Abstract
Objective
No clear consensus has been reached on the PTPN22 R620W polymorphism and anti-neutrophil cytoplasmic antibody (ANCA) disease, especially when stratified by ANCA specificity and disease phenotypes.
Methods
A metaanalysis was conducted on the PTPN22 R620W polymorphism across 4 studies in 1399 white patients with ANCA disease and 9934 normal control subjects.
Results
Overall, metaanalysis showed a statistically significant association between the A allele and ANCA disease in all subjects (OR 1.44, 95% CI 1.26–1.64, p < 0.00001), and stratification by disease category indicated the A allele was associated with granulomatosis with polyangiitis (Wegener’s; GPA; OR 1.72, 95% CI 1.35–2.20, p < 0.0001) and microscopic polyangiitis (MPA; OR 1.53, 95% CI 1.08–2.15, p = 0.02) as compared to controls. However, when stratified by ANCA specificity, the association of the A allele was statistically evident among those with proteinase 3 (PR3) ANCA disease (OR 1.74, 95% CI 1.25–2.430, p = 0.001), with the same trend but not statistically associated with myeloperoxidase ANCA disease (OR 1.94, 95% CI 0.64–5.85, p = 0.24). The marked associations were also demonstrated between this allele with lung (OR 1.69, 95% CI 1.21–2.36, p = 0.002), ENT (OR 2.03, 95% CI 1.45–2.84, p < 0.0001), skin (OR 2.55, 95% CI 1.69–3.84, p < 0.0001), and peripheral neuropathy involvement (OR 2.12, 95% CI 1.39–3.22, p = 0.0005).
Conclusion
The PTPN22 620W allele confers susceptibility to the occurrence and development of ANCA disease in whites, with specific evidence among subsets with GPA, MPA, and PR3 ANCA. (J Rheumatol First Release Dec 1 2014; doi:10.3899/jrheum.131430)
Keywords: ANTINEUTROPHIL CYTOPLASMIC ANTIBODY, MYELOPEROXIDASE GRANULOMATOSIS WITH POLYANGIITIS, MICROSCOPIC POLYANGIITIS PROTEINASE 3, PROTEIN TYROSINE PHOSPHATASE NONRECEPTOR 22
Antineutrophil cytoplasmic antibody (ANCA) disease is characterized by necrotizing inflammation of small blood vessels. ANCA is categorized into several disease groups, including granulomatosis with polyangiitis (Wegener’s; GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA), as proposed by the Chapel Hill Consensus Conference1.
The cause of ANCA disease is not clear. Although both environmental factors2 and epigenetic changes3 have been implicated, the molecular genetics of ANCA contributes significantly to the pathogenesis of the disease4. In 2004, a single nucleotide polymorphism (SNP) in the protein tyrosine phosphatase nonreceptor 22 (PTPN22) gene (R620W, rs2476601, C1858T) was identified; it caused a protein modification, which disrupted the regulatory domain of the phosphatase, conferring a gain-of-function phenotype. It has been demonstrated that this genetic variant is associated with numerous other autoimmune diseases including type 1 diabetes5,6,7,8,9, rheumatoid arthritis10,11,12,13,14,15, and systemic lupus erythematosus16,17,18. Later this genetic variant was demonstrated to be linked with ANCA disease in 4 studies19,20,21,22. However, as a rare autoimmune disease, the number of patients was relatively small within each individual study. Further, a genome-wide association study (GWAS) suggested that proteinase 3 (PR3) and myeloperoxidase (MPO) had distinct genetic origins4. The published genetic association results for the PTPN22 R620W polymorphism are controversial and inconclusive when the disease is stratified by clinical phenotype and ANCA specificity, a practice that might be due to small sample sizes, low statistical power, and/or clinical heterogeneity. Therefore, to overcome the limitations of individual studies and resolve inconsistencies, we turned to metaanalysis in the subgroup of ANCA disease. We investigated whether the PTPN22 R620W polymorphism contributes to the susceptibility of ANCA diseases and their subtypes using a metaanalysis approach.
MATERIALS AND METHODS
Identification and eligibility of relevant studies
To identify all studies that examined the association of PTPN22 R620W (rs 2476601) polymorphism with ANCA disease, we performed a systematic, computerized literature search of the PubMed database, Embase, and the Cochrane library (up to August 2013) using the following various combinations of keywords and subject terms: “tyrosine phosphatase non-receptor 22” OR “PTPN22”, “polymorphism” OR “polymorphisms” and “anti-neutrophil cytoplasmic antibody” OR “ANCA” OR “AAV” (ANCA-associated vasculitis).
We also retrieved additional studies through the MEDLINE option “related articles.” Search results were limited to research articles in English and studies on human subjects without country restrictions. The full text of the retrieved articles was scrutinized to decide whether information on the topic of interest was included. Studies included in the metaanalysis had to meet all the following criteria: (1) use of cases compared to unrelated controls (either retrospective or cross-sectional), (2) available genotype frequency, (3) a genotype distribution of the control population consistent with Hardy Weinberg equilibrium (HWE). All the patients met the criteria of the Chapel Hill consensus conference definition for GPA, MPA, and EGPA1.
Data extraction
To extract the information needed, all articles were separately collected and reviewed by 2 independent investigators (YC, KL) who checked for any discordance and reached a consensus. If they could not come to an agreement, a third investigator adjudicated the disagreements. The following information was collected from the studies on the genotype of PTPN22 R620W: first author, year of publication, full citation information, country, sample sizes of cases and controls, genotype numbers, allele frequency in both cases and controls, and p values of HWE in controls. Allele frequency in cases by disease category and ANCA specificity were also collected.
Statistical analysis
The strength of the association of Arg620Trp (R620W) with ANCA disease was measured by OR compared to controls, with corresponding 95% CI calculated according to the method of Woolf23. We examined the association between the W allele of PTPN22 R620W and ANCA disease risk (A vs G), the dominant genetic model (AA + GA vs GG), the recessive genetic model (AA vs GA + GG), and homozygote comparison (AA vs GG). In our study, 2 models of metaanalysis were used for dichotomous outcomes in the Review-Manager 5.2 software: the fixed-effects model and the random-effects model. The fixed-effects model used the Peto Mantel–Haenszel method, which assumes that studies are sampled from populations with the same effect size, and adjusts the study weights according to the in-study variance. The random-effects model used the Der Simonian and Laird’s method, which assumes that the studies are taken from populations with varying effect sizes, and calculates the study weights both from in-study and between-study variances, considering the extent of variation or heterogeneity. We performed a chi squared Q statistic test to assess between study heterogeneity24. Heterogeneity was considered significant for p < 0.10 because of the low power of the statistic. The inconsistency index I2 was also calculated to evaluate the variation that was caused by heterogeneity rather than by chance, and higher values of the index indicated the existence of heterogeneity25. A p value > 0.10 for the Q-test indicates a lack of heterogeneity among studies, and the pooled OR estimate of each study was calculated with the fixed-effects model26. Otherwise, the random-effects model was used27. The significance of the pooled OR was determined by the Z test and a p value of < 0.05 was considered significant. The same methods were used comparing subgroups based on ANCA specificity, disease specificity, and organ involvement to controls.
In addition, an estimate of potential publication bias was carried out by the funnel plot, with an asymmetric plot indicating possible publication bias. HWE was tested by the chi-square test for goodness-of-fit based on a Web program (www.ihg.gsf.de/cgi-bin/hw/hwa1.pl). Analyses were performed using the software Review-Manager 5.2. All p values were 2-sided.
RESULTS
Description of studies identified in metaanalysis
Figure 1 shows the flow chart summarizing the process of study selection and reasons for exclusion. The GWAS mentioned in introduction was not included because the PTPN22 R620W allele was not included in the GWAS study. Six relevant studies were identified that examined the association between polymorphisms of PTPN22 R620W and ANCA vasculitis. Two studies were excluded because they were metaanalyses used to determine the association between PTPN22 R620W and vasculitis, including Takayasu arteritis, Henoch-Schönlein purpura, Behçet disease, giant cell arteritis, and ANCA-associated27 or other autoimmune diseases28. Thus, 4 studies met the inclusion criteria19,20,21,22 and involved a total of 1399 patients and 9934 controls. There was 1 study each in Germany, the United Kingdom, Italy, and the United States. All cases and controls involved in these studies were white. The pooled overall frequencies of the A allele were 11.62% and 9.11% in ANCA cases and in controls, respectively. Detailed information on eligible studies, sample size, the distribution of R620W genotypes, A allele frequencies of cases and controls, and p values of HWE in controls are described in Tables 1a and 1b.
Figure 1.
Flow chart of selection of studies and specific reasons for exclusion from the metaanalysis.
Table 1A.
Characteristics of the individual studies included in the metaanalysis.
| Study | Country | Sample Sizes | Genotypes for Cases | Genotypes for Controls | A Allele Frequency (%) HWE p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Total | GG | GA | AA | GG | GA | AA | Case | Control | Control | ||
| Jagiello, et al (2005) | Germany | 199 | 399 | 598 | 142 | 52 | 5 | 323 | 72 | 4 | 15.58 | 10.03 | 0.9956 |
| Carr, et al (2009) | UK | 626 | 7412 | 8038 | 471 | 146 | 9 | 6044 | 1298 | 70 | 13.10 | 9.70 | 0.9732 |
| Martorana, et al (2012) | Italy | 344 | 945 | 1289 | 302 | 39 | 3 | 859 | 82 | 4 | 6.54 | 4.76 | 0.1828 |
| Cao, et al (2012) | USA | 230 | 1178 | 1408 | 179 | 48 | 3 | 984 | 186 | 8 | 11.74 | 8.57 | 0.8063 |
Table 1B Characteristics of the individual studies stratified by disease, ANCA type, and organ involvement.
| Subgroup | Sample Sizes | Allele for Cases | Allele for Controls | ||||
|---|---|---|---|---|---|---|---|
| Case | Control | Total | G | A | G | A | |
| GPA | 416 | 2522 | 2938 | 725 | 107 | 4672 | 372 |
| MPA | 212 | 2123 | 2335 | 382 | 42 | 3954 | 292 |
| PR3 | 200 | 2123 | 2323 | 355 | 45 | 3954 | 292 |
| MPO | 125 | 2123 | 2248 | 223 | 27 | 3954 | 292 |
| Lung | 202 | 2123 | 2325 | 360 | 44 | 3954 | 292 |
| ENT | 188 | 2123 | 2311 | 331 | 45 | 3954 | 292 |
| Skin | 92 | 2123 | 2215 | 154 | 30 | 3954 | 292 |
| Peripheral neuropathy | 98 | 2123 | 2221 | 168 | 28 | 3954 | 292 |
| Kidney | 278 | 2123 | 2401 | 504 | 52 | 3954 | 292 |
Jagiello, et al (2005), Martorana, et al (2012), Cao, et al (2012) were available for GPA subgroup. Martorana and Cao were available for MPA, PR3, MPO, lung, ENT, skin, peripheral neuropathy, and kidney subgroups. HWE: Hardy-Weinberg equilibrium; ANCA: antineutrophil cytoplasmic antibody; GPA: granulomatosis with polyangiitis (Wegener’s); MPA: microscopic polyangiitis; PR3: proteinase 3; MPO: myeloperoxidase.
PTPN22 R620W polymorphism conferred susceptibility to ANCA disease
For each study, we investigated the association between the PTPN22 R620W polymorphisms and ANCA disease risk, assuming different inheritance models of the 620W allele (Table 2). Overall, when all the eligible studies were pooled into the metaanalysis, significant associations between PTPN22 R620W polymorphism and ANCA susceptibility were observed in all genetic models, including allele comparison (A vs G: p < 0.00001, OR 1.44, 95% CI 1.26–1.64, Pheterogeneity 0.87; Figure 2a), dominant genetic model (AA + GA vs GG: p < 0.00001, OR 1.48, 95% CI 1.28–1.70, Pheterogeneity 0.89; Figure 2b), recessive genetic model (AA vs GA + GG: p = 0.03, OR 1.80, 95% CI 1.07–3.02, Pheterogeneity 0.92; Figure 2c), and homozygote comparisn (AA vs GG: p = 0.01, OR 1.94, 95% CI 1.15–3.25, Pheterogeneity 0.91; Figure 2d).
Table 2.
Metaanalysis of the association between the PTPN22 R620W polymorphism and AAV risk.
| Genotype Contrasts | AAV Phenotypes |
No. of Study |
Test of Heterogeneity | Test of Association | ||||
|---|---|---|---|---|---|---|---|---|
| Model | I2 (%) | p | OR | 95% CI | p | |||
| A vs G | AAV | 4 | F | 0 | 0.87 | 1.44 | 1.26–1.64 | 2.27 × 10−5 |
| GPA | 3 | F | 0 | 0.87 | 1.72 | 1.35–2.20 | 1.25 × 10−7 | |
| MPA | 2 | F | 0 | 0.86 | 1.53 | 1.08–2.15 | 0.02 | |
| PR3 | 2 | F | 0 | 0.74 | 1.74 | 1.25–2.43 | 0.001 | |
| MPO | 2 | R | 77 | 0.04 | 1.94 | 0.64–5.85 | 0.24 | |
| AA + GA vs GG | AAV | 4 | F | 0 | 0.89 | 1.48 | 1.28–1.70 | 5.10 × 10−5 |
| AA vs GA + GG | AAV | 4 | F | 0 | 0.92 | 1.80 | 1.07–3.02 | 0.03 |
| AA vs GG | AAV | 4 | F | 0 | 0.91 | 1.94 | 1.15–3.25 | 0.01 |
AAV: antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis; GPA: granulomatosis with polyangiitis; MPA: microscopic polyangiitis; MPO: myeloperoxidase; PR3: proteinase 3; F: fixed effects model; R: random effects model.
Figure 2.
Association between PTPN22 R620W polymorphism and antineutrophil cytoplasmic antibodies (ANCA) disease risk. Figure 2a shows the association between PTPN22 R620W polymorphism and ANCA disease in allele comparison (A vs G). Figure 2b shows the association between R620W polymorphism and ANCA disease under the dominant genetic model (AA + GA vs GG). Figure 2c shows the association between R620W polymorphism and ANCA disease under recessive genetic model (AA vs GA + GG). Figure 2d shows the association between R620W polymorphism and ANCA disease in homozygote comparison (AA vs GG). AVV: ANCA-associated vasculitis; M-H: Mantel-Haenszel test.
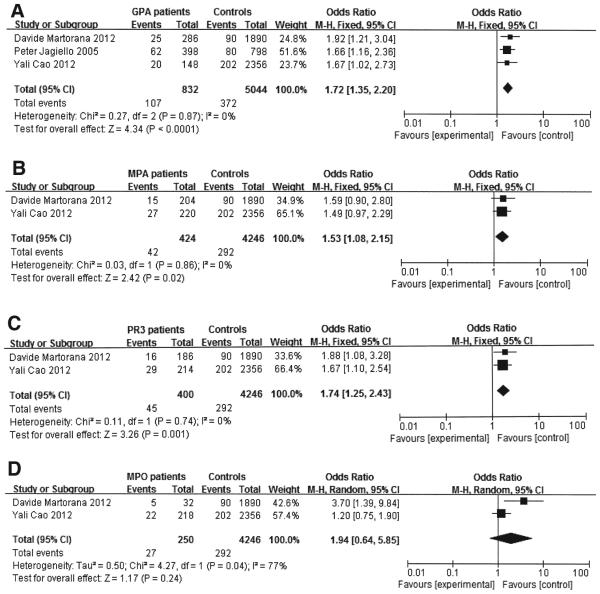
Significant association identified in the PTPN22 R620W polymorphism with both GPA and MPA
When all the available data were pooled into the metaanalysis (Table 2), a significant association between PTPN22 R620W polymorphism and GPA susceptibility was observed in allele comparison (A vs G p < 0.0001, OR 1.72, 95% CI 1.35–2.20, Pheterogeneity 0.87; Figure 3a). A similar result was also observed in the MPA subgroup (A vs G: p = 0.02, OR 1.53, 95% CI 1.08–2.15, Pheterogeneity 0.86; Figure 3b).
Figure 3.
Association between PTPN22 R620W polymorphism and antineutrophil cytoplasmic antibodies (ANCA) disease stratified by disease phenotype and ANCA serology. Figure 3a shows the association between PTPN22 R620W polymorphism and granulomatosis with polyangiitis (GPA) in allele comparison (A vs G). Figure 3b shows the association between PTPN22 R620W polymorphism and microscopic polyangiitis (MPA) in allele comparison (A vs G). Figure 3c shows the association between PTPN22 R620W polymorphism and proteinase 3 (PR3)-ANCA disease in allele comparison (A vs G). Figure 3d shows the association between PTPN22 R620W polymorphism and myeloperoxidase (MPO)-ANCA disease in allele comparison (A vs G). M-H: Mantel-Haenszel test.
PTPN22 R620W polymorphism was significantly correlated with PR3-ANCA serotype, but inconclusive with the MPO-ANCA serotype
We pooled the available data into the metaanalysis and found that there was a significant association between PTPN22 R620W polymorphism and PR3-ANCA in allele comparison (A vs G: p = 0.001, OR 1.74, 95% CI 1.25–2.43, Pheterogeneity 0.74; Figure 3c). However, carriage of this variant allele was inconclusive with MPO-ANCA serotype in allele comparison, with a large association but also a wide CI that crossed and equaled OR (1.0; A VS G: p = 0.24, OR 1.94, 95% CI 0.64–5.85, Pheterogeneity 0.04; Table 2, Figure 3d).
PTPN22 R620W polymorphism association with organ involvement
Further analysis of this polymorphism focused on its association with organ involvement. Organ involvement in the patients with AAV was assessed by physical examination, routine laboratory tests, and a standardized set of imaging studies. Two available datasets from Italy and the United States were pooled for this analysis. The 620W allele was significantly associated with lung involvement (A vs G: p = 0.002, OR 1.69, 95% CI 1.21–2.36, Pheterogeneity 0.35), ENT involvement (A vs G: p < 0.0001, OR 2.03, 95% CI 1.45–2.84, Pheterogeneity 0.90), skin involvement (A vs G: p < 0.00001, OR 2.55, 95% CI 1.69–3.84, Pheterogeneity 0.20) and peripheral neuropathy involvement (A vs G: p = 0.0005, OR 2.12, 95% CI 1.39–3.22, Pheterogeneity 0.68). This allele was positively but not statistically associated with kidney involvement (A vs G: p = 0.20, OR 1.44, 95% CI 0.82–2.53, Pheterogeneity 0.09;Table 3).
Table 3.
Metaanalysis of the association between the PTPN22 R620W polymorphism and organ involvement.
| Genotype Contrasts | Organ Involvement |
Ref No. | Test of Heterogeneity | Test of Association | ||||
|---|---|---|---|---|---|---|---|---|
| Model | I2 (%) | p | OR | 95% CI | p | |||
| A vs G | Lung | 2 | F | 0 | 0.35 | 1.69 | 1.21–2.36 | 0.002 |
| ENT | 2 | F | 0 | 0.90 | 2.03 | 1.45–2.84 | 3.33 × 10−4 | |
| Skin | 2 | F | 0 | 0.20 | 2.55 | 1.69–3.83 | 3.31 × 10−6 | |
| Peripheral neuropathy | 2 | F | 0 | 0.68 | 2.12 | 1.39–3.22 | 0.0005 | |
| Kidney | 2 | R | 66 | 0.09 | 1.44 | 0.82-2.53 | 0.20 | |
F: fixed effects model; R: random effects model.
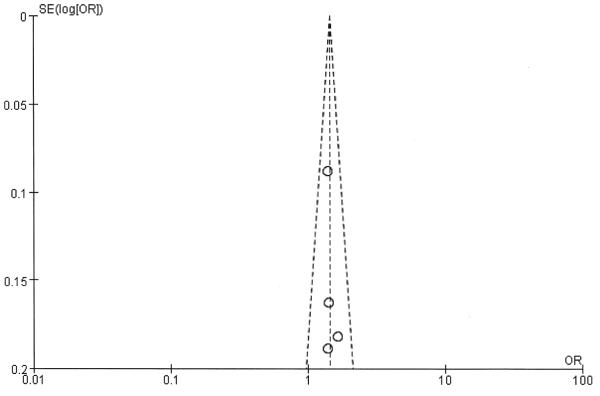
Heterogeneity and publication bias
The shape of the funnel plot did not reveal any evidence of obvious asymmetry, and no evidence of publication bias was found in the metaanalysis (Appendix 1).
APPENDIX 1.
Funnel plot for allele comparison.
DISCUSSION
This is the largest systematic review to date, to our knowledge, investigating the association between PTPN22 R620W polymorphism and ANCA disease. Compared with the metaanalysis of 3 studies by Lee, et al27, our metaanalysis included 4 studies with 1399 patients and 9934 controls. Our study also included stratified metaanalyses based on ANCA type and disease specificity. Consistent with the former metaanalysis, we observed a significant association between the PTPN22 R620W polymorphism and susceptibility to ANCA disease. The GWAS by Lyons, et al did not find the association between PTPN22 and ANCA disease4. However, they did not directly type rs2476601 in the GWAS study but instead typed rs6679677. Perhaps the 2 SNP could not tag each other perfectly. We had a large number of patients enrolled in our study and conducted further subgroup analysis, which showed the association of the disease-associated allele skewed toward PR3-ANCA disease, and suggested but did not detect an association for MPO-ANCA disease. Considering the small sample size and limited statistical power, future studies based on large-scale investigation are needed to further validate the association of PTPN22 620W polymorphism and MPO-ANCA disease in a white population.
Our metaanalysis reinforces the strength of the association between the R620W and susceptibility to both GPA and MPA. GPA and MPA are 2 distinct yet overlapping syndromes of AAV. It has been demonstrated that the strongest genetic associations were with the antigenic specificity of ANCA, rather than with the clinical syndrome4. The results of our metaanalysis were consistent with this conclusion. However, our results should be interpreted with caution because only 2 studies provided data in the subgroup of ANCA specificity. In addition, the results of the 2 above studies21,22 were positive for PR3-ANCA disease and neither was statistically significant for MPO-ANCA disease. This might be explained by the geographical and genetic factors involved in the pathogenesis of ANCA disease. Prevalence of ANCA type varies across geographic regions, with PR3-ANCA being more common in northern European populations and MPO-ANCA more common in southern European countries29,30,31. US cohorts suggested that PR3-ANCA was more prevalent in whites than in African Americans32. Additionally, MPO-ANCA is predominant in Asian countries33,34. The geographic distribution of this variant allele is highest in countries where PR3-ANCA is predominant. The allele is nearly absent in African American and Asian populations35.
In the present metaanalysis, the genotypic-phenotypic associations were also investigated. In the US study22, the A allele was particularly associated with lung, ENT, skin, and peripheral nervous system involvement. The Italian group21 instead found that this allele was enriched predominantly in GPA patients with ENT involvement, lung involvement, and skin lesions other than purpura. Our results seem to point to a particular association between the PTPN22 SNP and a PR3-ANCA disease pattern. It has been noted that patients with the most compelling evidence for necrotizing granulomatous inflammation are most likely to have PR3-ANCA36. Skin lesions and peripheral neuropathy may be granulomatous and are common phenotypes in PR3-ANCA disease37. Interestingly, PTPN22 is thought to be involved in granuloma formation38.
The results of our metaanalysis should be interpreted within the context of its limitations. First, we focused only on papers published in the English language, so some local literature biases were inevitable. Second, only a small number of studies were conducted on the subgroup of ANCA disease, which may mean that our investigation was underpowered. Third, our ethnic-specific metaanalysis was performed using data from white patients, and thus our results are only applicable to this ethnic group. Further studies are required in different ethnic populations, because the prevalence of the PTPN22 R620W polymorphism is ethnicity-dependent. Fourth, the results for publication bias should be interpreted with caution, given the relatively small number of available studies.
Our metaanalysis demonstrates that the PTPN22 R620W polymorphism confers susceptibility to ANCA disease in whites. In contrast, carriage of this variant allele was inconclusive with the MPO-ANCA serotype, which needs to be studied in larger samples.
Acknowledgments
Supported by the National Natural Science Foundation of China (grant number 81200535), the Scientific Research Foundation for Returned Chinese Scholars, Ministry of Human Resources and Social Security (grant number 2012), and China Japan Friendship Hospital Youth Science and Technology Excellence Project (grant number 2014-QNYC-A-01).
Contributor Information
Yali Cao, Department of Nephrology, China-Japan Friendship Hospital.
Kuo Liu, Department, China MeiTan General Hospital, National Mining Medical Center.
Zhigang Tian, Department of Surgery, Beijing LuHe Hospital.
Susan L. Hogan, UNC Kidney Center, Department of Medicine, UNC at Chapel Hill
Jiajin Yang, UNC Kidney Center, Department of Medicine, UNC at Chapel Hill.
Caroline J. Poulton, UNC Kidney Center, Department of Medicine, UNC at Chapel Hill
Ronald J. Falk, UNC Kidney Center, Department of Medicine, UNC at Chapel Hill
Wenge Li, Department of Nephrology, China-Japan Friendship Hospital.
REFERENCES
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Hogan SL, Cooper GS, Savitz DA, Nylander-French LA, Parks CG, Chin H, et al. Association of silica exposure with anti-neutrophil cytoplasmic autoantibody small-vessel vasculitis: a population-based, case-control study. Clin J Am Soc Nephrol. 2007;2:290–9. doi: 10.2215/CJN.03501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciavatta DJ, Yang J, Preston GA, Badhwar AK, Xiao H, Hewins P, et al. Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis. J Clin Invest. 2010;120:3209–19. doi: 10.1172/JCI40034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–23. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–8. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 6.Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 7.Ladner MB, Bottini N, Valdes AM, Noble JA. Association of the single nucleotide polymorphism C1858T of the PTPN22 gene with type 1 diabetes. Hum Immunol. 2005;66:60–4. doi: 10.1016/j.humimm.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Begovich AB, Caillier SJ, Alexander HC, Penko JM, Hauser SL, Barcellos LF, et al. The R620W polymorphism of the protein tyrosine phosphatase PTPN22 is not associated with multiple sclerosis. Am J Hum Genet. 2005;76:184–7. doi: 10.1086/427244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng W, She JX. Genetic association between a lymphoid tyrosine phosphatase (PTPN22) and type 1 diabetes. Diabetes. 2005;54:906–8. doi: 10.2337/diabetes.54.3.906. [DOI] [PubMed] [Google Scholar]
- 10.Van Oene M, Wintle RF, Liu X, Yazdanpanah M, Gu X, Newman B, et al. Association of the lymphoid tyrosine phosphatase R620W variant with rheumatoid arthritis, but not Crohn’s disease, in Canadian populations. Arthritis Rheum. 2005;52:1993–8. doi: 10.1002/art.21123. [DOI] [PubMed] [Google Scholar]
- 11.Orozco G, Sánchez E, González-Gay MA, López-Nevot MA, Torres B, Cáliz R, et al. Association of a functional single-nucleotide polymorphism of PTPN22, encoding lymphoid protein phosphatase, with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 2005;52:219–24. doi: 10.1002/art.20771. [DOI] [PubMed] [Google Scholar]
- 12.Hinks A, Barton A, John S, Bruce I, Hawkins C, Griffiths CE, et al. Association between the PTPN22 gene and rheumatoid arthritis and juvenile idiopathic arthritis in a UK population: further support that PTPN22 is an autoimmunity gene. Arthritis Rheum. 2005;52:1694–9. doi: 10.1002/art.21049. [DOI] [PubMed] [Google Scholar]
- 13.Simkins HM, Merriman ME, Highton J, Chapman PT, O’Donnell JL, Jones PB, et al. Association of the PTPN22 locus with rheumatoid arthritis in a New Zealand Caucasian cohort. Arthritis Rheum. 2005;52:2222–5. doi: 10.1002/art.21126. [DOI] [PubMed] [Google Scholar]
- 14.Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M, et al. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76:561–71. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AT, Li W, Liew A, Bombardier C, Weisman M, Massarotti EM, et al. The PTPN22 R620W polymorphism associates with RF positive rheumatoid arthritis in a dose-dependent manner but not with HLA-SE status. Genes Immun. 2005;6:129–33. doi: 10.1038/sj.gene.6364159. [DOI] [PubMed] [Google Scholar]
- 16.Robertson J, Wu J, Arends J, Glass W, 2nd, Southwood S, Sette A, et al. Characterization of the T-cell epitope that causes anti-GBM glomerulonephritis. Kidney Int. 2005;68:1061–70. doi: 10.1111/j.1523-1755.2005.00498.x. [DOI] [PubMed] [Google Scholar]
- 17.Reddy MV, Johansson M, Sturfelt G, Jönsen A, Gunnarsson I, Svenungsson E, et al. The R620W C/T polymorphism of the gene PTPN22 is associated with SLE independently of the association of PDCD1. Genes Immun. 2005;6:658–62. doi: 10.1038/sj.gene.6364252. [DOI] [PubMed] [Google Scholar]
- 18.Behrens TW, Graham RR, Kyogoku C, Baechler EC, Ramos PS, Gillett C, et al. Progress towards understanding the genetic pathogenesis of systemic lupus erythematosus. Novartis Found Symp. 2005;267:145–60. doi: 10.1002/047002139x.ch10. [DOI] [PubMed] [Google Scholar]
- 19.Jagiello P, Aries P, Arning L, Wagenleiter SE, Csernok E, Hellmich B, et al. The PTPN22 620W allele is a risk factor for Wegener’s granulomatosis. Arthritis Rheum. 2005;52:4039–43. doi: 10.1002/art.21487. [DOI] [PubMed] [Google Scholar]
- 20.Carr EJ, Niederer HA, Williams J, Harper L, Watts RA, Lyons PA, et al. Confirmation of the genetic association of CTLA4 and PTPN22 with ANCA-associated vasculitis. BMC Med Genet. 2009;10:121. doi: 10.1186/1471-2350-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martorana D, Maritati F, Malerba G, Bonatti F, Alberici F, Oliva E, et al. PTPN22 R620W polymorphism in the ANCA-associated vasculitides. Rheumatology. 2012;51:805–12. doi: 10.1093/rheumatology/ker446. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Yang J, Colby K, Hogan SL, Hu Y, Jennette CE, et al. High basal activity of the PTPN22 gain-of-function variant blunts leukocyte responsiveness negatively affecting IL-10 production in ANCA vasculitis. PLoS One. 2012;7:e42783. doi: 10.1371/journal.pone.0042783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–3. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 24.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petitti DB. Meta-analysis, decision analysis, and cost-effectiveness analysis. Oxford University Press; New York: 1994. pp. 15–20. [Google Scholar]
- 27.Lee YH, Choi SJ, Ji JD, Song GG. The protein tyrosine phosphatase nonreceptor 22 Cl858T polymorphism and vasculitis: a meta-analysis. Mol Biol Rep. 2012;39:8505–11. doi: 10.1007/s11033-012-1705-x. [DOI] [PubMed] [Google Scholar]
- 28.Zheng J, Ibrahim S, Petersen F, Yu X. Meta-analysis reveals an association of PTPN22 Cl858T with autoimmune diseases, which depends on the localization of the affected tissue. Genes Immun. 2012;13:641–52. doi: 10.1038/gene.2012.46. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Gay MA, Garcia-Porrua C, Guerrero J, Rodriguez-Ledo P, Llorca J. The epidemiology of the primary systemic vasculitides in northwest Spain: implications of the Oiapel Hill Consensus Conference definitions. Arthritis Rheum. 2003;49:388–93. doi: 10.1002/art.11115. [DOI] [PubMed] [Google Scholar]
- 30.Watts RA, Scott DG. Epidemiology of the vasculitides. Curr Opin Rheumatol. 2003;15:11–6. doi: 10.1097/00002281-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Reinhold-Keller E, Herlyn K, Wagner-Bastmeyer R, Gutfleisch J, Peter HH, Raspe HH, et al. No difference in the incidences of vasculitides between north and south Germany: first results of the German vasculitis register. Rheumatology. 2002;41:540–9. doi: 10.1093/rheumatology/41.5.540. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y, Schmitz JL, Yang J, Hogan SL, Bunch D, Hu Y, et al. DRB1*15 allele is a risk factor for PR3-ANCA disease in African Americans. J Am Soc Nephrol. 2011;22:1161–7. doi: 10.1681/ASN.2010101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M, Yu F, Zhang Y, Zhao MH. Antineutrophil cytoplasmic autoantibody-associated vasculitis in older patients. Medicine. 2008;87:203–9. doi: 10.1097/MD.0b013e31817c744b. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto S, Uezono S, Hisanaga S, Fukudome K, Kobayashi S, Suzuki K, et al. Incidence of ANCA-associated primary renal vasculitis in the Miyazaki Prefecture: the first population-based, retrospective, epidemiologic survey in Japan. Clin J Am Soc Nephrol. 2006;1:1016–22. doi: 10.2215/CJN.01461005. [DOI] [PubMed] [Google Scholar]
- 35.Vang T, Miletic AV, Bottini N, Mustelin T. Protein tyrosine phosphatase PTPN22 in human autoimmunity. Autoimmunity (Review) 2007;40:453–61. doi: 10.1080/08916930701464897. [DOI] [PubMed] [Google Scholar]
- 36.Lionaki S, Blyth ER, Hogan SL, Hu Y, Senior BA, Jennette CE, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64:3452–62. doi: 10.1002/art.34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geffriaud-Ricouard C, Noël LH, Chauveau D, Houhou S, Grünfeld JP, Lesavre P. Clinical spectrum associated with ANCA of defined antigen specificities in 98 selected patients. Clin Nephrol. 1993;39:125–36. [PubMed] [Google Scholar]
- 38.Lamprecht P, Gross WL. Current knowledge on cellular interactions in the WG-granuloma. Clin Exp Rheumatol. 2007;25(Suppl 44):S49–51. [PubMed] [Google Scholar]