Abstract
Mycobacterium tuberculosis remains a major public health burden. It is generally thought that while B cell- and antibody-mediated immunity plays an important role in host defense against extracellular pathogens, the primary control of intracellular microbes derives from cellular immune mechanisms. Studies on the immune regulatory mechanisms during infection with M. tuberculosis, a facultative intracellular organism, has established the importance of cell-mediated immunity in host defense during tuberculous infection. Emerging evidence suggest a role for B cell and humoral immunity in the control of intracellular pathogens, including obligatory species, through interactions with the cell-mediated immune compartment. Recent studies have shown that B cells and antibodies can significantly impact on the development of immune responses to the tubercle bacillus. In this review, we present experimental evidence supporting the notion that the importance of humoral and cellular immunity in host defense may not be entirely determined by the niche of the pathogen. A comprehensive approach that examines both humoral and cellular immunity could lead to better understanding of the immune response to M. tuberculosis.
Key works: Mycobacterium tuberculosis, tuberculosis, B cells, antibodies, FcγR
Introduction
Mycobacterium tuberculosis remains a significant public health burden worldwide. The World Health Organization reported that in 2012, there were 8.6 million incident cases of tuberculosis [1]. That same year, the disease killed 1.3 million people, 170,000 of whom died from multidrug-resistant infection [1] Co-evolution of the tubercle bacillus with the human host for centuries [2–4] has bestowed upon this pathogen a remarkable adaptability and tenacity for survival in an infected host, facilitated by a wide array of sophisticated mechanisms to modulate and to evade the host immune response [5, 6]. A naïve host develops primary tuberculosis upon the first encounter with M. tuberculosis [7]. Most of the infection is restricted and well contained at the primary site of bacterium-host interaction and the local draining lymph nodes, which together, are called the Ghon complex [7]. It is generally accepted despite being well controlled, the bacilli are not eradicated due to the unique ability of M. tuberculosis to enter a dormant state to establish a clinically silent latent infection that can subsequently reactivate to cause active diseases, sometimes decades later [8–10]. Post-primary tuberculosis, which occurs in a sensitized host, accounts for most of the cases that manifest active diseases, and is generally caused by exogenous reinfection or reactivation of latent bacilli [7]. The mechanisms underlying tuberculous reactivation remain to be clearly defined; but it is well established that a host with compromised immune function, such as individuals with HIV infection and those receiving tumor necrosis factor (TNF) blockade therapy, is at increased risks for developing disease recrudescence [11–13]. The latently infected constitute a reservoir of individuals that is critical for the perpetuation of the tubercle bacillus. These unique properties to persist in and transmit insidiously among the population render eradication of M. tuberculosis difficult [14]. In the post primary stage of infection, M. tuberculosis has the propensity to promote the development of caseating pneumonia in the sensitized host that can lead to tissue necrosis and eventual cavitation [7]. These immunopathological changes, whose underlying mechanisms have not been clearly characterized, enable effective bacterial transmission and therefore play an important role in the pathogenesis of the tubercle bacillus [7, 15].
A most effective way to combat an infectious disease is through immunization with efficacious vaccines [16]. For example, the existing measles vaccine costs approximately $17/disability-adjusted life year, making it one of the most cost-effective health interventions in developing countries [17]. The development of a reliable and effective vaccine against M. tuberculosis, however, has not been straightforward [18–20]. This difficulty is, at least in part, due to the complex life cycle of M. tuberculosis in the host [6], which elicits a spectrum of immunological responses not yet completely characterized; and the lack of a well-defined molecular and biochemical signature of protection against infection [19, 21]. The only anti-tuberculosis vaccine currently in use is bacillus Calmette-Guèrin (BCG) [22]. Although this vaccine effectively protects against severe childhood tuberculosis, its efficacy against adult pulmonary disease is inconsistent [23–26]. Concerted efforts of the tuberculosis community, however, together with advances in the fields of immunology and vaccinology [17, 27, 28], should hold promise for the rational design of effective vaccines against M. tuberculosis [18–20].
Characterization of the immune response to M tuberculosis has largely focused on cell-mediated immunity [18–20]. This approach is not without reasons. For example, the inconsistent efficacy of passive serum therapy in treating tuberculosis in the late nineteenth century, which was likely due to the use of non-standardized protocols and reagent, had cast doubt on the significance of humoral immunity in the control of M. tuberculosis [29, 30]. This doubt has been further bolstered by the generally accepted concept that while cell-mediated immunity plays a critical role in defense against intracellular pathogens, their extracellular counterparts are best controlled by B cell and humoral immune response [31–33]. Based on this latter concept, vaccine development against intracellular pathogens, including M. tuberculosis, has taken a mostly T cell-centric approach [34]. There exists, however, experimental evidence that humoral immunity can impact substantially on host defense against pathogens with a preferred intracellular niche (reviewed in [35, 36]). Taking a more comprehensive approach, encompassing both cell-mediated and B cell and humoral immunity, to characterizing immune responses to M. tuberculosis will likely gain new insights that can help design anti-tuberculosis strategies, including immunotherapies and vaccines.
The role of B cells and humoral immunity in regulating the immune response against intracellular pathogens
Accumulating evidence suggest that the concept of division of immunological labor in host defense against intracellular and extracellular microbes, as discussed above, is not absolute. It is becoming clear that B cells and immunoglobulins contribute significantly to shaping the immune response to and/or engendering protection against pathogens such as Chlamydia trachomatis, Coxiella Burnetti; Salmonella spp., Leishmania spp., Francisella tularensis, Plasmodium spp., Cryptococcus neoformans, Trypanosoma cruzi, and Ehrlichia chaffeensis [37–50], whose life cycle includes a significant intracellular sojourn of varying extent. In fct, a conjugate vaccine has been made that protects against the intracellular pathogen S. typhimurium by antibody-mediated immunity alone [51]. Of note, as obligatory an intracellular organism as Ehrlichia is [39] the life cycle of this bacterium has a transient extracellular phase that may lead to its susceptibility to the antimicrobial effects of antibodies that may be mediated through direct interaction between immunoglobulins and the target pathogen. This scenario is applicable to M. tuberculosis as emerging evidence support the notion that extracellular tubercle bacilli are likely not an uncommon entity in an infected host, particularly one (such as humans) in whom necrosis is part of the immunopathological process [52, 53]. Direct contact with target pathogens is, however, not requisite to the execution of the antimicrobial effects of antibodies. For example, certain contact-independent mechanisms against viruses, the quintessential intracellular pathogens, protect by attenuating viral transcription and replication via the binding of antibodies to specific antigens of the virion present on the membrane of infected cells [54–56]. It also has been reported that immunoglobulins, such as certain anti-DNA antibodies [57] and virus-specific IgA [58, 59], can gain entry into cells. Whether these latter mechanisms modulate the host response to intracellular bacteria remains to be determined. What is clear is that the multifaceted B cells, by virtue of their ability to present antigens, produced antibodies and cytokines, can exert significant effect on the T cell compartment that is deemed critical in defense against intracellular pathogens. [60, 61] (Fig. 1). Results from infectious diseases models involving a wide variety of organisms, including intracellular bacteria such as Chlamydia [62] and Fransicella [63], have provided ample evidence supporting a role for B cell and humoral immunity in regulating T cell memory [64–73] and recall immune response [60, 61] -- two immunological elements critical to vaccine efficacy [28, 74]. Characterization of the mechanisms by which B cells and the humoral immune response interact with cellular immunity during infection in general, and those caused by intracellular pathogens in particular, should provide information that can be useful for the design of antimicrobial strategies including vaccine development.
Figure 1. Mechanisms by which B cells shape the immune response to M. tuberculosis.
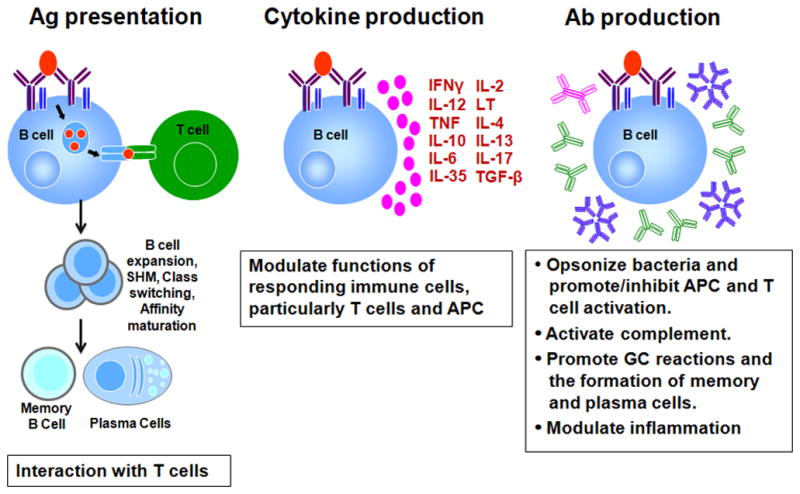
B cells could regulate the host response to M. tuberculosis by: 1) functioning as antigen presenting cells to interact with T cells: the primary site of this reaction is at the germinal center (GC). The interaction between GC B cells and Tfh (T follicular helper cells) culminates in B cell expansion, somatic hypermutation, affinity maturation, class switching, and the development of memory B cells and antibody-producing plasma cells; 2) producing cytokines that modulate responding immune cells including influencing the differentiation of T cells and hence effector functions; and 3) producing antibodies (Ab) which could modulate multiple aspects of both the innate and adaptive immune response. M. tuberculosis-specific antibodies can opsonize extracellular bacilli, form immune complex that fix complements, and engage Fcγ receptors of effector cells thereby modulating their functions and therefore, their effects on other immune cells, including T cells. Antibodies can also modulate the GC reactions as we as inflammation in infected tissues. LT: lymphotoxin; TGF: transforming growth factor; IL: interleukin; TNF: tumor necrosis factor; APC: antigen presenting cell; GC: germinal center; Ab: antibodies; Red oval: M. tuberculosis antigen; Pink circles: secreted cytokines; Green Y: IgG; Pink double Y: dimeric IgA; Blue snowflake: pentameric IgM; Maroon double Y: B cell receptor.
B cells regulate T cell immunity through antigen presentation
It has been well recognized that upon exposure to pathogens, CD4+ T cells of the responding host play a critical role in shaping B cell responses, including expansion, somatic hypermutation, antibody affinity maturation and class switching, the development of memory B cells and plasma cells, and cytokine production [75–77]. The significance of this interaction between B cells and follicular helper CD4+ T cells (Tfh), which takes place primarily in the germinal center (GC), has been underscored recently in the context of the development of broadly neutralizing antibodies against HIV [78–80]. Quantitative and qualitative analyses of the GC reaction in HIV infection models have revealed that the capacity to generate effective broadly neutralizing antibodies correlates with the Tfh response [78–80]. The roles B cells in modulating the T cell compartment are, however, less well characterized; and when studied, the results could be inconsistent [60, 81]. This inconsistency could be due to differences in the experimental systems employed for the studies as well as non-B cell immunological abnormality intrinsic in the commonly used B cell-deficient μMT mice [60, 81, 82]. The availability of Rituximab, a B cell-depleting monoclonal antibody that has seen increasing use in recent years to treat a wide variety of human diseases, has provided a venue for examining the role of B cells on T cells. indeed, studies using this system have provided evidence whose results support an important role for B cells could influence T cell functions in humans [60].
The importance of T cell help for antibody production by B cells was discovered over half a century ago [83, 84]. MHCII-restricted presentation by B cells and T-cell antigen specificity were subsequently described as important features of this collaborative interaction [85, 86]. These work set the stage for the characterization of GC in secondary lymphoid tissues, the anatomical locale in which CD4+ Tfh cells helps B cell activation, which culminates in the generation of memory B cells plasma cells [75–77] to establish long-term humoral immunity that is the basis for resistance against a number of viral pathogens [74, 87] (Fig. 1). These studies clearly demonstrate the proficient antigen-presenting capacity of B cells, establishing an important mechanism by which these lymphocytes can modulate T cell immunity [60, 81]. Upon capturing and internalizing by B cells via surface immunoglobulins, antigens can be processed and effectively presented as peptide:MHCII complexes [81, 85, 86, 88]. Studies including those involving in vivo mouse models have demonstrated that B cells, like dendritic cells, (generally considered the most potent antigen presenting cells [89]), can prime T cells [90–92]. Importantly, this capability has been stringently demonstrated in a system that lacks non-B cells antigen presenting cells [93]. In addition, using a CD11c-diphtheria toxin receptor transgenic mouse model that affords conditional depletion of dendritic cells, in conjunction with a system that enables quantifying the presentation of the Eα peptide using a monoclonal antibody specific for Eα:1-Ab complex, a recent report revealed that memory B cells and not dendritic cells, are the major antigen presenting cells required for the activation of antigen-specific memory Tfh cells in a recall response [73]. These two latter reports stringently demonstrate the antigen presenting capacity of B cells and the significance of this function in vivo. Results derived from an LCMV system involving the B cell-deficient μMT mouse strain have, however, revealed that memory Tfh cells are still capable of being activated during a recall response in the absence of B cells [94]. The apparently discrepant observations are likely due to the distinct experimental design and approach used for the studies. Indeed, likely for the same reasons, incongruency has been observed regarding the significance of the antigen-presenting capability of B cells in priming naïve T cells [81, 95]. Finally, experiments involving direct visualization of the immune response in the very initial phase of antigenic challenge (within minutes) have provided strong evidence suggesting that B cells participate in events that ensue in the early phase of an immune response, such as antigen presentation and T cell priming. [96, 97].
The antigen presenting capability of B cells has seen increasing translational utility over the past decades, particularly in the area of vaccine and immunotherapeutic design [98], and much of this development is related to the effect of this function on T cells [60, 81, 99]. This property of B cells has been exploited to engender enhanced immunity against tumors [100] and the efficacy of vaccines, including one designed against M. tuberculosis [101–103]. The anti-M. tuberculosis vaccine vector comprises a combination of two mucosal adjuvants: the B cell-targeting CTA1-DD (a non-toxic version of a potent mucosal adjuvant TCA1 (cholera toxin A1 subunit) fused in frame with a dimer of the D region of Staphylococcus aureus protein A) and the QuilA-containing dendritic cell-targeting immune-stimulating complex (ISCOM) [102]. Specific antigens incorporated with CTA1-DD/can be targeted to B cells via the DD portion of CTA1-DD, which facilitates binding of the adjuvant/antigen complex to the Fc portion of immunoglobulins on B cells for presentation, as well as enhances the germinal center reaction [102]. The immune enhancing effect of CTA1-DD is B cell-dependent [102]. A vaccine that incorporates CTA1-DD/ISCOM with fused ESAT-6/Ag85B displays Th1-ehancing effects and significantly boosts BCG-induced immunity [101]. In infectious diseases models, the antigen-presenting capability of B cells has been linked to modulation of T cell response and protection [67, 104]. These latter observations afford the possibilities of the development of anti-microbial strategies by targeting the antigen-presenting functions of B cells. But the ability of B cells in regulating T cell functions goes beyond antigen presentation. This versatility derives from the ability of B cells to generate cytokines and immunoglobulins, two factors that play important roles for the maturation of antigen presenting cells [105–110], and as a result, impact on the development of T cell immunity [111, 112].
B cell cytokines as potent T cell regulators
Cytokines are a most important regulator of the development of naïve CD4+ T cells upon engagement of their engagement with antigen presenting cells [113]. The significance of this regulatory function is perhaps best illustrated by the phenomenon of cytokine-driven T helper 1 (Th1)/Th2 dichotomy of T cell differentiation discovered almost three decades ago [114]. This principal of cytokine-dependent T cell development into distinct lineages was subsequently found applicable to biology of B lymphocytes [114, 115], immune cells that can produce a wide array of cytokines, either constitutively or when triggered through interaction with antigens, T cells or Toll-like receptor ligands [114–117]. The development of B cells into distinct subsets such as B effector-1 (Be1), B effector-2 (Be2), and IL-10-expressing cells, can be influenced by the cytokine milieu in which they interact with antigens and T cells [60, 114, 115, 118, 119]. Be1 cells, producers of interferon (IFN)-γ and interleukin (IL)-12, and TNF, IL-10, and IL-6, develop when B cells are primed in a Th1 environment. By contrast, priming in a Th2 cytokine milieu results in the development of Be2 subset, producers of IL-2, lymphotoxin, IL-4, and IL-13, as well as IL-10, and IL-6 [60, 114, 115, 118, 120]. Be1 and Be2 cells, by virtue of the distinct sets of cytokines they generate, can in turn direct the development of naïve CD4+ T cells into Th1 and Th2 effector T cells, respectively [72, 115]. Studies using human systems have revealed that IL-12- and IL-4-producing B cells can bias the in vitro development of a Th1 [121] and Th2 response [122], respectively. This ability of B cell subsets to regulate T cell differentiation affords a cross-regulatory interaction between B and T cell immunity [114, 115, 120].
Collectively, the results described above demonstrate an important role for the cytokines produced by B cells in modulating immunity. But our understanding of the role of B cell-derived cytokines in infectious diseases has only recently begun to expand [44, 72, 123–126]. Immunological characterization of a murine Heligomosomoides polygyrus model has revealed a complex role for B cells during this murine nematode infection [72]. The results showed that B cells promote the development of immune responses to H. polygyrus via multiple mechanisms: antibody and cytokine production and antigen presentation, demonstrating that in this model, IL-2 derived from effector B cells contribute to enhancing the differentiation and expansion of Th2 cells as well as generation of antibodies. In addition, B cell-derived TNF effectively sustains antibody production. Finally, the study revealed that the relevance of certain B cell functions might be dependent on specific phase of the immune response during the infection [72]. In a follow-up study, the same group went on to show that upon infection with H. polygyrus, B cell-derived lymphotoxin regulates the expression of CXCL13, which controls the co-localization of CXCR5+ dendritic cells and CD4+ T cells to the vicinity of the B cell area of the lymph nodes, thereby optimizing the development of a Th2 response that is essential for controlling the nematode infection [123]. More recently, IL-17-producing B cells have been identified during the innate phase of T. cruzi infection [44]. The development of these B cells is required for optimal control of the parasite and intriguingly, is driven by a T. cruzi trans-sialidase that modifies CD45, the mucin on the surface of B cells, resulting in signaling through the Bruton tyrosine kinase-dependent pathway and IL-17 production that is independent of ROR (retinoic acid receptor related orphan receptor)-γt and AHR (aryl hydrocarbon receptor) [44]. In a Pneumocystis murina model, it has been shown that B cell-derived TNF is essential for CD4+ T cell expansion and optimal control of the infection [124]. Interestingly, upon infection with Helicobacter suis in mice, it has been reported that B cell-derived IFNγ is critical for the development of gastric lymphoid follicles [126]. Similar structures exhibiting features of germinal centers have also been observed in the tuberculous lungs of a wide variety of hosts including humans [127–130] (see below). The roles of these lymphoid aggregates in modulating the local lung immune response during tuberculous infection remain to be determined.
Clearly, B cells possess multiple means to modulate the development of immune responses irrespective of their ability to synthesize antibodies, including the enhancement of T cell immunity. An current area of intense interest of B cell research focuses, however, on the IL-10-expressing B cells, which are potent negative regulators of immunity [60, 118, 119, 131–133], and have been implicated as an important modulator of autoimmunity, inflammation, infection, and cancer [60, 118, 119, 131]. There appears to be at least two subsets of IL-10-producing B cells; CD19+CD138-CD1dhi B cells produce both IL-10 and IL-6 (a mediator or pro-inflammatory functions of B cells); and CD138+ plasma cells, which have recently been shown to have an remarkable propensity to produce anti-inflammatory cytokines such as IL-10 and IL-35, but not IL-6, and are the main source of B-cell derived IL-10 and IL-35 during Salmonella infection and in an experimental autoimmune encephalomyelitis (EAE) model. [125, 134]. Thus, B cells are a rich source of cytokines that are relevant in a wide range of pathological states including infectious diseases. Splenic B cells immunomagnetically procured from tuberculous mice have the ability to produce a variety of cytokines (L. Kozakiewicz and J Chan, unpublished). The significance of these B cell-derived cytokines in regulating the immune response to M. tuberculosis remains to be clearly defined.
Modulation of T cell response by immunoglobulins
One important immune-regulatory capability of antibodies is mediated through the interaction of this major B cell product with antigens to form immune complexes that can bind to Fcγ receptor (FcγR) to modulate a wide spectrum of immune cell functions relevant to many disease states [135]. One much studied area is the effects of Fcγ receptor signaling on antigen presenting cells and the subsequent impact on their effector functions including the priming of T cells [136]. Indeed, the FcγR-immune complex reaction has been targeted for the design of a variety of therapeutics including vaccines against intracellular pathogens and cancers [62, 137]. The immune complex could engage stimulatory and/or inhibitory FcγRs, whose functions are determined by the ITAM or ITIM motifs in the cytoplasmic domain of the receptor, respectively [138, 139]. Signaling through FcγRIIB, the singular inhibitory FcγR, attenuates dendritic cells maturation and hence, impairs antigen presentation and activation of T cells. Engagement of the stimulatory FcγR’s promotes the antigen presenting cell priming of T cells [106, 109]. By virtue of its negative regulatory effects, FcγRIIB plays an important role in mediating peripheral T-cell tolerance [140]. Translationally, blocking the inhibitory FcγRIIB in mice results augments anti-cancer activity of T cells [105, 106, 109]; and signaling through the stimulatory FcγRs promotes either a Th1 or a Th2 response, depending on the immunological environment in which the T cell-dendritic cell interaction occurs [141].
The differential affinity for IgG subclasses for FcγRs, which translates into varied effector responses, has been exploited in the development of immunotherapeutics [142], including immunization strategies designed to augment cellular immune responses against intracellular pathogens [62, 143]. The observation in an IVIG model that the stimulatory FcγRIII can mediate immune suppression underscores the complexity of FcγR signaling [144]. Elucidation of the roles of the various FcγR in biological process including infection has been facilitated by generation of mouse strains with deficiency in specific receptors [145]. Not unexpectedly, deficiency in the γ chain common among the stimulatory receptors causes defective immunity against intracellular pathogens including influenza virus, Leishmania species, Plasmodium berghei, and S. enterica [42, 146–150]. The protective effect of a Cryptococcus neoformans-specific IgG1 monoclonal antibody has been shown to require intact stimulatory FcγR’s signaling [151]. Together, these results demonstrate the significance of the stimulatory FcγR for host defense against intracellular microbes. We have shown that signaling through FcγRs can modulate immunity upon infection with M. tuberculosis and significantly affect outcome [152]. These data thus identify antibody-mediated immunity as a potential target for the rationale design of immunotherapeutics such as vaccines against intracellular pathogens. [62, 143]. For example, a recombinant Sindbis virus-based vector has been engineered to express immunoglobulin kappa light chain-binding bacterial protein domains to effectively target FcγR-bearing cells (such as antigen-presenting cells) to manipulate immune activation [153]. A P. falciparum merozoite surface protein, which, in mice, can preferentially induce isotype class-switching to IgG2b [154], an immunoglobulin subclass with preferential affinity for stimulatory FcγRs [142], can be exploited to modulate the function of effector cells.
The contribution of antibodies and B cells to shaping the host immune response to M. tuberculosis
The effects of antibodies on the immune response to M. tuberculosis: Is there a protective role?
There is a growing body of work indicating a role for humoral immunity in promoting optimal protection against intracellular pathogen in general (reviewed in [36, 60, 61]), and M. tuberculosis in particular (reviewed in [61, 155–157]). In one patient study, analysis of serum from children with tuberculosis found that decreased levels of serum IgG to the mycobacterial cell wall glycolipid lipoarabinomannan (LAM), and other mycobacterial antigens, was associated with disseminated tuberculosis [158]. Similarly, in experimental systems, aerogenically infected mice that cannot secrete antibodies, were shown to have a diminished capacity to control M. tuberculosis infection and exhibited enhanced mortality ([159], Mehta S, Kozakiewizc L & Chan J; Unpublished). In another study, the lung neutrophilia and increased Th17 response were reversed with the administration of immune serum, suggesting that these effects can at least in part be ameliorated by immunoglobulin [160]. Worthy of note, the dysregulation of the IL-17/neutrophilic response also adversely affects the development of BCG-induced Th1 response in B cell-deficient mice by retarding DC migration from the site of vaccination to the draining lymph nodes as a result of tissue neutrophilia [160]. Understanding the mechanisms underlying the latter observation may yield useful information that can guide vaccine development.
Evidence has emerged over the last decade that administration of monoclonal antibodies against various M. tuberculosis components such as arabinomannan, LAM, heparin-binding hemagglutinin, and 16 kDa α-crystallin, can protect mice against the tubercle bacillus [161–165], as assessed by monitoring bacterial burden, the degree of dissemination, and changes in the level of inflammation [157]. The diverse effects of monoclonal antibody treatment revealed by these studies on disease pattern and progression suggest that the mechanisms underlying immunoglobulin-mediated protection are likely highly complex. For example, in mice intranasally infected with M. tuberculosis, treatment with monoclonal antibodies to the heparin-binding haemagglutinin (HBHA), a major mycobacterial adhesin, was found to limit dissemination to the spleen [162]. Likewise, administration of antibodies to arabinomannan was found to promote the long-term survival of M. tuberculosis-infected mice, although the mechanism of action was not clear [161, 164]. Pre-incubation of M. tuberculosis with the IgG3 arabinomanan-specific monoclonal antibody 9D8 did not appear to limit infection of macrophages, inhibit bacterial replication, or to mediate bacteriocidal activity [164]. Instead, the only observed difference between untreated and treated mice was in the organization of the lung granulomas that was associated with significantly prolonged survival, suggesting that the antibody conferred protection by enhancing or altering cellular immune responses [164]. Similar results were seen using an antibody to a different cell surface expressed antigen in an M. bovis mouse model [166]. Interestingly, administration of immune serum to deliver polyclonal antibodies against M. tuberculosis to SCID mice after anti-tuberculous drug treatment was shown to engender protection against disease recrudescence [167]; and IVIG therapy was reported to be effective in lowering tissue bacillary loads in C57BL/6 but not nude mice, intravenously infected with virulent M. tuberculosis [168]. These studies suggest that humoral immunity can be targeted to enhance anti-tuberculosis immune responses in the development of treatment and vaccines against the tubercle bacillus. Indeed, it was observed in a human study that sera from BCG-vaccinated individuals enhance BCG internalization by phagocytes, the IgG-dependent inhibitory effects of neutrophil and monocyte, as well as anti-specific T cell response [169]. Immunization with a humoral immunity-targeting vaccine based on an M. tuberculosis arabinomannan–protein conjugate was shown to elicit an antibody response more robust than that triggered by BCG; however, no improvement in survival was observed compared to recipients of the BCG group [170]. These studies suggest that it is feasible to direct production of M. tuberculosis-specific antibodies and more important, vaccine-induced humoral immunity can augment T cell response against the mycobacteria. Understanding of how immunoglobulins shape the immune response to M. tuberculosis might guide the design of effective anti-tuberculous strategies.
The M. tuberculosis cell wall is primarily composed of constituents rich in lipids and carbohydrates, molecules that are not typically presented by conventional MHC molecules. As a result, the T cell-independent (TI) antibody responses of the B1 and marginal zone B cell subsets, generally considered to be the primary source of natural IgM that recognize common bacterial antigens, could play a valuable role in enhancing recognition of these cell surface antigens by the immune system [171–173]. Natural antibodies, which are produced without any apparent antigenic stimulation, provide the first line of defense during an infection and predominantly consist of the IgM isotype. The pentameric structure of IgM allows for low affinity but high avidity interactions with phylogenetically conserved structures such as nucleic acids, phospholipids and carbohydrates [174, 175], and aids its function as an extremely potent activator of complement [176]. B-1 cells are the main source of natural IgM, whereas both B-1 and B-2 cells can produce immune IgM [177].
Both natural and immune IgM have been shown to play protective roles in a number of infection models (reviewed in [171, 177]), including intracellular bacterial infections such as Nocardia brassiliensis [178, 179] and F. tularensis [180–183], where humoral immunity is typically thought to be unimportant. These studies have established a role for antigen-specific, IgM-mediated protective effects against intracellular pathogens. Despite being considered a short-lived, early response antibody, several studies have recently demonstrated that IgM can provide long-lasting immunity. In studies with Ehrlichia muris, an obligate intracellular bacterium, mice chronically infected with the organism were protected from fatal challenge delivered at as late as 250 days after the initial infection; and this protection was mediated by a long-term antigen-specific IgM response derived from a population of CD138hiIgMhi bone marrow B cells that display characteristics of both plasmablasts and plasma cells, and perhaps from IgM-producing plasmablasts in the spleen [184, 185]. Long-lasting IgM-mediated protective immunity has also been shown to be protective against Borrelia hermsii and Streptococcus pneumoniae infections [186, 187]. In these cases protection was mediated via antigen-specific IgM antibodies produced by peritoneal cavity B-1b cells.
In addition to a direct role in mediating protective immunity, IgM has been shown to promote the generation of efficient neutralizing IgG antibody responses in a virus model [188]. More recently, Rapakka et al. demonstrated that, in a Pneumocystis model, IgM influenced trafficking of antigen presenting cells to the local draining lymph node, production of inflammatory cytokines by dendritic cells, and T cell differentiation [189]. In this model, IgM regulates the overall humoral response: in the absence of IgM, mucosal IgG and IgA were not detectable; this latter result suggests that IgM is required for normal programming of B cell adaptive antibody responses [189]. The reduced anti-specific IgG response to the T-cell dependent (TD) antigen NP-KLH observed in the μS−/− mouse strain, which selectively lacks secreted IgM [190], has been attributed to impaired antigen trapping on follicular dendritic cells due to deficiency of this immunoglobulin, which results in a comprised germinal center [190, 191]. Similarly, IgG responses to the TI antigen NP-Ficoll were also altered in the absence of secreted IgM [190, 191]. These studies directly show that IgM can regulate the specific antibody profile generated in response to both TD and TI antigens. Studies employing a TD antigen have provided evidence that IgM may regulate the overall humoral response via the complement pathway [192–194]. Clearly, IgM has the ability to modulate many aspects of the immune response. Studies using mice deficient in the recently discovered specific FcR (FcμR) for IgM [195] to characterize B cell responses have revealed (notwithstanding certain level of discrepancy) phenotypes similar to those observed in the μS−/− model, such as differences in B cell populations, altered germinal center reaction, dysregulation of humoral responses, impairment of B-cell proliferation upon ligation of BCR in vitro [196–198], suggesting that the effects of secreted IgM are mediated via signaling through the Fcμ receptor. Importantly, a human FcμR has also been identified, thus enhancing the relevance of this receptor [199].
Through neutralization activity, influencing adaptive immunity, regulating inflammation (via complement activation, FcγR signaling, and the generation of microbial products due to direct microbicidal activity), and altering microbial gene expression, antibodies play a significant role in modulating the immune response to pathogens [36, 200] (Fig. 1). Antibodies have also been show to modulate remodeling of the airway epithelium and vessels in chronic infection [201]. Adoptive transfer of B cells ameliorates the enhanced inflammatory response detected in the lungs of tuberculous B cell-deficient mice [202]. This attenuation of pulmonic inflammation is associated with partial reconstitution of serum immunoglobulin levels in the recipient animals but not with homing of the transferred B cells to the lungs [202], suggesting the inflammation-limiting function of antibodies may be operative in a tuberculous host [36, 200, 203]. Finally, in preliminary studies, we have observed that the IgM-defcient μS−/− mice [190] display enhanced susceptibility to M. tuberculosis (Unpublished, S Mehta, L Kozakiewicz, & J Chan). Given this latter observation, together with the abundance of carbohydrate and lipid moieties (targets of TI antibody responses) in the mycobacterial cell wall, the expanding data revealing unexpected roles for IgM in mediating protection to chronic intracellular bacterial infections (via its ability to regulate cell-mediated immunity, program adaptive humoral immunity, including IgG and memory responses), it seems prudent to investigate whether IgM plays a role in optimizing the host response to M. tuberculosis. This line of investigation could potentially pave the way to identifying protective IgM antibodies to specific cell wall components, which could be induced in new vaccine formulations to engender a more robust anti-tuberculous response.
Does FcγR contribute to the regulation of the anti-tuberculous immune response?
The apparent significance of antibodies in modulating the immune response in tuberculosis has prompted us to investigate if FcgR might contribute to shaping immunity to the tubercle bacillus. This line of investigation has yielded data suggesting that the immune response to M. tuberculosis can be modulated by antibody binding to FcγRs. In comparison to wild-type controls, mice lacking the inhibitory FcγRIIB are better able to control M. tuberculosis infection and generate stronger pulmonary Th1 responses, as indicated by the higher frequency of IFNγ+ CD4+ T cells in the lungs [152]. Additionally, in response to infection, FcγRIIB−/− macrophages produced more p40 component of the Th1-promoting cytokine IL-12, suggesting that FcγRIIB signaling can dampen the Th1 response to M. tuberculosis, at least partially, by attenuating IL-12 production. In contrast, mice lacking the common γ-chain of activating FcγRs demonstrated heightened susceptibility to low dose M. tuberculosis infection, with exacerbated immunopathology, increased mortality, and enhanced production of IL-10 [152]. Together these data suggest that B cells can regulate the CD4 Th1 response in acute tuberculosis through the engagement of FcγRs by immune complexes, and that signaling through specific FcγRs can divergently affect disease outcome, indicating that it may be possible to enhance anti-mycobacterial immunity by targeting FcγRs.
A number of functional polymorphisms have been identified in human Fcγ receptors that alter receptor expression, ligand affinity or signaling capacity, and recent studies have also demonstrated copy number variation in some low affinity Fcγ receptors [204, 205]. Two single nucleotide polymorphisms (SNPs) in particular, at amino acid 131 in FcγRIIa and amino acid 158 in FcγRIIIa, are associated with susceptibility to a number of infectious agents and both result in altered antibody binding [204, 205]. The FcγRIIa R131 allele does not bind human IgG2 (whereas H131 does) and is associated with increased infection and/or disease progression with some encapsulated bacteria and viruses [206–210], while the FcγRIIIa F158 allele has lower affinity for IgG1 and IgG3 antibodies relative to V158, and has been described as a heritable risk factor for some vasculitis diseases [211–213]. Only one study has looked for an association between FcγR polymorphisms and susceptibility to tuberculosis; specifically, the FcγRIIa R131H and FcγRIIIa F158V polymorphisms in active tuberculous patients versus healthy controls in the Moroccan population was investigated [214]. No significant association was found. It is not clear if there is no association between these polymorphisms and susceptibility to tuberculosis, or if these FcγR polymorphisms do not affect the development of tuberculous infection in this particular ethnic background or geographic location. Alternatively there may be other functional polymorphisms in FcγRs (which are largely uncharacterized) that affect susceptibility to TB.
Interestingly, expression of FCGR1A, one of the genes encoding the high affinity FcγRI, was found to be an important biomarker discriminating between active versus latent TB infections (LTBI) [215–218]. In a large multi-site study across four sub-Saharan countries, significantly increased FCGR1A expression was observed in active TB patients compared to LTBI donors, and was independent of HIV status or ethnic background [218]. FCGR1A was also identified as a biomarker for active tuberculosis in a European Caucasian population [215]. In concordance with these data, treatment for tuberculosis has been shown to significantly reduce FCGR1A expression, indicating the importance of FCGR1A in TB pathogenesis [219].
Given these data, it would be interesting to screen for an association between polymorphisms or copy number variation in FCGR1A and susceptibility to active tuberculosis. FcγR1 is less well studied than the other FcγRs and no polymorphisms have been described for FCGR1 that alter receptor affinity or function [220]. Using an in silico approach van der Poel et al., (2011) recently identified three non-synonymous SNPs in FCGR1, one of which was in the extracellular domain of FcγR1 and highly conserved within the FcγR family, suggesting it may be important for receptor structure/function [220].
B cells regulates the IL-10 in tuberculous lungs
Studies involving two different models of B-cell deficiency, we and others have provided evidence that B cells can regulate IL-10 production in the lungs of M. tuberculosis- infected mice [202, 221]. Interestingly, mice deficient in the common γ chain of stimulatory FcγRs demonstrate increased IL-10 production in the lungs in response to M. tuberculosis infection [152]. While a wide variety of immune cells can produce IL-10 [222], a feature of incompletely activated dendritic cells is IL-10 production [223]. Therefore, it is possible that B cells may indirectly influence the production of IL-10 by antigen-presenting cells by modulating their activation status via immune complex engagement of FcγRs. Regulatory T cells, which are another significant cellular source of IL-10, can also be regulated by B cells [132, 224]. Since IL-10 has been reported to detrimentally affect disease outcome in murine tuberculosis [225], it is possible that excess IL-10 production observed in the B cell- and γ chain-deficient mice contributes to the inability of these strains to optimally control M. tuberculosis infection [152, 202]. However, another possibility is that the increased production of IL-10 represents a compensatory mechanism to counter the exacerbated immunopathology seen in M. tuberculosis-infected B cell- and Fcγ-chain-deficient mice [152, 202]. Much work needs to be done to delineate how B cells and humoral immunity regulate the production of cytokines, in particular IL-10, at the site of tuberculous infection. The regulatory mechanisms are likely to be complex given the multiple immunological functions of B cells.
B cells modulates granulomatous inflammation during tuberculous infection
As discussed above, during acute infection caused by the Erdman strain, mice deficient in B cells exhibited sub-optimal anti-tuberculous immunity associated with exacerbated pulmonary pathology [202]. Interestingly, in an experimental tuberculosis model involving M. tuberculosis CDC1551, B cell-deficiency resulted in a delay in inflammatory progression [226]. In agreement with the latter finding, we have recently observed that the B cell-deficient, compared to wild-type controls, display attenuated lung granulomatous responses during the chronic phase of an infection established by the Erdman strain of M. tuberculosis (Unpublished, Y Chen, P Maglione & J Chan). This discrepancy suggests that B cell may function differentially in the acute and chronic phase of tuberculous infection. Thus, B cells are required in the acute phase of infection for the development of an optimal granulomatous response and anti-tuberculous immunity for the control of the tubercle bacillus; in the absence of B cells, infected animals exhibit dysregulation of the granulomatous response and as a result, increased pulmonary inflammation is required to contain bacterial growth [202]. During chronic infection, the immunologically active B-cell aggregates, likely to be the product of ectopic lymphoid neogenesis [227, 228] (see below), may play a role in sustaining effective local immunity so as to contain persistent bacilli and prevent disease reactivation. This leads to a sustained inflammatory state that eventually can result in the development of tissue-damaging immunopathology. As T cells exist within the B-cell aggregates in the tuberculous lungs of both human and mice [127, 128], the perpetuation of inflammation in chronic tuberculosis may occur in part through B cells acting as antigen-presenting cells, thereby activating lesional T cells. Thus, the inflammatory paradox observed in B-cell-deficient mice may be due to the role of B cells shifting from optimizing host defense during acute challenge to sustaining a tissue-damaging chronic inflammatory response during persistent infection, the latter process perhaps driven by the tubercle bacillus to facilitate its transmission to another host via escape from a cavity [229].
Among patients whose infection advances most severely, morbidity and mortality is at least partially due to a tissue-damaging host response [230, 231]. This severe immunopathological manifestation could be the result of an intense local immune response that is ineffective in controlling the infection, which in turn, leads to an exuberant compensatory recruitment of immune cells into infected tissues. This scenario is best illustrated by the pattern of disease progression in individuals with recrudescence tuberculosis, which is known to be associated with regions of intense pulmonary inflammation [231]. The mechanisms underlying the development of such exuberant inflammation in these patients are currently unknown. The pathological changes in the lungs of individuals with untreated active tuberculosis can be dominated by tissue neutrophilia [130]. Neutrophils are innate immune cells that provide early protection against acute pulmonary infection but can induce undesirable tissue-damaging inflammation [232]. In mice, as in humans, tissue neutrophilia is associated with a susceptibility phenotype to M. tuberculosis infection [233]. Although severe tissue-damaging host response is a well-recognized manifestation of tuberculosis in certain hosts, the limited pathological reaction of the localized Ghon complex seen in humans suggests that exuberant immunopathology need not be the requisite for adequate control of M. tuberculosis. Emerging experimental results strongly suggest that B cells and humoral immunity may play a role in modulating the level of granulomatous inflammation during tuberculosis infection [202, 226], and that this B-cell-based regulation could be infection phase-dependent. Understanding how B cells modulate the granulomatous inflammatory response may lead to strategies that can attenuate tissue damage caused by the tubercle bacillus.
B cells in germinal center-like structures in the tuberculous granuloma
B cells are a prominent component of the tuberculous granulomatous inflammation in the lungs of mice [127, 128, 202, 234–236], non-human primates [127, 237] and human [127–130], forming conspicuous aggregates with features of germinal center [127, 129, 130, 202, 236]. In the lungs of humans with tuberculosis, cellular proliferation is detected primarily in these B-cell aggregates [130]; and immunohistochemical characterization demonstrates the presence of CXCR5+ ICOS (inducible co-stimulatory receptor)+ T cells, PCNA (proliferating cell nuclear antigen)-expressing CD20+ B cells, and tissue expression of CXCL13 [127]. These observations strongly suggest that the B cell aggregates within tuberculous tissues are bona fide GC, thus likely represent the product of lymphoid neogenesis, a highly complex process described in a wide variety of chronic inflammatory disease states (reviewed in [227, 228]).
Germinal centers are transient sites in secondary lymphoid organs that provide a highly complex and dynamic immunological environment in which, upon antigenic challenge, B cells interact with Tfh to achieve B cell expansion, clonal selection, Ig class switching, somatic hypermutation, plasma cell commitment and memory B cell formation [75–77]. This reaction is thus critical for the development of effective and long-lived humoral immunity. In pathological states that perpetuate a chronic inflammatory environment, GC-like structures can form ectopically in tissues outside of secondary lymphoid organs through a process designated as lymphoid neogenesis [227, 228]. The pathological conditions associated with chronic inflammation that are conducive to the development of this tertiary lymphoid organ span a wide spectrum, ranging from persistent viral and bacterial infection to autoimmune disease such as rheumatoid arthritis, multiple sclerosis, chronic hepatitis C, and Sjogren’s syndrome [228]. Although much remains to be learned about these ectopic lymphoid organs, emerging evidence indicates that these structures resemble the traditional GC at the cellular, molecular, and structural level [228]. As a result, the ectopic lymphoid organ can carry out functions similar to those operative in the traditional germinal center, enabling B cells to undergo affinity maturation and develop into memory B cells and plasma cells [228]. In addition, evidence exists that these structures afford an environment for the priming of T cells by dendritic cells [228]. These observations provide compelling evidence that ectopic lymphoid structures in inflammatory tissues are immunologically versatile and active, and are therefore likely to play a role in modulating the local immune environment, thus affecting disease outcome. Existing data suggest that ectopic lymphoid organs appear to be in general, beneficial to the host in the context of infection and neoplasm, but detrimental in individuals with autoimmune diseases [228]. The apparent disease-dependent divergent effects of these tertiary lymphoid organs on outcome suggest that the biology of the ectopic germinal centers is likely to be complex.
Do the ectopic lymphoid organs in the lungs of an M. tuberculosis-infected host regulate the local immune response and as a result, modulate disease outcome? During acute M. tuberculosis infection, mice lacking B cells (hence no lymphoid aggregates in the lungs) exhibit aberrant granulomatous reaction associated with enhanced pulmonary pathology and suboptimal control of bacillary growth, suggesting a potential protective role for the ectopic pulmonic germinal center in the early phase of tuberculous infection [202]. This apparent beneficial effect could be the result of highly efficient interaction among the various immune cells (e.g. dendritic cells and T cells) present in and enabled by a lymphoid organ formed in situ in the tuberculous granulomatous tissues. Indeed, T cells and antigen presenting cells have been detected within or in the vicinity of the ectopic germinal centers in the lungs of M. tuberculosis-infected host [127, 128]. Results derived from an influenza pneumonia model have revealed that ectopic lymphoid nodules in the lungs of mice deficient in secondary lymphoid organs can prime a protective immune response [238, 239]. The observation that cellular proliferation occurs in the proximity of B-cell aggregates in tuberculous tissues has led to the formulation of the hypothesis that these structures serve to sustain the local granulomatous inflammation [130]. The perpetuation of this inflammatory state could be further exacerbated by the chronic nature of tuberculous infection. It is thus possible that as the infection progresses into the chronic state, promotion of the local immune responses by the ectopic GC, possibly driven by tissue tubercle bacilli, can result in an environment conducive to the development of tissue-damaging immunopathology, a process that plays critical role in transmission of the tubercle bacillus [229]. The significance of lymphoid neogenesis in the regulation of immune responses in tuberculosis warrants further investigation.
Concluding Remarks
The unique ability of the tubercle bacillus to establish a dormant state in the majority of those infected presents a major problem for effective control of tuberculosis [8–10]. Due to lack of drugs that can effectively kill latent bacilli, the ability of dormant organisms to reactivate when the immune status of the host is compromised, and the capability of this pathogen to cause severe pulmonic immunopathology that facilitate aerosol transmission, the huge reservoir of latently infected individuals represents a major obstacle to eradicating M. tuberculosis [14]. Compounding these difficulties are the emergence of drug resistance clinical isolates, the enhanced susceptibility to tuberculous infection of persons infected with the highly prevalent HIV, as well as those receiving immunosuppressive biologics, a treatment modality that is increasingly being used to treat a wide range of diseases (e.g. TNF blockade therapy), and the lack of correlates of protection that are requisite to the development of effective vaccines [14]. With respect to vaccine development, the fact that a previously infected host can acquire exogenous re-infection suggests that efficacious vaccines for the prevention of tuberculosis must be capable of engendering an immune response superior to that elicited by a natural infection [240]. This is a significant challenge. To be sure, much progress has been made over the years in the generation of anti-tuberculous vaccines, and in the process, much knowledge concerning the tubercle bacillus has been gained. However, the efforts have fallen short of producing an efficacious immunization protocol. It is thus not unreasonable to suggest that rational vaccine design should take a more comprehensive approach in characterizing the mechanisms of protection against the tubercle bacillus. Strategies employed to developing vaccines for tuberculosis have focus on T cell immunity. With emerging evidence that B cells and immunoglobulins can significantly modulate the immune response to M. tuberculosis, it seems prudent to expend efforts into characterizing this immunological compartment during the course of tuberculous infection. Knowledge gained from this effort should help gain more insight into the immune response to M. tuberculosis that can possibly be harnessed to optimize strategies used for vaccine design.
The mechanisms by which B cell and humoral immunity modulate the immune response to M. tuberculosis remain to be clearly defined. For example, can antibody-mediated immunity be effective for the prevention and treatment of tuberculosis? Studies designed to develop antibody-based therapy for toxin neutralization revealed that the use of a combination of distinct monoclonal antibodies with specificity against a single toxin molecule can achieve enhanced protective effect [241]. Intriguingly, analysis of this combinatorial system showed that disease enhancing monoclonal antibodies could improve protective efficacy of a disease ameliorating immunoglobulin [241]. This emergent property of immunoglobulins strongly suggest that analysis of an antibody singly cannot accurately predict how it functions in the presence of other antibodies, a scenario that more genuinely reflects a natural immune response to an antigen, which, in the context of humoral immunity, is virtually universally polyclonal. Although such a property presents a significant problem in designing antibody-based vaccines, understanding the mechanisms underlying this phenomenon could afford an approach by which the emergent property of immunoglobulins can be exploited to achieve maximal protection via the use of a combination of monoclonal antibodies in the treatment protocol. What is the role of natural antibodies in host defense against the tubercle bacillus and can they prevent infection? Are TI mycobacterial antigens, abundantly present in the M. tuberculosis cell wall, suitable targets for the development of therapeutics including vaccines? Given our recent observation that IgM-deficientμS−/− mice display increased susceptibility to M. tuberculosis, further characterization of the role of natural antibodies in defense against the tubercle bacillus seems worthwhile. What are the protective antibodies, if any, against M. tuberculosis and how can they be elicited for the treatment or prevention of tuberculosis? What are the roles of the ectopic GC in the tuberculous lungs? Are they beneficial or detrimental to the host? Is their function infection phase-specific, promoting protection during acute infection while driving immunopathology in the chronic phase? What antigens do these B cells recognize? Understanding the roles of these B cells could help design strategies to augment anti-tuberculous mechanisms and to ameliorate immunopathology so as to curb transmission. What is the nature of B cell memory that develops upon M. tuberculosis infection, and can they confer protection upon secondary challenge? Given the close interaction between GC B cells and Tfh [75–77], it would be logical to investigate the role of this T cell subset in shaping the immune response to the tubercle bacillus. Investigating into these questions should help gain knowledge about the basic biology of B cells and humoral immunity during M. tuberculosis infection, which in turn, should guide the design of strategies by which B cell and humoral immunity can be exploited for the development of novel anti-tuberculous protocols.
Highlights.
Review experimental evidence supporting the notion that the importance of humoral and cellular immunity in host defense may not be entirely determined by the niche of the pathogen (intracellular vs extracellular).
Provide evidence that antibodies contribute to the defense immune response against M. tuberculosis
Discuss the how the multifacted B cells and humoral immunity can interact with T cells and other effector cells to shape the development of immune responses to M. tuberculosis.
Advocate for consideration a comprehensive approach that embraces both humoral and cellular immunity so as to gain better understanding of the immune response to M. tuberculosis.
Acknowledgments
This work was supported by grants from the National Institutes of Health: JMA (AI-067665 and AI-105684, AI51519, Areas TB vaccine foundation); AC (AI033774, HL059842, and AI033142); JC (AI1094745, AI063537, AI098925), JF (AI1094745). We thank members of the Chan laboratory for discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. WHO Report 2013: Global Tuberculosis Control. 2013. [Google Scholar]
- 2.Bates JH, Stead WW. The history of tuberculosis as a global epidemic. Med Clin North Am. 1993;77:1205–1217. doi: 10.1016/s0025-7125(16)30188-2. [DOI] [PubMed] [Google Scholar]
- 3.Gagneux S. Host-pathogen coevolution in human tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2012;367:850–859. doi: 10.1098/rstb.2011.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013;45:1176–1182. doi: 10.1038/ng.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn JL, Chan J. Immune evasion by Mycobacterium tuberculosis: living with the enemy. Curr Opin Immunol. 2003;15:450–455. doi: 10.1016/s0952-7915(03)00075-x. [DOI] [PubMed] [Google Scholar]
- 6.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 7.Hunter RL. Pathology of post primary tuberculosis of the lung: an illustrated critical review. Tuberculosis (Edinb) 2011;91:497–509. doi: 10.1016/j.tube.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J, Flynn J. The immunological aspects of latency in tuberculosis. Clin Immunol. 2004;110:2–12. doi: 10.1016/s1521-6616(03)00210-9. [DOI] [PubMed] [Google Scholar]
- 9.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Modlin RL, Bloom BR. TB or not TB: that is no longer the question. Sci Transl Med. 2013;5:213sr216. doi: 10.1126/scitranslmed.3007402. [DOI] [PubMed] [Google Scholar]
- 11.Sperber SJ, Gornish N. Reactivation of tuberculosis during therapy with corticosteroids. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1992;15:1073–1074. doi: 10.1093/clind/15.6.1073. [DOI] [PubMed] [Google Scholar]
- 12.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 13.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 14.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271–286. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 15.Flynn JL, Chan J. What’s good for the host is good for the bug. Trends Microbiol. 2005;13:98–102. doi: 10.1016/j.tim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Impact of vaccines universally recommended for children--United States, 1990–1998. MMWR Morb Mortal Wkly Rep. 1999;48:243–248. [PubMed] [Google Scholar]
- 17.Koff WC, Burton DR, Johnson PR, Walker BD, King CR, Nabel GJ, et al. Accelerating next-generation vaccine development for global disease prevention. Science. 2013;340:1232910. doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen P, Kaufmann SH. Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med. 2014:4. doi: 10.1101/cshperspect.a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McShane H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos Trans R Soc Lond B Biol Sci. 2011;366:2782–2789. doi: 10.1098/rstb.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orme IM. Vaccine development for tuberculosis: current progress. Drugs. 2013;73:1015–1024. doi: 10.1007/s40265-013-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiner J, 3rd, Kaufmann SH. Recent advances towards tuberculosis control: vaccines and biomarkers. J Intern Med. 2014;275:467–480. doi: 10.1111/joim.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloom BR, Fine PEM. The BCG experience: Implications for future vaccines against tuberculosis. In: Bloom BR, editor. Tuberculosis: Pathogenesis, Protection, and Control. Washington, D.C: American Society for Microbiology; 1994. pp. 531–557. [Google Scholar]
- 23.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA : the journal of the American Medical Association. 1994;271:698–702. [PubMed] [Google Scholar]
- 24.Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 25.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 26.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 27.D’Argenio DA, Wilson CB. A decade of vaccines: Integrating immunology and vaccinology for rational vaccine design. Immunity. 2010;33:437–440. doi: 10.1016/j.immuni.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature immunology. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumararatne DS. Tuberculosis and immunodeficiency--of mice and men. Clin Exp Immunol. 1997;107:11–14. doi: 10.1046/j.1365-2249.1997.d01-910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11:514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins FM. Cellular antimicrobial immunity. CRC Crit Rev Microbiol. 1978;7:27–91. doi: 10.3109/10408417909101177. [DOI] [PubMed] [Google Scholar]
- 32.Mackaness GB. Cellular resistance to infection. The Journal of experimental medicine. 1962;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufmann SH. Elie Metchnikoff’s and Paul Ehrlich’s impact on infection biology. Microbes and infection/Institut Pasteur. 2008;10:1417–1419. doi: 10.1016/j.micinf.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Seder RA, Hill AV. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406:793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 35.Casadevall A. Antibody-mediated immunity against intracellular pathogens: two-dimensional thinking comes full circle. Infect Immun. 2003;71:4225–4228. doi: 10.1128/IAI.71.8.4225-4228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casadevall A, Pirofski LA. A new synthesis for antibody-mediated immunity. Nature immunology. 2012;13:21–28. doi: 10.1038/ni.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culkin SJ, Rhinehart-Jones T, Elkins KL. A novel role for B cells in early protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1997;158:3277–3284. [PubMed] [Google Scholar]
- 38.Langhorne J, Cross C, Seixas E, Li C, von der Weid T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc Natl Acad Sci U S A. 1998;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li JS, Winslow GM. Survival, replication, and antibody susceptibility of Ehrlichia chaffeensis outside of host cells. Infect Immun. 2003;71:4229–4237. doi: 10.1128/IAI.71.8.4229-4237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6(−/−) (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun. 2000;68:46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woelbing F, Kostka SL, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, et al. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- 44.Bermejo DA, Jackson SW, Gorosito-Serran M, Acosta-Rodriguez EV, Amezcua-Vesely MC, Sather BD, et al. Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORgammat and Ahr that leads to IL-17 production by activated B cells. Nat Immunol. 2013;14:514–522. doi: 10.1038/ni.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casadevall A, Pirofski L. Insights into mechanisms of antibody-mediated immunity from studies with Cryptococcus neoformans. Curr Mol Med. 2005;5:421–433. doi: 10.2174/1566524054022567. [DOI] [PubMed] [Google Scholar]
- 46.Gibson-Corley KN, Boggiatto PM, Bockenstedt MM, Petersen CA, Waldschmidt TJ, Jones DE. Promotion of a functional B cell germinal center response after Leishmania species co-infection is associated with lesion resolution. Am J Pathol. 2012;180:2009–2017. doi: 10.1016/j.ajpath.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nanton MR, Way SS, Shlomchik MJ, McSorley SJ. Cutting edge: B cells are essential for protective immunity against Salmonella independent of antibody secretion. J Immunol. 2012;189:5503–5507. doi: 10.4049/jimmunol.1201413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Pinto D, Saravia NG, McMahon-Pratt D. CD4 T cell activation by B cells in human Leishmania (Viannia) infection. BMC Infect Dis. 2014;14:108. doi: 10.1186/1471-2334-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szymczak WA, Davis MJ, Lundy SK, Dufaud C, Olszewski M, Pirofski LA. X-linked immunodeficient mice exhibit enhanced susceptibility to Cryptococcus neoformans Infection. MBio. 2013:4. doi: 10.1128/mBio.00265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang G, Peng Y, Schoenlaub L, Elliott A, Mitchell W, Zhang Y. Formalin-inactivated Coxiella burnetii phase I vaccine-induced protection depends on B cells to produce protective IgM and IgG. Infect Immun. 2013;81:2112–2122. doi: 10.1128/IAI.00297-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344:1263–1269. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- 52.Grosset J. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob Agents Chemother. 2003;47:833–836. doi: 10.1128/AAC.47.3.833-836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoff DR, Ryan GJ, Driver ER, Ssemakulu CC, De Groote MA, Basaraba RJ, et al. Location of intra- and extracellular M. tuberculosis populations in lungs of mice and guinea pigs during disease progression and after drug treatment. PloS one. 2011;6:e17550. doi: 10.1371/journal.pone.0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujinami RS, Oldstone MB. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979;279:529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- 55.Griffin DE, Metcalf T. Clearance of virus infection from the CNS. Curr Opin Virol. 2011;1:216–221. doi: 10.1016/j.coviro.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Metcalf TU, Griffin DE. Alphavirus-induced encephalomyelitis: antibody-secreting cells and viral clearance from the nervous system. J Virol. 2011;85:11490–11501. doi: 10.1128/JVI.05379-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanase K, Smith RM, Cizman B, Foster MH, Peachey LD, Jarett L, et al. A subgroup of murine monoclonal anti-deoxyribonucleic acid antibodies traverse the cytoplasm and enter the nucleus in a time-and temperature- dependent manner. Laboratory investigation; a journal of technical methods and pathology. 1994;71:52–60. [PubMed] [Google Scholar]
- 58.Mazanec MB, Coudret CL, Fletcher DR. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Nedrud JG. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci U S A. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur J Immunol. 2009;39:676–686. doi: 10.1002/eji.200839148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of T cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines. 2004;3:23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 63.Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol. 2008;180:5548–5557. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elkins KL, Bosio CM, Rhinehart-Jones TR. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Francisella tularensis live vaccine strain. Infect Immun. 1999;67:6002–6007. doi: 10.1128/iai.67.11.6002-6007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esser MT, Marchese RD, Kierstead LS, Tussey LG, Wang F, Chirmule N, et al. Memory T cells and vaccines. Vaccine. 2003;21:419–430. doi: 10.1016/s0264-410x(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 66.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 67.Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 68.Schultze JL, Michalak S, Lowne J, Wong A, Gilleece MH, Gribben JG, et al. Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cell signals CD40 ligand, interferon gamma, and IL-10: role of B cells in the maintenance of T cell responses. J Exp Med. 1999;189:1–12. doi: 10.1084/jem.189.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 70.Stephens R, Langhorne J. Priming of CD4+ T cells and development of CD4+ T cell memory; lessons for malaria. Parasite Immunol. 2006;28:25–30. doi: 10.1111/j.1365-3024.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 71.Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, et al. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ise W, Inoue T, McLachlan JB, Kometani K, Kubo M, Okada T, et al. Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc Natl Acad Sci U S A. 2014;111:11792–11797. doi: 10.1073/pnas.1404671111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 76.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 77.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 78.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vinuesa CG. HIV and T follicular helper cells: a dangerous relationship. J Clin Invest. 2012;122:3059–3062. doi: 10.1172/JCI65175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gray D, Gray M, Barr T. Innate responses of B cells. Eur J Immunol. 2007;37:3304–3310. doi: 10.1002/eji.200737728. [DOI] [PubMed] [Google Scholar]
- 82.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 83.Britton S, Mitchison NA, Rajewsky K. The carrier effect in the secondary response to hapten-protein conjugates. IV. Uptake of antigen in vitro and failure to obtain cooperative induction in vitro. Eur J Immunol. 1971;1:65–68. doi: 10.1002/eji.1830010203. [DOI] [PubMed] [Google Scholar]
- 84.Mitchison NA. T-cell-B-cell cooperation. Nature reviews Immunology. 2004;4:308–312. doi: 10.1038/nri1334. [DOI] [PubMed] [Google Scholar]
- 85.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 86.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 87.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 88.Vascotto F, Le Roux D, Lankar D, Faure-Andre G, Vargas P, Guermonprez P, et al. Antigen presentation by B lymphocytes: how receptor signaling directs membrane trafficking. Curr Opin Immunol. 2007;19:93–98. doi: 10.1016/j.coi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 89.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 90.Weber MS, Prod’homme T, Patarroyo JC, Molnarfi N, Karnezis T, Lehmann-Horn K, et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y, Wu Y, Ramarathinam L, Guo Y, Huszar D, Trounstine M, et al. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995;7:1353–1362. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- 92.Kurt-Jones EA, Liano D, HayGlass KA, Benacerraf B, Sy MS, Abbas AK. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988;140:3773–3778. [PubMed] [Google Scholar]
- 93.Rodriguez-Pinto D, Moreno J. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. Eur J Immunol. 2005;35:1097–1105. doi: 10.1002/eji.200425732. [DOI] [PubMed] [Google Scholar]
- 94.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez-Pinto D. B cells as antigen presenting cells. Cell Immunol. 2005;238:67–75. doi: 10.1016/j.cellimm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 96.Catron DM, Itano AA, Pape KA, Mueller DL, Jenkins MK. Visualizing the first 50 hr of the primary immune response to a soluble antigen. Immunity. 2004;21:341–347. doi: 10.1016/j.immuni.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 97.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 98.Hackett CJ, Rotrosen D, Auchincloss H, Fauci AS. Immunology research: challenges and opportunities in a time of budgetary constraint. Nat Immunol. 2007;8:114–117. doi: 10.1038/ni0207-114. [DOI] [PubMed] [Google Scholar]
- 99.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nature immunology. 2011;12:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schultze JL, Grabbe S, von Bergwelt-Baildon MS. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 2004;25:659–664. doi: 10.1016/j.it.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 101.Andersen CS, Dietrich J, Agger EM, Lycke NY, Lovgren K, Andersen P. The combined CTA1-DD/ISCOMs vector is an effective intranasal adjuvant for boosting prior Mycobacterium bovis BCG immunity to Mycobacterium tuberculosis. Infect Immun. 2007;75:408–416. doi: 10.1128/IAI.01290-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Helgeby A, Robson NC, Donachie AM, Beackock-Sharp H, Lovgren K, Schon K, et al. The combined CTA1-DD/ISCOM adjuvant vector promotes priming of mucosal and systemic immunity to incorporated antigens by specific targeting of B cells. J Immunol. 2006;176:3697–3706. doi: 10.4049/jimmunol.176.6.3697. [DOI] [PubMed] [Google Scholar]
- 103.Hon H, Oran A, Brocker T, Jacob J. B lymphocytes participate in cross-presentation of antigen following gene gun vaccination. J Immunol. 2005;174:5233–5242. doi: 10.4049/jimmunol.174.9.5233. [DOI] [PubMed] [Google Scholar]
- 104.McClellan KB, Gangappa S, Speck SH, Virgin HWt. Antibody-independent control of gamma-herpesvirus latency via B cell induction of anti-viral T cell responses. PLoS Pathog. 2006;2:e58. doi: 10.1371/journal.ppat.0020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dhodapkar KM, Kaufman JL, Ehlers M, Banerjee DK, Bonvini E, Koenig S, et al. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci U S A. 2005;102:2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J Exp Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mizoguchi A, Ogawa A, Takedatsu H, Sugimoto K, Shimomura Y, Shirane K, et al. Dependence of intestinal granuloma formation on unique myeloid DC-like cells. J Clin Invest. 2007;117:605–615. doi: 10.1172/JCI30150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sugimoto K, Ogawa A, Shimomura Y, Nagahama K, Mizoguchi A, Bhan AK. Inducible IL-12-producing B cells regulate Th2-mediated intestinal inflammation. Gastroenterology. 2007;133:124–136. doi: 10.1053/j.gastro.2007.03.112. [DOI] [PubMed] [Google Scholar]
- 111.Liu Y, Janeway CA., Jr Cells that present both specific ligand and costimulatory activity are the most efficient inducers of clonal expansion of normal CD4 T cells. Proc Natl Acad Sci U S A. 1992;89:3845–3849. doi: 10.1073/pnas.89.9.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 113.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mosmann T. Complexity or coherence? Cytokine secretion by B cells. Nat Immunol. 2000;1:465–466. doi: 10.1038/82707. [DOI] [PubMed] [Google Scholar]
- 115.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 116.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Current directions in autoimmunity. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 118.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 119.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259:259–272. doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harris DP, Goodrich S, Gerth AJ, Peng SL, Lund FE. Regulation of IFN-gamma production by B effector 1 cells: essential roles for T-bet and the IFN-gamma receptor. J Immunol. 2005;174:6781–6790. doi: 10.4049/jimmunol.174.11.6781. [DOI] [PubMed] [Google Scholar]
- 121.Wagner M, Poeck H, Jahrsdoerfer B, Rothenfusser S, Prell D, Bohle B, et al. IL-12p70-dependent Th1 induction by human B cells requires combined activation with CD40 ligand and CpG DNA. J Immunol. 2004;172:954–963. doi: 10.4049/jimmunol.172.2.954. [DOI] [PubMed] [Google Scholar]
- 122.Johansson-Lindbom B, Ingvarsson S, Borrebaeck CA. Germinal centers regulate human Th2 development. J Immunol. 2003;171:1657–1666. doi: 10.4049/jimmunol.171.4.1657. [DOI] [PubMed] [Google Scholar]
- 123.Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol. 2012;13:681–690. doi: 10.1038/ni.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Opata MM, Ye Z, Hollifield M, Garvy BA. B cell production of tumor necrosis factor in response to Pneumocystis murina infection in mice. Infect Immun. 2013;81:4252–4260. doi: 10.1128/IAI.00744-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang L, Yamamoto K, Nishiumi S, Nakamura M, Matsui H, Takahashi S, et al. Interferon-gamma-producing B cells induce the formation of gastric lymphoid follicles after Helicobacter suis infection. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.66. [DOI] [PubMed] [Google Scholar]
- 127.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, et al. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 129.Ulrichs T, Kosmiadi GA, Jorg S, Pradl L, Titukhina M, Mishenko V, et al. Differential organization of the local immune response in patients with active cavitary tuberculosis or with nonprogressive tuberculoma. J Infect Dis. 2005;192:89–97. doi: 10.1086/430621. [DOI] [PubMed] [Google Scholar]
- 130.Ulrichs T, Kosmiadi GA, Trusov V, Jorg S, Pradl L, Titukhina M, et al. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204:217–228. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- 131.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 132.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 133.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 134.Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 135.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 136.Amigorena S, Bonnerot C. Fc receptors for IgG and antigen presentation on MHC class I and class II molecules. Semin Immunol. 1999;11:385–390. doi: 10.1006/smim.1999.0196. [DOI] [PubMed] [Google Scholar]
- 137.Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239–245. doi: 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 138.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 139.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 140.Desai DD, Harbers SO, Flores M, Colonna L, Downie MP, Bergtold A, et al. Fc gamma receptor IIB on dendritic cells enforces peripheral tolerance by inhibiting effector T cell responses. J Immunol. 2007;178:6217–6226. doi: 10.4049/jimmunol.178.10.6217. [DOI] [PubMed] [Google Scholar]
- 141.Bandukwala HS, Clay BS, Tong J, Mody PD, Cannon JL, Shilling RA, et al. Signaling through Fc{gamma}RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation. J Exp Med. 2007;204:1875–1889. doi: 10.1084/jem.20061134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 143.Shi J, McIntosh RS, Pleass RJ. Antibody- and Fc-receptor-based therapeutics for malaria. Clin Sci (Lond) 2006;110:11–19. doi: 10.1042/CS20050136. [DOI] [PubMed] [Google Scholar]
- 144.Park-Min KH, Serbina NV, Yang W, Ma X, Krystal G, Neel BG, et al. FcgammaRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26:67–78. doi: 10.1016/j.immuni.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 145.Moore T, Ekworomadu CO, Eko FO, MacMillan L, Ramey K, Ananaba GA, et al. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J Infect Dis. 2003;188:617–624. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- 146.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 147.Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, et al. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191:1063–1068. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Menager N, Foster G, Ugrinovic S, Uppington H, Verbeek S, Mastroeni P. Fcgamma receptors are crucial for the expression of acquired resistance to virulent Salmonella enterica serovar Typhimurium in vivo but are not required for the induction of humoral or T-cell-mediated immunity. Immunology. 2007;120:424–432. doi: 10.1111/j.1365-2567.2006.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Uppington H, Menager N, Boross P, Wood J, Sheppard M, Verbeek S, et al. Effect of immune serum and role of individual Fcgamma receptors on the intracellular distribution and survival of Salmonella enterica serovar Typhimurium in murine macrophages. Immunology. 2006;119:147–158. doi: 10.1111/j.1365-2567.2006.02416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yoneto T, Waki S, Takai T, Tagawa Y, Iwakura Y, Mizuguchi J, et al. A critical role of Fc receptor-mediated antibody-dependent phagocytosis in the host resistance to blood-stage Plasmodium berghei XAT infection. J Immunol. 2001;166:6236–6241. doi: 10.4049/jimmunol.166.10.6236. [DOI] [PubMed] [Google Scholar]
- 151.Yuan R, Clynes R, Oh J, Ravetch JV, Scharff MD. Antibody-mediated modulation of Cryptococcus neoformans infection is dependent on distinct Fc receptor functions and IgG subclasses. J Exp Med. 1998;187:641–648. doi: 10.1084/jem.187.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maglione PJ, Xu J, Casadevall A, Chan J. Fc gamma receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J Immunol. 2008;180:3329–3338. doi: 10.4049/jimmunol.180.5.3329. [DOI] [PubMed] [Google Scholar]
- 153.Klimstra WB, Williams JC, Ryman KD, Heidner HW. Targeting Sindbis virus-based vectors to Fc receptor-positive cell types. Virology. 2005;338:9–21. doi: 10.1016/j.virol.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 154.Tongren JE, Corran PH, Jarra W, Langhorne J, Riley EM. Epitope-specific regulation of immunoglobulin class switching in mice immunized with malarial merozoite surface proteins. Infect Immun. 2005;73:8119–8129. doi: 10.1128/IAI.73.12.8119-8129.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: Implications for vaccine development. Cell Host Microbe. 2013;13:250–262. doi: 10.1016/j.chom.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kozakiewicz L, Phuah J, Flynn J, Chan J. The Role of B Cells and Humoral Immunity in Mycobacterium tuberculosis Infection. Adv Exp Med Biol. 2013;783:225–250. doi: 10.1007/978-1-4614-6111-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Glatman-Freedman A. The role of antibody-mediated immunity in defense against Mycobacterium tuberculosis: advances toward a novel vaccine strategy. Tuberculosis (Edinb) 2006;86:191–197. doi: 10.1016/j.tube.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 158.de L, Costello AM, Kumar A, Narayan V, Akbar MS, Ahmed S, Abou-Zeid C, et al. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans R Soc Trop Med Hyg. 1992;86:686–692. doi: 10.1016/0035-9203(92)90192-f. [DOI] [PubMed] [Google Scholar]
- 159.Torrado E, Fountain JJ, Robinson RT, Martino CA, Pearl JE, Rangel-Moreno J, et al. Differential and Site Specific Impact of B Cells in the Protective Immune Response to Mycobacterium tuberculosis in the Mouse. PloS one. 2013;8:e61681. doi: 10.1371/journal.pone.0061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kozakiewicz L, Chen Y, Xu J, Wang Y, Dunussi-Joannopoulos K, Ou Q, et al. B Cells Regulate Neutrophilia during Mycobacterium tuberculosis Infection and BCG Vaccination by Modulating the Interleukin-17 Response. PLoS Pathog. 2013;9:e1003472. doi: 10.1371/journal.ppat.1003472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, et al. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–194. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 163.Reljic R, Clark SO, Williams A, Falero-Diaz G, Singh M, Challacombe S, et al. Intranasal IFNgamma extends passive IgA antibody protection of mice against Mycobacterium tuberculosis lung infection. Clin Exp Immunol. 2006;143:467–473. doi: 10.1111/j.1365-2249.2006.03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Williams A, Reljic R, Naylor I, Clark SO, Falero-Diaz G, Singh M, et al. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111:328–333. doi: 10.1111/j.1365-2567.2004.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chambers MA, Gavier-Widén D, Glyn Hewinson R. Antibody bound to the surface antigen MPB83 of Mycobacterium bovis enhances survival against high dose and low dose challenge. FEMS Immunol Med Microbiol. 2004;41:93–100. doi: 10.1016/j.femsim.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 167.Guirado E, Amat I, Gil O, Diaz J, Arcos V, Caceres N, et al. Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect. 2006;8:1252–1259. doi: 10.1016/j.micinf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 168.Roy E, Stavropoulos E, Brennan J, Coade S, Grigorieva E, Walker B, et al. Therapeutic efficacy of high-dose intravenous immunoglobulin in Mycobacterium tuberculosis infection in mice. Infect Immun. 2005;73:6101–6109. doi: 10.1128/IAI.73.9.6101-6109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.de Valliere S, Abate G, Blazevic A, Heuertz RM, Hoft DF. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun. 2005;73:6711–6720. doi: 10.1128/IAI.73.10.6711-6720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hamasur B, Haile M, Pawlowski A, Schroder U, Williams A, Hatch G, et al. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine. 2003;21:4081–4093. doi: 10.1016/s0264-410x(03)00274-3. [DOI] [PubMed] [Google Scholar]
- 171.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nature reviews Immunology. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 172.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 173.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hardy RR, Hayakawa K. CD5 B cells, a fetal B cell lineage. Adv Immunol. 1994;55:297–339. doi: 10.1016/s0065-2776(08)60512-x. [DOI] [PubMed] [Google Scholar]
- 175.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 176.Cooper NR. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol. 1985;37:151–216. doi: 10.1016/s0065-2776(08)60340-5. [DOI] [PubMed] [Google Scholar]
- 177.Racine R, Winslow GM. IgM in microbial infections: Taken for granted? Immunol Lett. 2009;125:79–85. doi: 10.1016/j.imlet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Salinas-Carmona MC, Pérez-Rivera I. Humoral Immunity through Immunoglobulin M Protects Mice from an Experimental Actinomycetoma Infection by Nocardia brasiliensis. Infect Immun. 2004;72:5597–5604. doi: 10.1128/IAI.72.10.5597-5604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Salinas-Carmona MC, Torres-LÓPez E. Role of Passive Humoral Immunity in Experimental Mycetoma by Nocardia brasiliensis. Ann N Y Acad Sci. 1996;797:263–265. doi: 10.1111/j.1749-6632.1996.tb52972.x. [DOI] [PubMed] [Google Scholar]
- 180.Cole LE, Yang Y, Elkins KL, Fernandez ET, Qureshi N, Shlomchik MJ, et al. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proceedings of the National Academy of Sciences. 2009;106:4343–4348. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun. 2000;68:1988–1996. doi: 10.1128/iai.68.4.1988-1996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19:4465–4472. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 183.Savitt AG, Mena-Taboada P, Monsalve G, Benach JL. Francisella tularensis infection-derived monoclonal antibodies provide detection, protection, and therapy. Clin Vaccine Immunol. 2009;16:414–422. doi: 10.1128/CVI.00362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Racine R, Chatterjee M, Winslow GM. CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection. J Immunol. 2008;181:1375–1385. doi: 10.4049/jimmunol.181.2.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Racine R, McLaughlin M, Jones DD, Wittmer ST, MacNamara KC, Woodland DL, et al. IgM Production by Bone Marrow Plasmablasts Contributes to Long-Term Protection against Intracellular Bacterial Infection. The Journal of Immunology. 2011;186:1011–1021. doi: 10.4049/jimmunol.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b Lymphocytes Confer T Cell-Independent Long-Lasting Immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 187.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b Cells Exhibit Distinct Developmental Requirements and Have Unique Functional Roles in Innate and Adaptive Immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 188.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, et al. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med. 2010;207:2907–2919. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 191.Ehrenstein MR, O’Keefe TL, Davies SL, Neuberger MS. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proceedings of the National Academy of Sciences. 1998;95:10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Applequist SE, Dahlström J, Jiang N, Molina H, Heyman B. Antibody Production in Mice Deficient for Complement Receptors 1 and 2 Can Be Induced by IgG/Ag and IgE/Ag, But Not IgM/Ag Complexes. The Journal of Immunology. 2000;165:2398–2403. doi: 10.4049/jimmunol.165.5.2398. [DOI] [PubMed] [Google Scholar]
- 193.Heyman B, Pilström L, Shulman MJ. Complement activation is required for IgM-mediated enhancement of the antibody response. J Exp Med. 1988;167:1999–2004. doi: 10.1084/jem.167.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rutemark C, Bergman A, Getahun A, Hallgren J, Henningsson F, Heyman B. Complement Receptors 1 and 2 in Murine Antibody Responses to IgM-Complexed and Uncomplexed Sheep Erythrocytes. PloS one. 2012;7:e41968. doi: 10.1371/journal.pone.0041968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 2010;22:149–156. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]
- 196.Choi S-C, Wang H, Tian L, Murakami Y, Shin D-M, Borrego F, et al. Mouse IgM Fc Receptor, FCMR, Promotes B Cell Development and Modulates Antigen-Driven Immune Responses. The Journal of Immunology. 2013;190:987–996. doi: 10.4049/jimmunol.1202227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Honjo K, Kubagawa Y, Jones DM, Dizon B, Zhu Z, Ohno H, et al. Altered Ig levels and antibody responses in mice deficient for the Fc receptor for IgM (FcμR) Proceedings of the National Academy of Sciences. 2012;109:15882–15887. doi: 10.1073/pnas.1206567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ouchida R, Mori H, Hase K, Takatsu H, Kurosaki T, Tokuhisa T, et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proceedings of the National Academy of Sciences. 2012;109:E2699–E2706. doi: 10.1073/pnas.1210706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang D-W, et al. Identity of the elusive IgM Fc receptor (FcμR) in humans. J Exp Med. 2009;206:2779–2793. doi: 10.1084/jem.20091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv Immunol. 2006;91:1–44. doi: 10.1016/S0065-2776(06)91001-3. [DOI] [PubMed] [Google Scholar]
- 201.Aurora AB, Baluk P, Zhang D, Sidhu SS, Dolganov GM, Basbaum C, et al. Immune complex-dependent remodeling of the airway vasculature in response to a chronic bacterial infection. J Immunol. 2005;175:6319–6326. doi: 10.4049/jimmunol.175.10.6319. [DOI] [PubMed] [Google Scholar]
- 202.Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007;178:7222–7234. doi: 10.4049/jimmunol.178.11.7222. [DOI] [PubMed] [Google Scholar]
- 203.Clynes R. Protective mechanisms of IVIG. Curr Opin Immunol. 2007;19:646–651. doi: 10.1016/j.coi.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 204.Bournazos S, Woof JM, Hart SP, Dransfield I. Functional and clinical consequences of Fc receptor polymorphic and copy number variants. Clin Exp Immunol. 2009;157:244–254. doi: 10.1111/j.1365-2249.2009.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li X, Gibson A, Kimberly R. Human FcR Polymorphism and Disease. In: Daeron M, Nimmerjahn F, editors. Fc Receptor. Spriger International Publishing; 2014. pp. 275–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cooke GS, Aucan C, Walley AJ, Segal S, Greenwood BM, Kwiatkowski DP, et al. Association of Fcgamma receptor IIa (CD32) polymorphism with severe malaria in West Africa. Am J Trop Med Hyg. 2003;69:565–568. [PubMed] [Google Scholar]
- 207.Forthal DN, Landucci G, Bream J, Jacobson LP, Phan TB, Montoya B. FcgammaRIIa genotype predicts progression of HIV infection. J Immunol. 2007;179:7916–7923. doi: 10.4049/jimmunol.179.11.7916. [DOI] [PubMed] [Google Scholar]
- 208.Platonov AE, Kuijper EJ, Vershinina IV, Shipulin GA, Westerdaal N, Fijen CA, et al. Meningococcal disease and polymorphism of FcgammaRIIa (CD32) in late complement component-deficient individuals. Clin Exp Immunol. 1998;111:97–101. doi: 10.1046/j.1365-2249.1998.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.van der Pol WL, Huizinga TW, Vidarsson G, van der Linden MW, Jansen MD, Keijsers V, et al. Relevance of Fcgamma receptor and interleukin-10 polymorphisms for meningococcal disease. J Infect Dis. 2001;184:1548–1555. doi: 10.1086/324662. [DOI] [PubMed] [Google Scholar]
- 210.Yoshie H, Kobayashi T, Tai H, Galicia JC. The role of genetic polymorphisms in periodontitis. Periodontol 2000. 2007;43:102–132. doi: 10.1111/j.1600-0757.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 211.Biezeveld M, Geissler J, Merkus M, Kuipers IM, Ottenkamp J, Kuijpers T. The involvement of Fc gamma receptor gene polymorphisms in Kawasaki disease. Clin Exp Immunol. 2007;147:106–111. doi: 10.1111/j.1365-2249.2006.03266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dijstelbloem HM, Scheepers RH, Oost WW, Stegeman CA, van der Pol WL, Sluiter WJ, et al. Fcgamma receptor polymorphisms in Wegener’s granulomatosis: risk factors for disease relapse. Arthritis Rheum. 1999;42:1823–1827. doi: 10.1002/1529-0131(199909)42:9<1823::AID-ANR5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 213.Morgan AW, Robinson JI, Barrett JH, Martin J, Walker A, Babbage SJ, et al. Association of FCGR2A and FCGR2A-FCGR3A haplotypes with susceptibility to giant cell arteritis. Arthritis research & therapy. 2006;8:R109. doi: 10.1186/ar1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sadki K, Lamsyah H, Rueda B, Akil E, Sadak A, Martin J, et al. Analysis of MIF, FCGR2A and FCGR3A gene polymorphisms with susceptibility to pulmonary tuberculosis in Moroccan population. Journal of genetics and genomics = Yi chuan xue bao. 2010;37:257–264. doi: 10.1016/S1673-8527(09)60044-8. [DOI] [PubMed] [Google Scholar]
- 215.Jacobsen M, Repsilber D, Gutschmidt A, Neher A, Feldmann K, Mollenkopf HJ, et al. Candidate biomarkers for discrimination between infection and disease caused by Mycobacterium tuberculosis. J Mol Med (Berl) 2007;85:613–621. doi: 10.1007/s00109-007-0157-6. [DOI] [PubMed] [Google Scholar]
- 216.Maertzdorf J, Ota M, Repsilber D, Mollenkopf HJ, Weiner J, Hill PC, et al. Functional correlations of pathogenesis-driven gene expression signatures in tuberculosis. PloS one. 2011;6:e26938. doi: 10.1371/journal.pone.0026938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Maertzdorf J, Repsilber D, Parida SK, Stanley K, Roberts T, Black G, et al. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes and immunity. 2011;12:15–22. doi: 10.1038/gene.2010.51. [DOI] [PubMed] [Google Scholar]
- 218.Sutherland JS, Loxton AG, Haks MC, Kassa D, Ambrose L, Lee JS, et al. Differential gene expression of activating Fcgamma receptor classifies active tuberculosis regardless of human immunodeficiency virus status or ethnicity. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20:O230–238. doi: 10.1111/1469-0691.12383. [DOI] [PubMed] [Google Scholar]
- 219.Cliff JM, Lee JS, Constantinou N, Cho JE, Clark TG, Ronacher K, et al. Distinct phases of blood gene expression pattern through tuberculosis treatment reflect modulation of the humoral immune response. J Infect Dis. 2013;207:18–29. doi: 10.1093/infdis/jis499. [DOI] [PubMed] [Google Scholar]
- 220.van der Poel CE, Spaapen RM, van de Winkel JG, Leusen JH. Functional characteristics of the high affinity IgG receptor, FcgammaRI. J Immunol. 2011;186:2699–2704. doi: 10.4049/jimmunol.1003526. [DOI] [PubMed] [Google Scholar]
- 221.Junqueira-Kipnis AP, Kipnis A, Henao Tamayo M, Harton M, Gonzalez Juarrero M, Basaraba RJ, et al. Interleukin-10 production by lung macrophages in CBA xid mutant mice infected with Mycobacterium tuberculosis. Immunology. 2005;115:246–252. doi: 10.1111/j.1365-2567.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 223.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 224.Olson TS, Bamias G, Naganuma M, Rivera-Nieves J, Burcin TL, Ross W, et al. Expanded B cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease. J Clin Invest. 2004;114:389–398. doi: 10.1172/JCI20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Redford PS, Murray PJ, O’Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4:261–270. doi: 10.1038/mi.2011.7. [DOI] [PubMed] [Google Scholar]
- 226.Bosio CM, Gardner D, Elkins KL. Infection of B cell-deficient mice with CDC 1551, a clinical isolate of Mycobacterium tuberculosis: delay in dissemination and development of lung pathology. J Immunol. 2000;164:6417–6425. doi: 10.4049/jimmunol.164.12.6417. [DOI] [PubMed] [Google Scholar]
- 227.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 228.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14:447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 229.Hunter RL. On the pathogenesis of post primary tuberculosis: the role of bronchial obstruction in the pathogenesis of cavities. Tuberculosis (Edinb) 2011;91 (Suppl 1):S6–10. doi: 10.1016/j.tube.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 230.Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005;192:1201–1209. doi: 10.1086/444545. [DOI] [PubMed] [Google Scholar]
- 232.Moraes TJ, Zurawska JH, Downey GP. Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol. 2006;13:21–27. doi: 10.1097/01.moh.0000190113.31027.d5. [DOI] [PubMed] [Google Scholar]
- 233.Eruslanov EB, Lyadova IV, Kondratieva TK, Majorov KB, Scheglov IV, Orlova MO, et al. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun. 2005;73:1744–1753. doi: 10.1128/IAI.73.3.1744-1753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gonzalez-Juarrero M, Turner OC, Turner J, Marietta P, Brooks JV, Orme IM. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect Immun. 2001;69:1722–1728. doi: 10.1128/IAI.69.3.1722-1728.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Turner J, Frank AA, Brooks JV, Gonzalez-Juarrero M, Orme IM. The progression of chronic tuberculosis in the mouse does not require the participation of B lymphocytes or interleukin-4. Exp Gerontol. 2001;36:537–545. doi: 10.1016/s0531-5565(00)00257-6. [DOI] [PubMed] [Google Scholar]
- 236.Khader SA, Rangel-Moreno J, Fountain JJ, Martino CA, Reiley WW, Pearl JE, et al. In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J Immunol. 2009;183:8004–8014. doi: 10.4049/jimmunol.0901937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Phuah JY, Mattila JT, Lin PL, Flynn JL. Activated B cells in the granulomas of nonhuman primates infected with Mycobacterium tuberculosis. Am J Pathol. 2012;181:508–514. doi: 10.1016/j.ajpath.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 239.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 240.Kaufmann SH. The contribution of immunology to the rational design of novel antibacterial vaccines. Nat Rev Microbiol. 2007;5:491–504. doi: 10.1038/nrmicro1688. [DOI] [PubMed] [Google Scholar]
- 241.Chow SK, Smith C, MacCarthy T, Pohl MA, Bergman A, Casadevall A. Disease-enhancing antibodies improve the efficacy of bacterial toxin-neutralizing antibodies. Cell Host Microbe. 2013;13:417–428. doi: 10.1016/j.chom.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


