quiet rest; panic vs. healthy | 0.83 |
| Acute BDNF measured after regular programmed exercise | |||||||||||
| Zoladz et al., 2008 | Healthy | 13 | 0 | 22.7 | plasma | 5 weeks | Endurance cycling (4d/wk), test: cycloergometer to exhaustion | Moderate | Supervised | n/a | 0.96 |
| Seifert et al., 2010 | Healthy | 12 | 0 | 29.8 | plasma | 12 weeks (3 months) | Cycling, running, swimming, rowing | Moderate (70% VO2max) | Supervised | Training vs. sedentary | 0.00 |
| Yarrow et al., 2010 | Healthy | 20 | 0 | 21.9 | serum | 5 weeks | Resistance training (3d/wk) | Traditional: 75% 1RM; eccentric: 50–120% 1RM | Supervised | Traditional vs. eccentric enhanced resistance training | 3.53 |
| Ruscheweyh et al., 2011 | Healthy | 62 | 69.4 | 60.2 | serum | 24 weeks (6 months) | Nordic walking and gymnastics | Moderate (Nordic walking: 50–60% VO2max, gymnastics: 30–40% VO2max) | Supervised | Nordic walking vs. gymnastics vs. control | 0.18 |
| Bos et al., 2013 | Healthy | 24 | 62.5 | 32.1 | serum | 12 weeks | Walking and running | Moderate (75% VO2max) | Supervised | Urban vs. rural environment | −0.32 |
| Schulz et al., 2004 | MS | 28 | 68 | 39.5 | serum | 8 weeks | Individualized aerobic training (2d/wk) | Moderate (60% VO2max) | Home-based | Training vs. control | 0.14 |
| Castellano et al., 2008 | MS | 22 | 72.7 | 40 | serum | 4 weeks, 8 weeks | Cycling (3d/wk) | Moderate (60% VO2max) | Supervised | MS vs. control | 0.48 |
| Bansi et al., 2012 | MS | 52 | 67.3 | 51.1 | serum | 3 weeks | Cycling (5d/wk) | Moderate (60% VO2max) | Supervised | Land ergometer vs. aquatic bike training | 0.43 |
| Resting BDNF measured after regular programmed exercise | |||||||||||
| Zoladz et al., 2008 | Healthy | 13 | 0 | 22.7 | plasma | 5 weeks | Endurance cycling (4d/wk) | moderate | Supervised | n/a | 0.79 |
| Schiffer et al., 2009 | Healthy | 27 | UN | 23 | plasma | 12 weeks | Endurance: running; strength: curl, press, rows, crunches, etc. | Endurance: high (80% VO2max); strength: 70–80% 1RM | Supervised | Endurance vs. strength vs. control | −0.23 |
| Goekint et al., 2010 | Healthy | 16 | 21.7 | 21.2 | serum | 10 weeks | Strength exercises (3d/wk) | Not reported | Supervised | Training vs. sedentary | 0.33 |
| Seifert et al., 2010 | Healthy | 12 | 0 | 29.8 | plasma | 12 weeks (3 months) | Cycling, running, swimming, rowing | Moderate (70% VO2max) | Supervised | Training vs. sedentary | 1.53 |
| Erickson et al., 2011 | Healthy | 120 | 66.7 | 66.6 | serum | 52 weeks (1 year) | Aerobic walking | Moderate (60% VO2max) | Supervised | Exercise vs. stretching control | 0.19 |
| Griffin et al., 2011 | Healthy | 47 | 0 | 22 | serum | 3 weeks, 5 weeks | Aerobic cycling (3d/wk) | Moderate | Supervised | Exercise vs. sedentary | 0.22 |
| Swift et al., 2012 | Diabetes | 150 | 55.1 | 56.9 | serum | 36 weeks (9 months) | Aerobic exercise, resistance training, combined (3d/wk) | Moderate (50–80% VO2max) | Supervised | Aerobic exercise, resistance training, combined, stretching control | −0.17 |
| Toups et al., 2011 | MDD | 70 | 78.6 | 49.4 | serum | 12 weeks | unspecified | 16 KKW (high expenditure) or 4KKW (low expenditure) | Supervised | High vs. low energy expenditure | 0.02 |
| Schulz et al., 2004 | MS | 28 | 68 | 39.5 | serum | 8 weeks | Individualized aerobic training (2d/wk) | Moderate (max 75% VO2max) | Home-based | Training vs. control | 0.59 |
| Baker et al., 2010 | MCI | 33 | 51.5 | 70 | plasma | 24 weeks (6 months) | Treadmill, bike, elliptical (4d/wk) | Moderate to high (75–85% VO2max) | Supervised | High intensity exercise vs. stretching control | 0.94 |
| Bansi et al., 2012 | MS | 52 | 67.3 | 51.1 | serum | 3 weeks | Cycling (5d/wk) | Moderate (60% VO2max) | Supervised | Land ergometer or aquatic bike | 0.22 |
| Corripio et al., 2012 | Obese | 120 | 44.2 | 7.9 | plasma | 104 weeks (2 years) | Unspecified (3d/wk) | Moderate | Home-based | n/a | 0.25 |
| Araya et al., 2013 | Obese | 15 | 60 | 38.3 | plasma & serum | 12 weeks (3 months) | Treadmill, bike (3d/wk) | Moderate (65% VO2max) | Supervised | n/a | 0.81 |
Note. MDD = major depressive disorder; MS = multiple sclerosis; MCI = mild cognitive impairment, KKW = kcal/kg/week; RM = repetition maximum; UN = unknown.
Effect of Acute Exercise on BDNF Levels
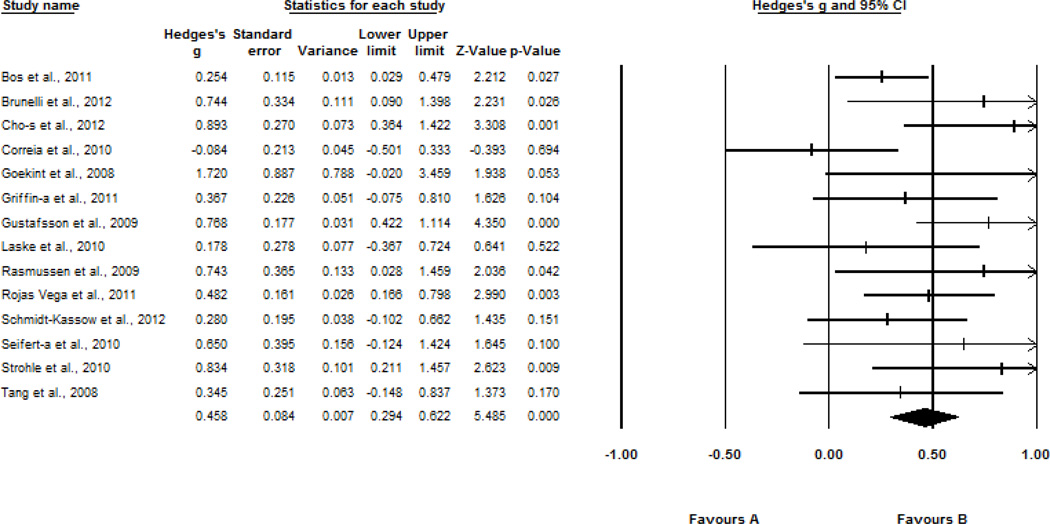
Figure 2 shows the effect sizes for the 14 studies analyzing the effect of acute exercise on change in BDNF levels. For these studies, BDNF levels were measured prior to and following (from immediately to 60 minutes after) a single exercise session in the laboratory. Pre- to post-exercise change in BDNF levels across a single exercise session reflected a moderate effect size (Hedges’ g = 0.46, SE = 0.08, 95% CI = 0.29–0.62, z = 5.49, p < 0.001).
Figure 2.
Effect sizes of the association between exercise and BDNF levels following acute exercise. s, serum; a, acute exercise.
Effect of Programmed Regular Exercise on BDNF Levels
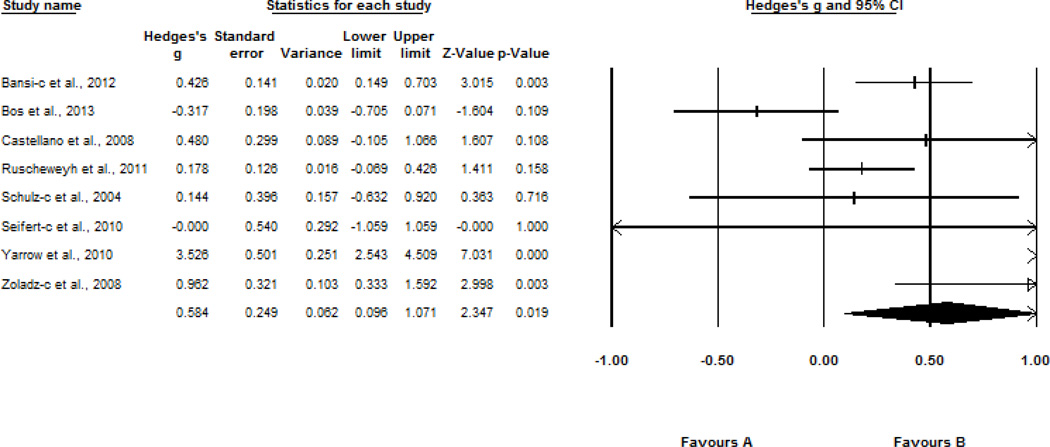
The effect of programmed regular exercise on BDNF levels was examined for two different outcome variables. First, we examined the effect of a program of regular exercise, ranging from 3 to 24 weeks, on changes in BDNF levels across a single session of exercise. In a sample of 8 studies, pre- to post-exercise change in BDNF levels intensified following a program of regular exercise (Hedges’ g = 0.58, SE = 0.25, 95% CI = 0.10–1.07, z = 2.35, p = 0.02). Results are displayed in Figure 3.
Figure 3.
Effect sizes of the association between acute exercise and BDNF levels following programmed regular exercise. c, change after programmed exercise.
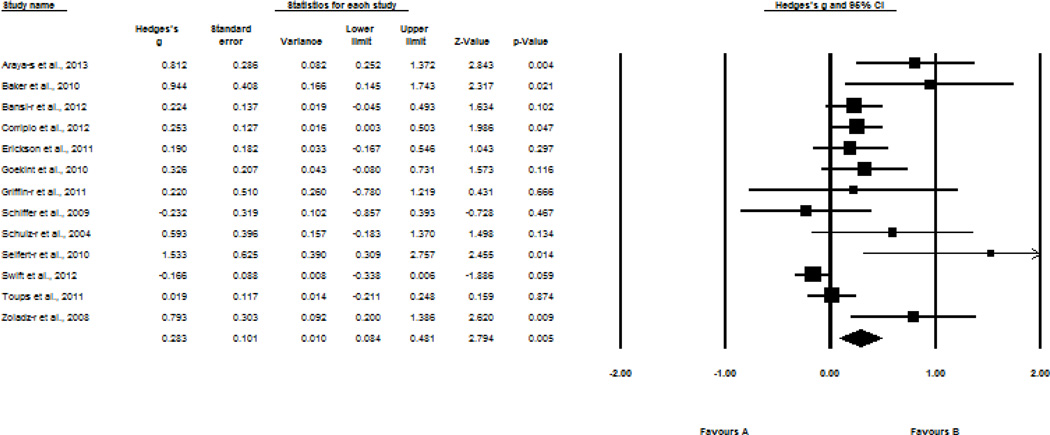
Second, we examined the effect of a program of regular exercise, ranging from 3 weeks to 2 years, on resting BDNF levels. In a sample of 13 studies, programs of exercise also exhibited changes in resting levels of BDNF; however, this result was on the order of a small effect size (Hedges’ g = 0.28, SE = 0.10, 95% CI = 0.08–0.48, z = 2.79, p = 0.005). Results are displayed in Figure 4.
Figure 4.
Effect sizes of the association between exercise and resting BDNF levels following programmed regular exercise. s, serum; r, resting BDNF levels after programmed exercise.
Effects of Strength Training on BDNF Levels
Removing a resistance paradigm (i.e., arm and elbow strength) from examination of effects of acute exercise on BDNF levels did not significantly impact the results (Hedges’ g = 0.49, SE = 0.08, 95% CI = 0.34–0.64, z = 6.48, p < 0.001). However, removing a resistance training intervention from the effect of programmed exercise on acute BDNF levels reduced the effect size previously observed (Hedges’ g = 0.26, SE = 0.15, 95% CI = −0.03–0.54, z = 1.78, p = 0.075), potentially due to the very large effect size obtained in the removed study (g = 3.53). Finally, removing one strength training study and two strength/resistance training study groups did not significantly affect the observed effect for resting BDNF levels following programmed exercise (Hedges’ g = 0.29, SE = 0.10, 95% CI = 0.09–0.50, z = 2.82, p = 0.005).
When examining the five studies which included strength/resistance training groups, the effect did not reach significance (Hedges’ g = 0.57, SE = 0.41, 95% CI = −0.24–1.37, z = 1.39, p = 0.17), perhaps due to two studies exhibiting moderate to strong positive effects and three studies exhibiting negative effects.
Moderators of Exercise Effects on BDNF Levels
Of the moderators of interest, the proportion of women in studies, participant age, and assay type (i.e., serum or plasma) were not confounded with the type of exercise program. Across all studies, we found a significant negative correlation between effect size and percentage of women in studies (r(33) = −0.38, p = 0.03). Effect sizes were smaller for studies with a greater proportion of women and retained significance when the one outlying effect size estimate (g = 3.53) was excluded from the analysis (r(32) = −0.37, p = 0.04). Across all studies, participant age was not significantly related to changes in BDNF levels following exercise (r(32) = −0.24, p = 0.19), nor was assay type (serum or plasma; Q(1) = 0.54, p = 0.46).
The potential moderators of diagnostic status and exercise frequency were specific to the type of exercise paradigm; hence, the latter variables were examined only within the relevant paradigms, albeit at low power. Within studies of resting BDNF levels, exercise frequency category was not significantly related to BDNF levels (Q(1) = 0.001, p = 0.98). Of note, effect sizes for two psychiatric samples studied (major depressive disorder and panic disorder) were at least that of healthy samples for both acute exercise (0.49 for psychiatric versus 0.40 for healthy; Q(1) = 0.86, p = 0.36) and resting BDNF following regular exercise (0.40 for psychiatric versus 0.17 for healthy).
Publication Bias
Publication bias was evaluated separately for each of the three paradigms examined.
Acute exercise
Evaluation of publication bias indicated that 191 studies reporting a null effect would be required to shift the observed effect to a non-significant level. A fail-safe N of 191 is substantially greater than the number of studies needed for a robust effect (N = 80) according to guidelines suggested by Rosenthal (1991), indicating that our effect is robust for this paradigm. Also, the funnel plot is for this paradigm is roughly symmetrical by visual inspection (see Supplemental Materials).
Programmed regular exercise
The fail-safe N of 50 for the effect of programmed regular exercise on BDNF levels immediately following exercise equaled the number of studies needed for a robust effect (N = 50), and the funnel plot appeared roughly symmetrical by visual inspection. Finally, the fail-safe N of 54 for the effect of programmed regular exercise on resting BDNF levels was marginally less than that needed for a robust effect (N = 80). The funnel plot also appears asymmetrical with more studies with small sample sizes appearing above the mean than below the mean, indicating that studies with smaller sample sizes are more likely to get published if the effect sizes are greater. Funnel plots are presented in Supplemental Materials.
Discussion
This meta-analysis provides reliable evidence that both acute and regular exercise have a significant impact on BDNF levels. Evidence from 14 studies indicated that a single session of exercise increases BDNF levels, reflecting a moderate effect size. Moreover, regular exercise intensifies the magnitude of these effects with increased BDNF responsivity, reflecting a moderate effect size, following a regular program of exercise relative to those completing acute exercise alone. Both of these findings are reliable as evaluated by fail-safe N and funnel plot analyses. Finally, 13 studies demonstrated that programs of regular exercise also impact resting BDNF levels; however, this effect was more modest than that seen immediately after exercise, and this effect was not considered robust.
Considering these findings, there is reliable evidence from human studies indicating that each episode of exercise results in a “dose” of BDNF activity and that the magnitude of this “dose” can be enhanced over time by regular exercise. The relative importance of these episodic BDNF doses, relative to the more subtle increases in resting BDNF levels seen across an exercise program, is not clear. Much more information is needed on the time course of benefits from exercise, particularly cognitive benefits, and the potential importance of BDNF activity at the time of learning, rather than as a general prime in the days before a learning task (Korol et al., 2013). Moreover, the staying power of brief exercise interventions needs to be elucidated, particularly given evidence in animal models that cognitive gains from brief exercise training (e.g., 4 weeks) can be lost within several weeks (e.g., Hopkins et al., 2011).
More generally, animal studies provide evidence for a variety of mechanisms by which BDNF enhancement from exercise results in improved cognition. For example, as little as one week of exercise improves subsequent learning in animals, an effect that is eliminated by blockade of BDNF in the hippocampus (Vaynman et al., 2004). Also, exercise-induced BDNF activity can reduce the threshold for successful encoding and memory (Intlekofer et al., 2013) and has been hypothesized to place the brain in a state of readiness for plasticity (Cotman et al., 2007). The precise mechanism of this readiness is made challenging by the host of pre-synaptic enhancement of neurotransmitter release and post-synaptic N-methyl-D-aspartate receptor (NMDA) and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor changes as well as important downstream activity (e.g., cAMP response element-binding protein; CREB) associated with exercise-induced BDNF activity (Christie et al., 2008; Vaynman et al., 2004), all of which could play a role in long-term potentiation effects. In addition, in terms of longer-term structural changes, exercise appeared to reverse hippocampal degeneration and declining hippocampal network efficiency in aged animals, with enhancement of presynaptic densities and greater connectivity (Siette et al., 2013). These changes appear to explain the observed improvements in recognition memory associated with exercise in animals (Cotman et al., 2007). In addition to the rescue of hippocampal neurons from the negative effects of aging, exercise-induced BDNF activity helps reduce the effects of specific stressors, including oxidative DNA changes (Yang et al., 2014) and disruption of synaptic plasticity from sleep deprivation (Zagaar et al., 2013). Also, relative to models for cognition, models for exercise-induced BDNF effects on mood have undergone only limited development, drawing from the stress-protective aspects of BDNF, as well as the effects of antidepressant medications on BDNF (for review, Duman and Monteggia, 2006), but otherwise not offering detailed mechanisms. Greater specification of the role of BDNF in mood disorders awaits additional study.
Concerning human studies, we had limited power to examine moderating influences of interpersonal and exercise characteristics, due to the limited sample size within each of the exercise paradigms investigated. When examining moderators across paradigms, sex significantly moderated the effect of exercise on BDNF levels, such that BDNF did not increase as much in females following exercise as in males. Given these data, we encourage further evaluation of this discrepancy in BDNF production and the use of sex as a covariate to hone the evaluation of exercise effects on BDNF, as well as outcomes assumed to be mediated by BDNF activity.
Also of interest is the magnitude of exercise effects on BDNF levels for psychiatric populations and healthy populations. In our meta-analysis, effect sizes between these populations were in similar ranges for acute exercise, but raised the question whether regular exercise may have greater effects in psychiatric than healthy participants, given a tentative 2:1 difference in mean effect sizes for BDNF changes across exercise (0.40 for psychiatric versus 0.17 for healthy). More studies are needed to see if these potential differences become reliable. Yet, our tentative results raise the possibility that programs of exercise may help rescue the low resting BDNF levels often observed in depressed patients (Brunoni et al., 2008; Piccinni et al., 2008) and exercise produces effects in a similar range to those of antidepressants (d = 0.62 for antidepressants, Sen et al., 2008). Future research may also focus on differences in exercise effects on BDNF in populations with medical conditions. In our meta-analysis, we did not find significant differences between healthy individuals and those with medical conditions (e.g., obesity, MS, diabetes), but conclusions are limited due to few studies examining medical populations.
Future studies also need to examine the effects of strength/resistance training on BDNF levels in comparison to effects of aerobic exercise on BDNF levels. Our meta-analysis had limited power to assess these affects due to the small number of studies including strength training. These studies also included considerable variation in the strength and direction of effects, with three studies indicating negative effects of strength training (Correia et al., 2010; Schiffer et al., 2009; Swift et al., 2012) on BDNF levels and two studies indicating positive effects (Goekint et al., 2010; Yarrow et al., 2010), with one of these studies showing a very large positive effect (Yarrow et al., 2010). Given the importance of both aerobic and strength exercises in an exercise program, it would be beneficial to identify BDNF changes across both paradigms.
Other potential moderators for future study include exercise intensity (e.g., mild, moderate, vigorous), sedentary status (e.g., sedentary, regular exercise completer, athlete), and genotype (e.g., val66met) as well as further investigating the potential negative association between age and effect size. Exercise intensity was reported in several studies as estimated % of VO2max. However, several studies failed to report intensity and many studies only evaluated moderate intensity exercise. Future studies should clearly report exercise intensity level, utilizing estimated metabolic equivalents (METs) in order to better uniformly describe results. In addition, future studies should consider investigating the effect of varying degrees of exercise intensity on BDNF production. Sedentary status (e.g., as quantified by the Physical Activity Recall Questionnaire: (Blair et al., 1985); or the International Physical Activity Questionnaire: (Craig et al., 2003)) and genotype expressions were rarely reported in detail in the studies examined, therefore, making these variables difficult to assess as moderator variables. The val66met polymorphism in particular may be important for understanding age-related cognitive decline and degree of BDNF response to exercise (Erickson et al., 2012; Phillips et al., 2014).
Despite these unanswered questions, the available evidence indicates that exercise should be considered as a successful strategy for enhancing BDNF activity. Accordingly, use of exercise to enhance cognitive abilities and living skills has recently been successful in dementia patients according to meta-analytic review (Forbes et al., 2013), with preliminary promising evidence in Parkinson’s disease (Ahlskog, 2011), and schizophrenia (Oertel-Knochel et al., 2014). The fact that exercise offers wide ranging physical health benefits (Alford, 2010) in addition to effects on mood (Asmundson et al., 2013; Josefsson et al., 2014; Stathopoulou et al., 2006) and cognition (Erickson et al., 2012; Smith et al., 2010) encourages the regular application of exercise as a particularly broad spectrum intervention.
Supplementary Material
Highlights.
Meta-analysis of brain-derived neurotrophic factor (BDNF) levels following exercise
We found a moderate effect of increased BDNF following a single session of exercise
Regular exercise intensified effect of a single session of exercise on BDNF levels
We found a small effect of increased resting BDNF levels after regular exercise
Sex moderated effect of exercise on BDNF levels with smaller effects for females
Acknowledgement
The first author’s contribution to this work was supported by grant F31 MH100773 from the National Institute of Mental Health.
Role of Funding Source
The first author’s contribution to this work was supported by grant F31 MH100773 from the National Institute of Mental Health. The National Institutes of Health had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication. The National Institute of Mental Health had no role other than financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
In the past 3 years Dr. Otto has served as a paid consultant for MicroTransponder Inc., Concert Pharmaceuticals, and ProPhase; provided expert consensus opinion for Otsuka Pharmaceuticals; received royalty support for use of the SIGH-A from ProPhase; and received book royalties from Oxford University Press, Routledge, and Springer. The other authors have no conflicts to report.
Contributors
Kristin L. Szuhany was involved in the retrieval and review of articles, data checking, data analysis, and preparation and revision of the manuscript, tables, and figures. Matteo Bugatti was involved in retrieval and review of articles, data entering, data analysis, and review of the manuscript. Michael W. Otto was involved in the review and revision of the manuscript as well as suggested avenues of data analysis. All authors have approved the final manuscript.
References
- Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77:288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford L. What men should know about the impact of physical activity on their health. International journal of clinical practice. 2010;64:1731–1734. doi: 10.1111/j.1742-1241.2010.02478.x. [DOI] [PubMed] [Google Scholar]
- Altar CA. Neurotrophins and depression. Trends in pharmacological sciences. 1999;20:59–62. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Gelfo F, De Bartolo P, Caltagirone C, Petrosini L. BDNF concentrations are decreased in serum and parietal cortex in immunotoxin 192 IgG-Saporin rat model of cholinergic degeneration. Neurochemistry international. 2011;59:1–4. doi: 10.1016/j.neuint.2011.04.010. [DOI] [PubMed] [Google Scholar]
- *.Araya AV, Orellana X, Godoy D, Soto L, Fiedler J. Effect of exercise on circulating levels of brain-derived neurotrophic factor (BDNF) in overweight and obese subjects. Hormone and metabolic research. 2013;45:541–544. doi: 10.1055/s-0032-1333237. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Fetzner MG, Deboer LB, Powers MB, Otto MW, Smits JA. Let's get physical: a contemporary review of the anxiolytic effects of exercise for anxiety and its disorders. Depression and anxiety. 2013;30:362–373. doi: 10.1002/da.22043. [DOI] [PubMed] [Google Scholar]
- *.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of neurology. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bansi J, Bloch W, Gamper U, Kesselring J. Training in MS: influence of two different endurance training protocols (aquatic versus overland) on cytokine and neurotrophin concentrations during three week randomized controlled trial. Multiple sclerosis (Houndmills, Basingstoke, England) 2013;19:613–621. doi: 10.1177/1352458512458605. [DOI] [PubMed] [Google Scholar]
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. American Journal of Epidemiology. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- Bornstein M, Hedges LV, Higgins J, Rothstein H. Comprehensive meta-analysis, version 2. Englewood, NJ: Biostat Inc.; 2005. [Google Scholar]
- *.Bos I, De Boever P, Vanparijs J, Pattyn N, Panis LI, Meeusen R. Subclinical effects of aerobic training in urban environment. Medicine and science in sports and exercise. 2013;45:439–447. doi: 10.1249/MSS.0b013e31827767fc. [DOI] [PubMed] [Google Scholar]
- *.Bos I, Jacobs L, Nawrot TS, de Geus B, Torfs R, Int Panis L, et al. No exercise-induced increase in serum BDNF after cycling near a major traffic road. Neuroscience letters. 2011;500:129–132. doi: 10.1016/j.neulet.2011.06.019. [DOI] [PubMed] [Google Scholar]
- *.Brunelli A, Dimauro I, Sgro P, Emerenziani GP, Magi F, Baldari C, et al. Acute exercise modulates BDNF and pro-BDNF protein content in immune cells. Medicine and science in sports and exercise. 2012;44:1871–1880. doi: 10.1249/MSS.0b013e31825ab69b. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- *.Castellano V, White LJ. Serum brain-derived neurotrophic factor response to aerobic exercise in multiple sclerosis. Journal of the neurological sciences. 2008;269:85–91. doi: 10.1016/j.jns.2007.12.030. [DOI] [PubMed] [Google Scholar]
- *.Cho HC, Kim J, Kim S, Son YH, Lee N, Jung SH. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO(2)max performance in healthy college men. Neuroscience letters. 2012;519:78–83. doi: 10.1016/j.neulet.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: how physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular medicine. 2008;10:47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological bulletin. 1992;112:155. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- *.Correia PR, Pansani A, Machado F, Andrade M, Silva AC, Scorza FA, et al. Acute strength exercise and the involvement of small or large muscle mass on plasma brain-derived neurotrophic factor levels. Clinics (Sao Paulo, Brazil) 2010;65:1123–1126. doi: 10.1590/S1807-59322010001100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Corripio R, Gonzalez-Clemente JM, Jacobo PS, Silvia N, Lluis G, Joan V, et al. Plasma brain-derived neurotrophic factor in prepubertal obese children: results from a 2-year lifestyle intervention programme. Clinical endocrinology. 2012;77:715–720. doi: 10.1111/j.1365-2265.2012.04431.x. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in neurosciences. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise. 2003;195:3508–1381. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2012;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AP. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed- and random-effects methods. Psychological methods. 2001;6:161–180. doi: 10.1037/1082-989x.6.2.161. [DOI] [PubMed] [Google Scholar]
- Forbes D, Thiessen EJ, Blake CM, Forbes SC, Forbes S. Exercise programs for people with dementia. The Cochrane database of systematic reviews. 2013;12:CD006489. doi: 10.1002/14651858.CD006489.pub3. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M, et al. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Medicine & Science in Sports & Exercise. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. 10.249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- *.Goekint M, De Pauw K, Roelands B, Njemini R, Bautmans I, Mets T, et al. Strength training does not influence serum brain-derived neurotrophic factor. European journal of applied physiology. 2010;110:285–293. doi: 10.1007/s00421-010-1461-3. [DOI] [PubMed] [Google Scholar]
- *.Goekint M, Heyman E, Roelands B, Njemini R, Bautmans I, Mets T, et al. No influence of noradrenaline manipulation on acute exercise-induced increase of brain-derived neurotrophic factor. Medicine and science in sports and exercise. 2008;40:1990–1996. doi: 10.1249/MSS.0b013e31817eee85. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. The Journal of neuroscience. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Stein LM, Lopes RP, Teixeira AL, Bauer ME. Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression--a preliminary report. Biological psychiatry. 2008;64:281–285. doi: 10.1016/j.biopsych.2008.02.023. [DOI] [PubMed] [Google Scholar]
- *.Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiology & behavior. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- *.Gustafsson G, Lira CM, Johansson J, Wisen A, Wohlfart B, Ekman R, et al. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry research. 2009;169:244–248. doi: 10.1016/j.psychres.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press; 1985. [Google Scholar]
- Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, et al. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Nitecki R, Bucci DJ. Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience. 2011;194:84–94. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Larsen KT, Ried-Larsen M, Moller NC, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: a review. Scandinavian journal of medicine & science in sports. 2014;24:1–10. doi: 10.1111/sms.12069. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hunter JE, Schmidt FL. Methods of meta-analysis: Correcting error and bias in research findings: Sage. 2004 [Google Scholar]
- Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:2027–2034. doi: 10.1038/npp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson T, Lindwall M, Archer T. Physical exercise intervention in depressive disorders: meta-analysis and systematic review. Scandinavian journal of medicine & science in sports. 2014;24:259–272. doi: 10.1111/sms.12050. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neuroscience letters. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Komulainen P, Pedersen M, Hanninen T, Bruunsgaard H, Lakka TA, Kivipelto M, et al. BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA Study. Neurobiology of learning and memory. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE, Scavuzzo CJ. Use it and boost it with physical and mental activity. Hippocampus. 2013;23:1125–1135. doi: 10.1002/hipo.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- *.Laske C, Banschbach S, Stransky E, Bosch S, Straten G, Machann J, et al. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. The International Journal of Neuropsychopharmacology. 2010;13:595–602. doi: 10.1017/S1461145709991234. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nature reviews Neuroscience. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Progress in neurobiology. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Oertel-Knochel V, Mehler P, Thiel C, Steinbrecher K, Malchow B, Tesky V, et al. Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. European archives of psychiatry and clinical neuroscience. 2014 doi: 10.1007/s00406-014-0485-9. [DOI] [PubMed] [Google Scholar]
- Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Frontiers in cellular neuroscience. 2014;8:170. doi: 10.3389/fncel.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinni A, Marazziti D, Catena M, Domenici L, Del Debbio A, Bianchi C, et al. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. Journal of affective disorders. 2008;105:279–283. doi: 10.1016/j.jad.2007.05.005. [DOI] [PubMed] [Google Scholar]
- *.Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental physiology. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Rethorst CD, Trivedi MH. Evidence-based recommendations for the prescription of exercise for major depressive disorder. Journal of psychiatric practice. 2013;19:204–212. doi: 10.1097/01.pra.0000430504.16952.3e. [DOI] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin C-Y, Kramár EA, Rogers GA, Gall CM, et al. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. Journal of Neurophysiology. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig M, Nordbrandt S, Geertsen SS, Nielsen JB. The effects of cardiovascular exercise on human memory: a review with meta-analysis. Neuroscience and biobehavioral reviews. 2013;37:1645–1666. doi: 10.1016/j.neubiorev.2013.06.012. [DOI] [PubMed] [Google Scholar]
- *.Rojas Vega SR, Kleinert J, Sulprizio M, Hollmann W, Bloch W, Struder HK. Responses of serum neurotrophic factors to exercise in pregnant and postpartum women. Psychoneuroendocrinology. 2011;36:220–227. doi: 10.1016/j.psyneuen.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research: Sage. 1991 [Google Scholar]
- Rosenthal R, Rubin DB. [Selection Models and the File Drawer Problem]: Comment: Assumptions and Procedures in the File Drawer Problem. Statistical Science. 1988;3:120–125. [Google Scholar]
- *.Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, et al. Physical activity and memory functions: an interventional study. Neurobiology of aging. 2011;32:1304–1319. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- *.Schiffer T, Schulte S, Hollmann W, Bloch W, Struder HK. Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Hormone and metabolic research. 2009;41:250–284. doi: 10.1055/s-0028-1093322. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Schmidt-Kassow M, Schadle S, Otterbein S, Thiel C, Doehring A, Lotsch J, et al. Kinetics of serum brain-derived neurotrophic factor following low-intensity versus high-intensity exercise in men and women. Neuroreport. 2012;23:889–893. doi: 10.1097/WNR.0b013e32835946ca. [DOI] [PubMed] [Google Scholar]
- *.Schulz KH, Gold SM, Witte J, Bartsch K, Lang UE, Hellweg R, et al. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. Journal of the neurological sciences. 2004;225:11–18. doi: 10.1016/j.jns.2004.06.009. [DOI] [PubMed] [Google Scholar]
- *.Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, et al. Endurance training enhances BDNF release from the human brain. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298:R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biological psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siette J, Westbrook RF, Cotman C, Sidhu K, Zhu W, Sachdev P, et al. Age-specific effects of voluntary exercise on memory and the older brain. Biological psychiatry. 2013;73:435–442. doi: 10.1016/j.biopsych.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic medicine. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulou G, Powers MB, Berry AC, Smits JAJ, Otto MW. Exercise Interventions for Mental Health: A Quantitative and Qualitative Review. Clinical Psychology: Science and Practice. 2006;13:179–193. [Google Scholar]
- *.Strohle A, Stoy M, Graetz B, Scheel M, Wittmann A, Gallinat J, et al. Acute exercise ameliorates reduced brain-derived neurotrophic factor in patients with panic disorder. Psychoneuroendocrinology. 2010;35:364–368. doi: 10.1016/j.psyneuen.2009.07.013. [DOI] [PubMed] [Google Scholar]
- *.Swift DL, Johannsen NM, Myers VH, Earnest CP, Smits JA, Blair SN, et al. The effect of exercise training modality on serum brain derived neurotrophic factor levels in individuals with type 2 diabetes. PloS one. 2012;7:e42785. doi: 10.1371/journal.pone.0042785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neuroscience letters. 2008;431:62–65. doi: 10.1016/j.neulet.2007.11.019. [DOI] [PubMed] [Google Scholar]
- *.Toups MS, Greer TL, Kurian BT, Grannemann BD, Carmody TJ, Huebinger R, et al. Effects of serum Brain Derived Neurotrophic Factor on exercise augmentation treatment of depression. Journal of psychiatric research. 2011;45:1301–1306. doi: 10.1016/j.jpsychires.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European journal of neuroscience. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- *.Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain, behavior, and immunity. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman AS, Young DE, He X, Chen TC, Wagenaar RC, Stern CE, et al. Interaction between serum BDNF and aerobic fitness predicts recognition memory in healthy young adults. Behavioural brain research. 2014;259:302–312. doi: 10.1016/j.bbr.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014;16:161–174. doi: 10.1007/s12017-013-8270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Yarrow JF, White LJ, McCoy SC, Borst SE. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF) Neuroscience letters. 2010;479:161–165. doi: 10.1016/j.neulet.2010.05.058. [DOI] [PubMed] [Google Scholar]
- Zagaar M, Dao A, Alhaider I, Alkadhi K. Regular treadmill exercise prevents sleep deprivationinduced disruption of synaptic plasticity and associated signaling cascade in the dentate gyrus. Molecular and cellular neurosciences. 2013;56:375–383. doi: 10.1016/j.mcn.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2010;61:533–541. [PubMed] [Google Scholar]
- Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2008;59(Suppl 7):119–132. * All references marked with an asterisk were included in the meta-analysis.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.