Abstract
Background
There are currently no well-defined, evidence-based guidelines for management of end stage heart failure in patients over 65 and decisions to utilize mechanical circulatory support with left ventricular assist device (LVAD), either as a bridge to transplant or destination therapy, or isolated heart transplant (HTx) remain controversial. We aim to compare outcomes following implementation of thee heart replacement strategies in this high-risk population.
Methods
We conducted a retrospective, cohort study of all patients between the age of 65–72 receiving a continuous-flow LVAD as bridge to transplant or destination therapy or isolated HTx at our center between 2005–2012. Patients were stratified according to treatment strategy into 3 groups; Group D (destination LVAD, n=23), Group B (bridge to transplant LVAD, n=43), and Group H (HTx alone, n=47). Primary outcomes of interest were survival to discharge and 2-year overall survival.
Results
Patients in Group D were significantly older, had a higher prevalence of ischemic cardiomyopathy, and a higher pulmonary vascular resistance than patients in Groups B or H. There were no significant differences between groups in survival to discharge (87% D vs. 83.7% B vs. 87.2% H, p=0.88) or 2 year overall survival (75.7% D vs. 68.7% B vs. 80.9% H, log-rank p=0.47). Incidence rates of readmission were 1.1 events/patient*year in Group D and 0.5 events/patient*year in Group H.
Conclusions
There was no significant difference in perioperative, short, and medium-term survival between treatment groups. However, LVAD patients had a higher incidence of readmission. Larger trials are needed to refine differences in long-term survival, quality of life, and resource utilization for elderly patients requiring heart replacement therapy.
Keywords: Circulatory Support, Geriatric, Heart Failure, Heart Transplantation
INTRODUCTION
Advanced heart failure remains a burdensome disease that is responsible for significant morbidity and mortality as well as approximately $39 billion annually in healthcare costs in the United States (1). While it is widely accepted that heart transplantation (HTx) with or without bridge-to-transplant (BTT) left ventricular assist device (LVAD) is the gold standard for end-stage heart failure in younger patients eligible for transplant, definitive guidelines are lacking for heart replacement therapy in the high-risk population of heart failure patients older than 65 years of age. Undoubtedly, with the combination of a growing elderly population, an increasing burden of ischemic heart disease, and improving medical therapy, the prevalence of end-stage heart failure in older patients will continue to rise in the foreseeable future.
Numerous studies have evaluated HTx in this population in comparison to younger patients and have found mixed results regarding the equivalence of survival outcomes (2–6). Some centers have employed alternate waiting list strategies for elderly patients with worsened overall survival compared to non-high-risk cohorts (7–8). Similarly, multiple studies have compared VAD therapy [both BTT and destination therapy (DT)] in the elderly population with the standard population and generally found decreased, although acceptable, survival in older patients (9–12). With increasing DT experience (13), it is possible that durable LVAD therapy could afford elderly patients with comparable outcomes compared to HTx, thus obviating the need to utilize precious organ resources in this high-risk population. However, to date, no study has specifically compared outcomes following isolated HTx vs. continuous-flow (CF) LVAD as DT or BTT in the elderly. Therefore, our aim in this study is to compare post-operative survival and resource utilization between these three heart replacement strategies in patients older than 65 years. These findings may impact current guidelines that lack robust evidence-based support.
METHODS
Patient Selection
All patients undergoing either durable CF-LVAD (both DT and BTT) placement or isolated heart transplantation at our institution between 2005–2012 were reviewed. Patients who were between the ages of 65 and 72 years at the index operation were selected for the study cohort and included in the analysis. Patients older than 72 years in the CF-LVAD cohort were excluded because they are ineligible for heart transplantation at our center. Patients were stratified according to their initial, as-treated therapy: Group D (DT-LVAD, n=23), Group B (BTT-LVAD, n=43), and group H (isolated HTx, n=47). This study was approved by the Columbia University Institutional Review Board and need for informed consent was waived.
All advanced heart failure patients are evaluated by a multidisciplinary team to determine their need for heart replacement therapy. In this age group, if the patients meets criteria for a heart transplant, they are listed on the alternate transplant waiting list to be paired with an extended-criteria donors. The decision to institute mechanical circulatory support (MCS) in those listed for transplant (BTT) or in those ineligible for transplant (DT) is based on presenting characteristics including severity of heart failure and end-organ function as well as physician preference. At our center, there are no pre-defined protocols that address the decision to implement durable MCS, but rather a consensus agreement is made on a case-by-case basis after discussion with the heart failure team.
Heart failure regimen for patients implanted with LVAD includes a neurohormonal antagonist, diuretics, and an anti-arrhythmic agent if needed, and anticoagulation strategy includes warfarin and aspirin. Goal international normalized ratio is between 2–3 and adjustments in anticoagulation regimen are managed by the LVAD clinic. For patients in the transplant group, all transplants were performed with bi-caval anastomoses. Immunosuppression regimen includes azathioprine and methylprednisolone induction followed by maintenance with a calcineurin inhibitor (cyclosporine or tacrolimus), mycophenolate mofetil, and prednisone. Upon discharge, these patients are followed regularly by the advanced heart failure clinic.
Data Collection
All data used for analysis was collected retrospectively from the electronic medical record. Variables collected included baseline characteristics, pre-operative comorbidities, pre-operative echocardiographic findings, right heart catheterization hemodynamic variables, need for pre-operative MCS, operative details, post-operative complications, and basic readmission data (dates and reason for admission). Readmission was defined as any unplanned readmission. Routine endomyocardial biopsies in transplant patients were not considered as readmissions unless they were complicated. Survival data was determined using hospital medical records for in-hospital deaths and the Social Security Death Index for out-of-hospital deaths.
Statistical Analysis
Data was analyzed using SPSS version 21 (IBM corp., Armonk, NY). Continuous variables are reported as mean ± standard deviation and compared using one-way ANOVA tests or independent samples t-test where applicable. Post-hoc analysis was performed using the Bonferroni method when comparison of ANOVA results was desired. Categorical variables are reported as frequency and percentage of total group and compared using Pearson’s chi-squared test or Fisher’s Exact test where applicable.
Kaplan-Meier analysis was used to compare 2-year survival and freedom from 1 and 2 readmissions at 2 years. Time zero was on the day of implant for patients receiving an LVAD and on the day of transplant for patients in Group H. For survival analysis, all patients were censored on the date of death or at the conclusion of the study period. Patients in Group B were not censored on the day of transplant in order to compare overall outcomes following implementation of heart replacement therapy. The log-rank test was used to determine significant differences in survival and readmission curves. Cox proportional-hazard regression analysis was used to determine pre-operative risk factors predictive of late death. All pre-operative variables with a p-value of ≤ 0.2 on univariable analysis were entered into a multivariable regression model to determine independent predictors of death. Results are presented as hazard ratios (HR) and 95% confidence intervals. For analysis of readmission data, Group D is compared with Group H given that patients from Group B undergoing transplant could potentially have readmissions as both “LVAD” patients and as “transplant” patients. All p-values ≤ 0.05 are considered statistically significant.
RESULTS
Patient Demographics and Baseline Characteristics
Demographics, etiology of heart failure, comorbidities, and baseline clinical data are presented in Table 1. Patients in Group D were significantly older and had a higher prevalence of coronary artery disease and ischemic etiology of heart failure. Likewise, there was a higher incidence of dilated cardiomyopathy in Groups B and H than in Group D. There were no other significant differences in etiology of heart failure or comorbidities between groups. There was a statistical difference in the left ventricular end diastolic dimension between Group B and Group H (B vs. H, p=0.02), but no other differences in echocardiographic variables. Patients in Groups D and B had significantly higher mean pulmonary artery (D vs. H, p<0.001, B vs. H, p=0.03, D vs. B, p=0.06) and pulmonary capillary wedge pressure (D vs. H; p=0.002, B vs. H; p=0.01, D vs. B; p=0.87) than Group H. Patients in Group D had significantly higher pulmonary vascular resistance than both Groups B and H (D vs. B; p=0.01, D vs. H; p=0.01, B vs. H; p=1.00). Finally, patients in groups D and B required the use of a pre-operative intra-aortic balloon pump more frequently than patients in Group H (p<0.001), and patients in Group B had a significantly greater requirement for pre-operative biventricular assist device or extracorporeal membrane oxygenation than either group D or H (p=0.03). There was no difference in pre-operative inotrope dependence between groups.
Table 1.
Baseline Patient Characteristics
| Group D | Group B | Group H | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Total, n | 23 | 43 | 47 | |
| Age, years | 69.7 ± 2.2 | 67.2± 2.1 | 67.1 ± 2.1 | <0.001 |
| Male, n (%) | 19 (82.6) | 38 (88.4) | 46 (97.9) | 0.08 |
| BSA, m2 | 1.82 ± 0.20 | 1.94± 0.28 | 1.94 ± 0.21 | 0.12 |
| NYHA class | 3.5 ± 0.5 | 3.5± 0.7 | 3.5± 0.5 | 0.99 |
| Etiology of Heart Failure, n(%) | ||||
| Ischemic | 20 (87.0) | 24 (55.8) | 29 (61.7) | 0.04 |
| Dilated | 2 (8.7) | 17 (39.5) | 12 (25.5) | 0.03 |
| Restrictive | 0 (0) | 1 (2.3) | 3 (6.4) | 0.34 |
| Valvular | 1 (4.3) | 2 (2.3) | 2 (4.3) | 0.86 |
| Other | 0 (0) | 0 (0) | 1 (2.1) | 0.49 |
| Co-morbidities, n(%) | ||||
| Coronary artery disease | 21 (91.3) | 27 (62.8) | 32 (68.1) | 0.05 |
| Myocardial infarction | 14 (60.9) | 20 (46.5) | 18 (38.3) | 0.21 |
| Diabetes | 9 (39.1) | 16 (37.2) | 17 (36.2) | 0.97 |
| Hypertension | 15 (65.2) | 18 (41.9) | 21 (44.7) | 0.17 |
| Hyperlipidemia | 12 (52.2) | 15 (34.9) | 14 (29.8) | 0.18 |
| Stroke/TIA | 1 (4.3) | 5 (11.6) | 3 (6.4) | 0.51 |
| Peripheral arterial disease | 2 (8.2) | 3 (7.0) | 6 (12.8) | 0.64 |
| Chronic Kidney Disease | 14 (60.9) | 15 (34.9) | 17 (36.2) | 0.09 |
| COPD | 3 (13.0) | 5 (11.6) | 3 (6.4) | 0.59 |
| Prior cardiac surgery | 13 (56.5) | 18 (41.9) | 28 (59.6) | 0.22 |
| Echocardiographic Data | ||||
| LV ejection fraction, % | 19.5 ± 5.3 | 20.4± 4.4 | 21.9 ± 9.1 | 0.34 |
| LVEDD, cm | 6.5 ± 0.8 | 6.8± 0.8 | 6.2 ± 1.2 | 0.02 |
| RV function a | 2.3 ± 0.7 | 2.1± 0.6 | 2.0 ± 0.6 | 0.37 |
| Catheterization Data | ||||
| Mean PAP, mmHg | 41.3 ± 7.6 | 35.5± 9.7 | 30.3 ± 9.9 | <0.001 |
| PCWP, mmHg | 27.5 ± 7.6 | 25.3± 9.0 | 20.2 ± 7.2 | 0.001 |
| CVP, mmHg | 15.7 ± 8.6 | 12.5± 6.9 | 9.8 ± 5.7 | 0.005 |
| Cardiac Output, L/min | 2.7 ± 0.6 | 3.4± 1.1 | 3.2 ± 1.0 | 0.08 |
| Cardiac index, L/min/m2 | 1.4 ± 0.3 | 1.7± 0.5 | 1.7 ± 0.5 | 0.04 |
| PVR, dyn*s/cm5 | 448.2 ± 170.5 | 284.1± 136.7 | 288.3 ± 184.4 | 0.006 |
| RVSWI, gm/m2/beat | 7.1 ± 3.3 | 7.8± 3.6 | 7.7 ± 2.5 | 0.83 |
| Pre-operative Support, n(%) | ||||
| Inotrope | 18 (78.3) | 41 (95.3) | 39 (83.0) | 0.09 |
| Intra-aortic balloon pump | 7 (30.4) | 12 (27.9) | 0 (0) | <0.001 |
| Centrimag BiVAD/ECMO | 0 (0) | 4 (9.3) | 0 (0) | 0.03 |
RV function scale (1 = normal, 2= mild to moderate dysfunction, 3= severe dysfunction)
Abbreviations: BSA=body surface area, COPD=chronic obstructive pulmonary disease, CVP=central venous pressure, ECMO=extracorporeal membrane oxygenation, LVEDD=left ventricular end diastolic dimension, PAP=pulmonary arterial pressure, PCWP=pulmonary capillary wedge pressure, PVR=pulmonary vascular resistance, RVSWI=right ventricular stroke work index, TIA=transient ischemic attack
Contraindication to transplant for patients in Group D is presented in Table 2. The most common reasons for deferral to destination therapy was irreversible pulmonary hypertension (34.8%) and advanced age (26.1%). In one case, the patient decided she would rather undergo destination LVAD therapy than heart transplantation despite being eligible for a transplant.
Table 2.
Group D Contraindications to Transplant
| Contraindication | N (%) |
|---|---|
| Irreversible pulmonary hypertension | 8 (34.8) |
| Advanced age | 6 (26.1) |
| Creatinine> 2.5 mg/dL | 3 (13.1) |
| Multiple comorbidities | 3 (13.1) |
| Lack of social support | 1 (4.3) |
| Patient preference | 1 (4.3) |
| Recent history of malignancy | 1 (4.3) |
Surgical Characteristics
Device characteristics and operative details for all 3 groups are presented in Table 3. The Thoratec HeartMate II was the most commonly implanted device in both groups (87% in Group D, 81.4% in Group B). During implant, cardiopulmonary bypass was used in all but 2 cases, which were done under a very brief period of paced ventricular fibrillation. Of concomitant procedures performed during implant, there were significantly more mitral valve repairs in Group D compared to Group B (p=0.02). There were no significant differences in other concomitant procedures performed between Group D and Group B. In Group H, mean graft ischemic time was 192 ± 55 minutes and mean donor age was 39.7 ± 13.3 years.
Table 3.
Heart Replacement Therapy Surgical Characteristics
| Group D | Group B | |
|---|---|---|
| Device Type, n (%) | ||
| Thoratec Heart Mate II | 20 (87.0) | 35 (81.4) |
| HeartWare | 3 (13.0) | 1 (2.3) |
| DuraHeart | 0 (0) | 3 (7.0) |
| Ventrassist | 0 (0) | 3 (7.0) |
| DeBakey VAD | 0 (0) | 1 (2.3) |
| Concomitant Procedures, n (%) | ||
| Aortic valve repair | 8 (34.8) | 12 (27.9) |
| Mitral valve repair | 8 (34.8) | 4 (9.3) |
| Tricuspid valve repair | 4 (17.4) | 14 (32.6) |
| PFO closure | 3 (13.0) | 4 (9.3) |
| CABG | 1 (4.3) | 1 (2.3) |
| Group H | ||
| Graft ischemic time, mins | 192 ± 55 | |
| Donor age, years | 39.7 ± 13.3 | |
Abbreviations: CABG=coronary artery bypass grafting, PFO=patent foramen ovale
Outcomes
Survival and post-operative complications, and readmission data are presented in Table 4 and Figure 1. There was no significant difference in survival to discharge (87% Group D, 83.7% Group B vs. 87.2% Group H, p=0.88), 1-year survival (82.6% D vs. 76.7% B vs. 83.0% H, p=0.73), or estimated 2-year survival by Kaplan-Meier analysis (75.7% D vs. 68.9% B vs. 80.9% H, p=0.47). Of patients in Group B, 79% eventually underwent heart transplant at a mean duration of LVAD support of 233 ± 157 days, 7% were alive on their initial device at the end of the study period, and 14% died on the device while awaiting HTx. Mean follow-up time was significantly longer in Group H than Groups D and B (p<0.001). On multivariable Cox proportional-hazard regression analysis, New York Heart Association classification [HR 2.35 (1.00 – 5.50), p = 0.05], pre-operative need for extracorporeal membrane oxygenation [HR 12.09 (1.52–96.47), p = 0.02], and female gender [HR 3.2 (1.20–8.55, p = 0.02] were independent predictors of late death.
Table 4.
Patient Outcomes
| Group D | Group B | Group H | p-value | |
|---|---|---|---|---|
| Survival | ||||
| Discharge, n (%) | 20 (87.0) | 36 (83.7) | 41 (87.2) | 0.88 |
| 1-year, n (%) | 19 (82.6) | 33 (76.7) | 39 (83.0) | 0.73 |
| Post-operative Complications, n (%) | ||||
| Re-operation for bleeding | 3 (13.0) | 8 (18.6) | 11 (23.4) | 0.58 |
| Need for CRRT | 3 (13.0) | 7 (16.3) | 7 (14.9) | 0.94 |
| Respiratory Failure | 3 (13.0) | 6 (14.0) | 8 (17.0) | 0.88 |
| Infection | 6 (26.1) | 14 (32.6) | 12 (25.5) | 0.74 |
| Any arrhythmia | 8 (34.8) | 17 (39.5) | 5 (10.6) | 0.005 |
| Stroke | 2 (8.7) | 2 (4.7) | 2 (4.3) | 0.72 |
| Post-op MCS requirement a | 1 (4.3) | 4 (9.3) | 4 (8.5) | 0.77 |
| RV failure | 2 (8.7) | 8 (18.6) | 2 (4.3) | 0.08 |
| Device malfunction | 1 (4.3) | 0 (0) | --- | 0.35 |
| Graft Rejection (≥2R on biopsy) | --- | --- | 1 (2.1) | --- |
| Readmission Data | ||||
| 1 RA in 1st year, n (%) | 14 (70.0) | --- | 26 (63.4) | 0.61 |
| >1 RA in 1st year, n (%) | 7 (35.0) | --- | 13 (31.7) | 0.79 |
| Time to 1st RA, days | 243 ± 236 | --- | 380 ± 626 | 0.26 |
| Time to 2nd RA, days | 407 ± 253 | --- | 716 ± 779 | 0.09 |
| Incidence of RA, event/pt*yr | 1.1 | --- | 0.5 | --- |
| Follow-up Data | ||||
| Mean follow-up, years | 1.8 ± 1.2 | 2.5 ± 2.0 | 4.2 ± 2.7 | <0.001 |
Centrimag RVAD in Group D/B
Abbreviations: CRRT=continuous renal replacement therapy, MCS=mechanical circulatory support, RA=readmission
Figure 1.
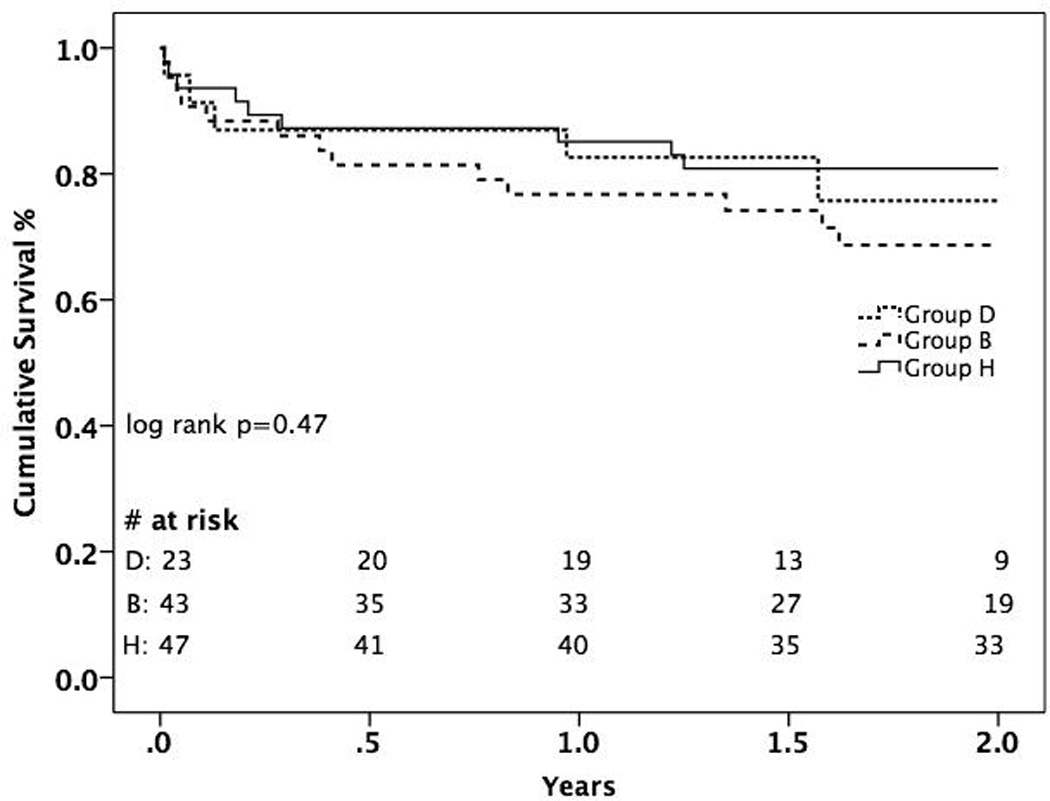
Kaplan-Meier Analysis of 2-year Survival Stratified by Treatment Group
There were a total of 45 readmissions in group D and 104 readmissions in Group H over the study period making the incidence rate of readmission 1.1 events/patient*year in Group D and 0.5 events/patient*year in Group H. There were no significant differences in patients requiring 1 readmission in the 1st year, >1 readmission in the 1st year, or time to 1st or 2nd readmission between groups. Kaplan-Meier analysis showed no significant difference in 2-year freedom from 1 (p=0.75, data not shown) or 2 readmissions between groups (p=0.77, Figure 2). The most common causes of readmission in Group D were bleeding from any source (n=11), infection (n=10), and cardiac related (volume overload/arrhythmia) and neurological symptoms (n=8 for each category). In comparison, readmissions in Group H were due to infection (n=40), graft rejection (n=15), and cardiac causes other than rejection (n=12).
Figure 2.
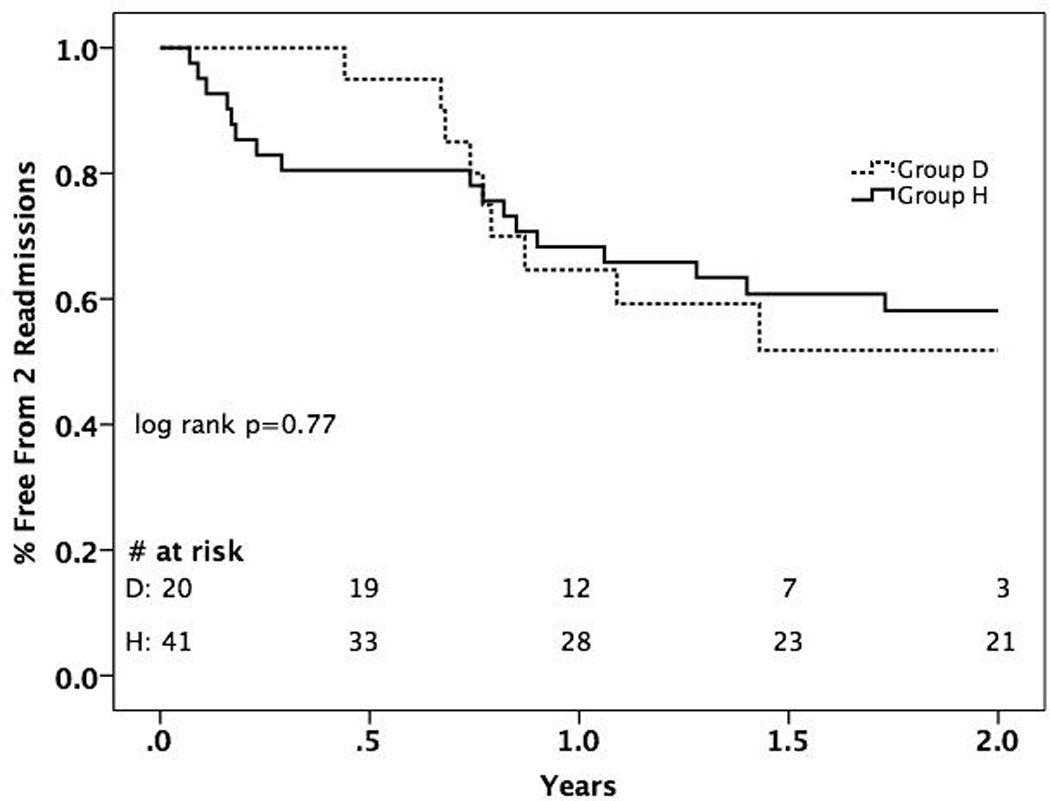
Kaplan-Meier Analysis of 2-Year Freedom from 2 Readmissions Between Destination Therapy LVAD and Isolated Heart Transplant Groups
DISCUSSION
End-stage heart failure in the elderly remains a difficult entity to treat given high rates of patient co-morbidities and the lack of well-defined therapeutic guidelines for this population. Multiple prior studies have compared either HTx or CF-LVAD in older patients with younger patients receiving the same treatment with mixed results regarding outcomes. Although transplantation remains the gold standard for younger patients with advanced heart failure who are eligible for transplant, it is not clear that older patients derive the same survival benefit from transplant and may, in fact, have similar outcomes when treated with chronic VAD therapy. However, there are currently no direct comparison studies addressing post-operative outcomes following all three heart replacement strategies (HTx, BTT LVAD, and DT LVAD) in patients over 65 years of age. With equivocal post-transplant outcomes (2–8), increasing wait times for patients listed for HTx (14), and increasing experience with destination LVAD therapy (13), the optimal treatment strategy for this unique population remains elusive.
In this study, we compared outcomes after DT LVAD, BTT LVAD, or isolated HTx in patients between age 65 and 72 years old and found no significant difference in short and medium-term survival between all 3 groups. Furthermore, we found no difference in 2-year freedom from 1 or 2 readmissions or time to readmission between groups, however, the overall incidence of readmission is higher in LVAD patients. These results suggest that transplant and durable LVAD therapy, including DT, may provide equivalent medium-term survival benefit for elderly patients requiring heart replacement. Additionally, LVAD patients appear to utilize a greater number of post-operative healthcare resources.
In addition to an assessment of patient outcomes, our study also highlights the inherent differences between patients who undergo LVAD implant and those who receive an isolated heart transplant. Based on our echocardiographic, hemodynamic, and pre-operative mechanical circulatory support data, it is evident that LVAD patients present for implant in a more clinically advanced state of heart failure than isolated transplant patients. Given this fact, our initial hypothesis was that isolated HTx would lead to improved survival over LVAD, however, this was not borne out in our analysis. Thus, while the decision to pursue early LVAD versus prolonged inotropic support while waiting for isolated HTx should be based on the preoperative characteristics of the patient and is the subject of a different analysis, it appears that once heart replacement therapy is implemented, either through HTx or LVAD implant, there is no statistical difference in medium-term survival between treatment strategies in this population.
Although the difference in 2-year survival curves between the 3 comparison groups was not statistically significant, it appears that survival in Group B begins to separate from the curves of Group D and Group H at approximately 6 months. It would make clinical sense that subjecting an elderly patient to 2 major cardiac operations would expose those patients to an increased risk of morbidity and mortality compared to patients undergoing only 1 major operation. In addition to increased risk to the patient, resource utilization and healthcare costs are likely significantly higher for patients who undergo both an LVAD implant and HTx compared to one or the other. Although speculative, should this trend continue into the long-term, it would suggest that once an elderly patient reaches the point of needing LVAD placement, DT should be pursued over BTT. Further follow-up is required to validate this preliminary observation.
We found no differences between proportion of Groups D or H that required 1 or >1 readmission in the 1st year after surgery, time to 1st or 2nd readmission, or 2-year freedom from 1 and 2 readmission. However, we did note a higher incidence rate of readmission in LVAD patients over the entire study follow-up period. This suggests that late readmissions in the LVAD group are responsible for the higher incidence, which can also be gleaned from evaluation of Figure 2 that shows a crossing of curves at approximately 300 days. The expectation is that HTx patients should improve significantly following a successful transplant and should eventually require readmission less frequently. Conversely, patients with a DT LVAD will continue to require re-hospitalization for the same co-morbidities that led to their ineligibility for transplant as well as for LVAD-related complications, thus leading to persistently high readmission rates. With further refinement of LVAD therapy including device design, anticoagulation strategy, and infection prophylaxis, it is likely that resource utilization for LVAD patients will decrease.
To date, there is no published comparison of these 3 treatment strategies in elderly patients, however, other studies have compared similar populations using a similar design approach (10, 15, 16). The most relevant to our analysis was published by the Duke University group and compared 60 DT LVAD patients with 93 extended-criteria alternate list HTx patients. Analysis showed no difference in 30-day mortality (2.5% HTx vs. 6.7% LVAD) and 1-year survival (82.2% HTx vs. 77.5% LVAD). At 3 years, the extended criteria HTx group had significantly better survival (73% HTx vs. 50% LVAD). However, once performing propensity matching and removing pulsatile-flow LVADs from the DT group, they found no difference in survival at 3 years (estimated survival ~75% in both groups). There are some important differences between our study and the Duke study, namely that all LVAD patients in our analysis received 2nd generation CF devices and that we only included elderly patients instead of all extended-criteria transplant candidates which includes younger patients as well. However, despite these differences, our short and medium-term survival data appear to be similar.
The main limitation of our study was its retrospective, single-center nature that reflects the biases of our heart failure group in treating elderly patients. Also, it is clear from our analysis that LVAD patients and HTx patients represent two different populations of patients pre-operatively. Therefore, one must be cautious about making comparisons between them. The purpose of this analysis was to evaluate survival differences between patients after the implementation of heart replacement therapy, and the decision regarding which therapy to employ must be based on the presenting characteristics of each patient and is the subject of a different analysis. Finally, there was a significant difference in follow-up time between groups. However, despite these limitations, we feel that our data allows for accurate analysis of the population.
In conclusion, we have shown that there is no difference in perioperative or medium-term survival following the implementation of the three available heart replacement strategies in elderly patients. While this finding does not allow us to determine which therapy is optimal in this population given the baseline differences between groups, it does suggest that destination VAD therapy offers a comparable survival benefit to transplantation in end-stage heart failure patients over 65 years. Refinement of treatment guidelines in these high-risk patients will require large, multi-center trials with long-term follow-up as well as analysis of quality of life and healthcare expenditures.
Footnotes
DISCLOSURES
Drs. Naka and Jorde serve as advisors to the Thoratec Corporation.
REFERENCES
- 1.Norton C, Georgeiopoulous VV, Kalogeropoulos AP, Butler J. Epidemiology and Cost of Advanced Heart Failure. Prog Cardiovasc Dis. 2011;54(2):78–85. doi: 10.1016/j.pcad.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Peraira JR, Segovia J, Fuentes R, et al. Differential Characteristics of Heart Transplantation in Patients Older than 60 Years. Transplant Proc. 2003;35:1959–1961. doi: 10.1016/s0041-1345(03)00650-x. [DOI] [PubMed] [Google Scholar]
- 3.Borkon AM, Muehlebach GF, Jones PG, et al. An Analysis of the Effect of Age on Survival After Heart Transplant. J Heart Lung Transplant. 1999;18(7):668–674. doi: 10.1016/s1053-2498(99)00024-8. [DOI] [PubMed] [Google Scholar]
- 4.Favaloro R, Diez M, Bertolotti A, et al. Orthotopic Heart Transplantation in Elderly Patients: A 10-Year Experience at a Single Center. Transplant Proc. 2004;36:1692–1694. doi: 10.1016/j.transproceed.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 5.Daneshvar D, Czer LSC, Phan A, Schwartz ER, et al. Heart Transplantation in Patients Aged 70 Years and Older: A Two-Decade Experience. Transplant Proc. 2011;43:3851–3856. doi: 10.1016/j.transproceed.2011.08.086. [DOI] [PubMed] [Google Scholar]
- 6.Demers P, Moffatt S, Oyer PE, et al. Long-term results of heart transplantation in patients older than 60 years. J Thorac Cardiovasc Surg. 2003;126(1):224–231. doi: 10.1016/s0022-5223(03)00055-2. [DOI] [PubMed] [Google Scholar]
- 7.Sandner SE, Zimpfer D, Zrunck P, et al. Age and Outcome After Continuous-Flow Left Ventricular Assist Device Implantation as Bridge to Transplantation. J Heart Lung Transplant. 2009;28(4):367–372. doi: 10.1016/j.healun.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Russo MJ, Davies RR, Hong KN, et al. Matching High-Risk Recipients With Marginal Donor Hearts Is a Clinically Effective Strategy. Ann Thorac Surg. 2009;87:1066–1071. doi: 10.1016/j.athoracsur.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felker GM, Milano CA, Yager JEE, et al. Outcomes With an Alternate List Strategy for Heart Transplantation. J Heart Lung Transplant. 2005;24(11):1781–1785. doi: 10.1016/j.healun.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum AN, John R, Liao KK, et al. Survival in Elderly Patients Supported With Continuous Flow Left Ventricular Assist Device as Bridge to Transplantation or Destination Therapy. J Card Fail. 2014;20(3):161–167. doi: 10.1016/j.cardfail.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Atluri P, Goldstone AB, Kobrin DM, et al. Ventricular Assist Device Implant in the Elderly is Associated With Increased, but Respectable Risk: A Multi-Institutional Study. Ann Thorac Surg. 2013;96:141–147. doi: 10.1016/j.athoracsur.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamson RM, Stahovich M, Chillcott S, et al. Clinical Strategies and Outcomes in Advanced Heart Failure Patients Older Than 70 Years of Age Receiving the HeartMate II Left Ventricular Assist Device: A Community Hospital Experience. J Am Coll Cardiol. 2011;57(25):2487–2495. doi: 10.1016/j.jacc.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter MS, Rogers JG, Milano CA, et al. Advanced Heart Failure Treated with Continous-Flow Left Ventricular Assist Device. New Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 14.Colvin-Adamas M, Smithy JM, Heubner BM, et al. OPTN/SRTR 2012 Annual Data Report: Heart. Am J Transplant. 2014;14(s1):113–138. doi: 10.1111/ajt.12583. [DOI] [PubMed] [Google Scholar]
- 15.Daneshmand MA, Rajagopal K, Lima B, et al. Left Ventricular Assist Device Destination Therapy Versus Extended Criteria Cardiac Transplant. Ann Thorac Surg. 2010;89:1205–1210. doi: 10.1016/j.athoracsur.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 16.Williams ML, Trivedi JR, McCants KC, et al. Heart Transplant vs Left Ventricular Assist Device in Heart Transplant-Eligible Patients. Ann Thorac Surg. 2011;91:1330–1334. doi: 10.1016/j.athoracsur.2011.01.062. [DOI] [PubMed] [Google Scholar]


