Abstract
The GABAB receptor agonist baclofen has been studied extensively in preclinical models of alcohol use disorders, yet results on its efficacy have been uncertain. Racemic baclofen, which is used clinically, can be broken down into separate enantiomers of the drug. Baclofen has been shown to produce enantioselective effects in behavioral assays including those modeling reflexive and sexual behavior. The current studies sought to characterize the enantioselective effects of baclofen in two separate models of ethanol consumption. The first was a Drinking-in-the-Dark procedure that provides “binge-like” ethanol access to mice by restricting access to a two hour period, three hours into the dark cycle. The second was a two-bottle choice procedure that utilized selectively bred High Alcohol Preferring 1 (HAP1) mice to model chronic ethanol access. HAP1 mice are selectively bred to consume pharmacologically relevant amounts of ethanol in a 24-hour two-bottle choice paradigm. The results showed that baclofen yields enantioselective effects on ethanol intake in both models, and that these effects are bidirectional. Total ethanol intake was decreased by R(+)- baclofen, while total intake was increased by S(-)-baclofen in the binge-like and chronic drinking models. Whereas overall binge-like saccharin intake was significantly reduced by R(+)- baclofen, chronic intake was not significantly altered. S(-)- baclofen did not significantly alter saccharin intake. Neither enantiomer significantly affected locomotion during binge-like reinforcer consumption. Collectively, these results demonstrate that baclofen produces enantioselective effects on ethanol consumption. More importantly, the modulation of consumption is bidirectional. The opposing enantioselective effects may explain some of the variance seen in published baclofen literature.
Keywords: rodent model, drinking-in-the-dark, selected line, baclofen, ethanol consumption
Introduction
We have previously demonstrated that systemic administration of the GABAB receptor agonist baclofen increased ethanol drinking in C57BL/6J (B6) mice using the limited-access ethanol drinking procedure Drinking in the Dark (DID) (Moore et al., 2007). However, varied response to baclofen is common in the general ethanol intake and self-administration literature. Whereas baclofen decreases responding and amount of self-administered ethanol in operant paradigms (Besheer, Lepoutre, & Hodge, 2004; Janak & Michael Gill, 2003; Maccioni et al., 2012; Walker & Koob, 2007), reduces breakpoint to obtain ethanol (Maccioni et al., 2008), and decreases ethanol intake (Colombo et al., 2000; Colombo et al., 2003; Stromberg, 2004), baclofen administration has also been shown to increase ethanol intake (Czachowski, Legg, & Stansfield, 2006; Petry, 1997; Smith, Boyle, & Amit, 1999). Thus, although GABAB receptors appear to have a clear role in the modulation of ethanol intake-related behavior, the nature of this role remains uncertain.
A potential explanation for the variance seen in the baclofen literature is the composition of racemic baclofen. The absolute configurations R- and S- baclofen comprise the racemate, with the R(+)- enantiomer being the more potent of the two (Falch et al., 1986; Witczuk, Khaunina, & Kupryszewski, 1980). Experiments examining R- and S- baclofen separately have shown that R-is more effective than S- at moderating sexual behavior, motor movements, locomotion, spinal cord segmental inhibition, reflexes, and blood pressure (Fromm, Shibuya, Nakata, & Terrence, 1990; Olpe et al., 1978; Paredes & Agmo, 1989). The activity ratio of R:S varies between behavioral assays, making it important to consider enantioselective aspects of drug action when considering therapeutic uses of the drug (Jamali, Mehvar, & Pasutto, 1989).
The initial goal of the current studies was to evaluate whether baclofen has enantioselective effects on limited-access ethanol intake in B6 mice using the DID procedure. DID is a binge-like model of ethanol intake that produces pharmacologically relevant levels of consumption in a two-hour period (Rhodes, Best, Belknap, Finn, & Crabbe, 2005; Rhodes et al., 2007). Mice consume considerable amounts of ethanol with blood ethanol concentrations (BECs) exceeding 100 mg/dl. Since its development, DID has been widely used to test a broad range of pharmacological targets for alcohol use disorders (AUDs) (Gupta et al., 2008; Hendrickson, Zhao-Shea, & Tapper, 2009; Kamdar et al., 2007; Moore et al., 2007; Sparta et al., 2008).
Our second goal for the current studies was to explore the pharmacological mechanisms behind chronic ethanol intake by separately assessing if these drugs also worked to modulate two-bottle choice intake in High Alcohol Preferring (HAP) selectively bred mice. HAP mice were selectively bred in three replicates for high intake of 10% ethanol solution in a 24 hour free-choice drinking paradigm (Grahame, Li, & Lumeng, 1999; Oberlin, Best, Matson, Henderson, & Grahame, 2011). Line 1 (HAP1) mice, which are the furthest along in the selection process, were used in the current experiments. These mice represent a model of excessive chronic ethanol intake, displaying ethanol consumption of over 20 g/kg/day. Examination of drinking rhythms in these mice have demonstrated that intake in HAP1 mice occurs at a rate of 1.5 g/kg/hour immediately following lights off and is sustained throughout the 12-hour dark period, with HAP1 mice reaching average BECs over 200 mg/dl eight hours into the dark cycle (Matson & Grahame, 2011). Little testing for pharmacological AUD treatments has been carried out in this model of chronic ethanol intake. O'Tousa et al. (2013) showed that triple monoamine reuptake inhibitors reduced binge-like and chronic drinking, while the GABAA α1 receptor subunit preferring drug 3-propoxy-β-carboline hydrochloride (3-PBC) reduced binge-like drinking in line 2 and 3 HAP mice. The ability of 3-PBC to reduce chronic drinking was not assessed.
We initiated the current studies in B6 and HAP1 mice with two general hypotheses. The first of these was that the R(+)- baclofen enantiomer would reduce total ethanol intake in both paradigms. Based on baclofen effects seen in the first hour of drinking by Moore et al. (2007), we also hypothesized that these effects would be time-dependent. Our second hypothesis was that the S(-)- baclofen enantiomer would not affect ethanol intake in either drinking paradigm, based on its minimal efficacy in other behavioral assays (Paredes & Agmo, 1989).
Method
Animals
For all DID studies, male B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were delivered at 7 weeks of age and were allowed two weeks to acclimate to the reverse light cycle before testing started. For all two-bottle choice studies, HAP1 male and female animals from the 47th, 51st and 53rd generations were obtained from Dr. Nicholas Grahame at IUPUI. All animals were single housed 7 days prior to onset of testing, and between 9 and 10 weeks of age at onset of testing. Lights were maintained on a 12-hr reverse light-dark schedule with lights out at 8, 9, or 11am. Animals had access to food and water at all times, apart from during DID ethanol presentation when water was not available. All procedures were approved by the IUPUI School of Science Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals (The National Academic Press, 2003).
Drugs
Ethanol (195 proof) was obtained from Pharmco, Inc. (Brookefield, CT). Ethanol solutions (10% v/v and 20% v/v) were made using tap water. Saccharin was obtained from Sigma Aldrich (St. Louis, MO) and was dissolved in a 0.32% w/v solution using tap water. R(+)- and S(-)- baclofen were obtained from Sigma Aldrich (St. Louis, MO) and dissolved in 0.9% saline to concentrations of 0, 1, 3, and 10 mg/kg for R(+)- studies and 0, 1, 3, 10, and 30 mg/kg for S(-)- studies. All solutions were delivered via intraperitoneal injections in a volume of 0.1ml per 10 g of body weight.
Method
Drinking-in-the-Dark
Male B6 mice were used in the DID studies. DID was slightly modified from that of Rhodes et al. (2005) and Moore et al. (2007). Briefly, each day 3 hours into the dark cycle, animals were provided daily access to a 20% ethanol or 0.32% saccharin solution for two hours. Ethanol was presented in 10ml graduated plastic tubes. Water was not available during the two hours of ethanol access. Days 1-3 constituted an acquisition phase, with total 2hr fluid intake volumes recorded each day. On Day 4 all animals received a saline injection immediately prior to 2hr ethanol access. On Day 5 animals received a drug injection (drugs and doses described below) immediately prior to 2hr ethanol access. Animals were matched to drug groups based on Day 4 ethanol consumption. Fluid intakes were recorded at the 1hr and 2hr time intervals on Days 4 and 5. Blood samples were taken on Day 5 immediately following 2hr access for determination of BECs (see Retro-orbital sinus blood section).
Two-bottle choice drinking
HAP1 mice were used in the two-bottle choice studies. Both male and female mice were included in these studies due to the limited availability of animals. Mice received two weeks of drinking acquisition which closely followed methods described by Oberlin et al. (2011) and Matson and Grahame (2011). Briefly, 25ml graduated glass cylinders were filled with 10% v/v ethanol or distilled water. One ethanol and one water bottle were placed on each cage at all times during the acquisition phase. Volumes were recorded every other day immediately prior to lights off. Bottles were only removed to replace ethanol or water with fresh fluid when fluid levels were less than 10mL or if the fluid had not been changed within the past four days. To assess drug effects on alternative reinforcers, all studies were also run using identical methodology, but with 0.32% saccharin in place of 10% ethanol.
HAP mice consume low levels of ethanol during the light part of the light/dark cycle but ramp up drinking at lights-off and sustain a high level of drinking throughout the entire dark period (Matson & Grahame, 2011). We therefore chose to inject drug immediately at lights off. On Day 15 all animals received a saline injection to habituate them to the injection procedure. On Day 16 animals received a drug injection (drug and doses described below) immediately prior to lights out. Consumption on Days 15 & 16 was recorded at 1, 2, and 3 hours post-injection. On the test days, ethanol or saccharin was removed one hour prior to injection to assure ethanol clearance and reintroduced immediately following injection using 10mL graduated bottles. This was the only time animals went without ethanol or saccharin. In each experiment, Day 16 drug doses were assigned by matching animals to groups based on total consumption following the Day 15 saline injection. Retro-orbital sinus blood samples were taken from ethanol consuming animals immediately after the end of the drinking period on drug test days for determination of BECs (see Retro-orbital sinus blood section).
R(+)- baclofen DID
Thirty-nine male B6 mice were used in the R(+)- baclofen DID dose-response study. On Day 5 animals received a 0, 1, 3, or 10 mg/kg dose of R(+)- baclofen. These doses were chosen based on previously published literature which found both increases and decreases in drinking at each separate dose (Czachowski et al., 2006; Moore et al., 2007). B6 mice were first tested for baclofen effects on ethanol intake. The same animals were subsequently used to test effects of baclofen on saccharin intake following a two week water-drinking wash-out period.
S(-)- baclofen DID
One hundred thirteen male B6 mice were included in the S(-)- baclofen DID dose-response study. On Day 5 animals received a 0, 1, 3, 10, or 30 mg/kg dose of S(-)- baclofen. As for R(+)-, these doses were chosen based on previous literature (Czachowski et al., 2006; Moore et al., 2007). The higher 30 mg/kg dose was also included because S(-)- baclofen has been shown to be less potent than R(+)- baclofen (Falch et al., 1986; Witczuk et al., 1980). We included the larger dose to capture a broader range of effects on ethanol intake. Sixty-five of these animals were used to test the effects of baclofen on ethanol intake, while the other 50 were used to assess the effects of baclofen on saccharin intake.
Baclofen and free choice drinking
Twenty eight HAP1 mice (12 male, 16 female) were included to assess the effects of R(+)- and S(-)- baclofen on two-bottle choice drinking. Due to limited animal availability, only the doses that were effective in moderating drinking in B6 mice were used so that a similar between-subjects design could be employed. On Day 16 animals received a saline (0 mg/kg), 10 mg/kg S(-)-, or 10 mg/kg R(+)- baclofen dose. Following a one-week water drinking wash-out period, the ability of each baclofen enantiomer to modulate saccharin intake was tested in the same animals. Mice did not necessarily receive the same dose of baclofen twice, as dose groups were determined by matching intake following the Day 15 saline injection.
Locomotor activity
Forty-six B6 males were included in a study to characterize the locomotor effects of R(+)- and S(-)- baclofen during access to 20% ethanol or 0.32% saccharin during DID. However, these animals were not naïve. The 23 animals that received ethanol had previous 7 day DID experience with 2% sucrose, but were ethanol naïve. The 23 animals that received saccharin had previous 7 day DID experience with 20% ethanol, but were saccharin naïve. All animals had received one dose of the adenosine A2A antagonist MSX-3 or the A1 antagonist DPCPX three or more weeks before locomotor testing. Animals were matched to drug treatment group based on baseline consumption, previous drug exposure, and previous dose exposure. The same 5 day DID paradigm as described in the DID method section was used. On Day 5, animals received a 10 mg/kg dose of R(+)- or S(-)- baclofen. The control groups received a saline injection of equal fluid volume titrated to their body weight. The 10 mg/kg doses of R(+)- and S(-)- baclofen were chosen because they were known to have an effect on ethanol consumption. To reduce animal usage, only the doses shown to have an effect on drinking were used to characterize locomotor activity.
Locomotor activity was recorded using Opto M3 13“x9” Mouse Cages (Columbus Instruments, Columbus, OH). The animal's cage is placed directly into the monitor and locomotor scores determined based on sensor beam breaks. Each cage monitor was started immediately following introduction of ethanol or saccharin each day. Locomotor scores were collected in 5 minute bins during the two hours of reinforcer access.
Retro-orbital sinus blood sampling
Retro-orbital sinus blood samples were collected using 50 μl microcapillary tubes (Fisher Science, Pittsburgh, PA) immediately after the final ethanol drinking session for each experiment. Samples were centrifuged and plasma was decanted and stored at −80 °C until the time of BEC determination. An Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA) was used to determine blood ethanol content.
Statistical Analysis
Data were analyzed using IBM SPSS version 20 (Armonk, NY). For all studies, acquisition data were analyzed using a repeated measures ANOVA with day as the within-subjects variable. Tukey's post-hoc tests were used when appropriate for the acquisition data. For analysis of baclofen's effects on DID drinking, Day 5 total intake, 1hr intake, 2hr intake, and BEC data were analyzed by one-way ANOVAs with dose as the between-subjects factor. One way ANOVAs at each hour were used in favor of a Time*Dose repeated measures ANOVA to match the 1 hour analysis used by Moore et al. (2007). Dunnett's post-hoc tests were used when appropriate with the 0 mg/kg group set as the control.
Locomotor activity was analyzed using a repeated measures Day*Compound*Time Bin ANOVA with compound as the between-subjects factor and day (saline versus drug) and five minute time bins as the within-subjects factors.
Analyses of the two-bottle choice data included the 0 mg/kg control, 10 mg/kg R(+)-, and 10 mg/kg S(-)- baclofen doses. Sex was initially included as a factor in all analyses; however the omnibus ANOVA indicated no main or interactive effects of sex on intake. Therefore data were collapsed on this factor. An omnibus Time*Dose repeated measures ANOVA with time as the within-subjects factor and dose as a between subjects factor was used to analyze ethanol and saccharin intake, as well as respective water intake. Ethanol and saccharin preference were analyzed using a one-way ANOVA with Dose as the between groups factor. Preference was calculated by dividing the amount of ethanol or saccharin consumed by total fluid consumed. BECs for the ethanol animals were compared using a one-way ANOVA with dose as the between subjects factor. Dunnett's post-hoc tests were used when appropriate with 0 mg/kg set as the control. For DID and two-bottle choice tests, bivariate correlations were run between total test day intake and BECs for ethanol experiments. Significance was set at p < .05 for all overall tests and was corrected for all necessary post-hoc tests.
Results
DID in B6 mice and R(+)- baclofen: Ethanol
Acquisition of ethanol intake is shown in Fig. 1A. The analysis revealed that intake was stable across days 1-4; F(3,158) = 1.03, p > .05. A significant effect of R(+)-dose on Day 5 total intake was revealed; F(3,38) = 4.546, p < .01, where the 10 mg/kg dose of R(+)- baclofen reduced ethanol intake compared to the 0 mg/kg group (p < .05) (Fig. 1B). ANOVAs analyzing the individual hourly intake revealed that this effect was specific to the first hour of intake; F(3,38) = 3.855, p < .05. There was no significant effect of dose in the second hour of intake (p > .05) (Fig. 1C). R(+)- baclofen also had a significant effect on BECs; F(3,38) = 3.870, p < .05. The 10 mg/kg dose of baclofen significantly reduced BECs compared to the 0 mg/kg group (p < .05) (Fig. 1D). BECs were strongly correlated with total 2 hour ethanol intake; r(39) = .922, p < .001 (data not shown).
Figure 1. R(+)- baclofen reduces binge-like reinforcer intake.
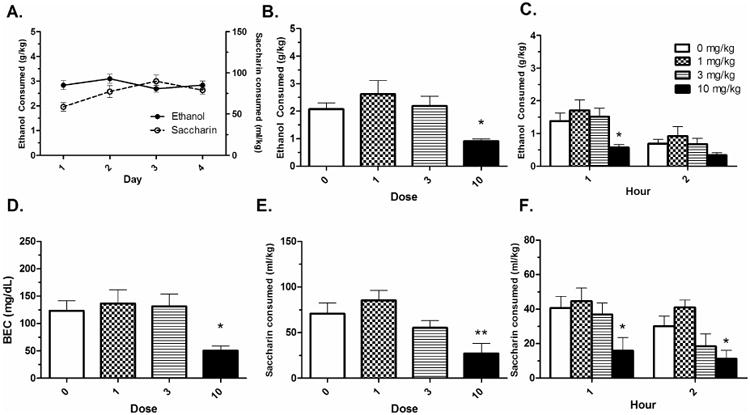
A) Acquisition of ethanol and saccharin intake. B) The R(+)- baclofen enantiomer reduced total binge-like ethanol intake in B6 mice at the 10 mg/kg dose. C) The effect on intake was specific to the first hour of consumption. D) The 10 mg/kg R(+)- dose also reduced BECs. E) Total binge-like saccharin intake was also reduced at the 10 mg/kg R(+)- baclofen dose. F) This result was significant at both hours. Means ± SEM are depicted. Asterisk (*) indicates different than 0 mg/kg at p < .05. Double asterisk (**) indicates different than 0 mg/kg at p < .01. Group n's = 9-10.
DID in B6 mice and R(+)- baclofen: Saccharin
Acquisition of saccharin intake is shown in Fig. 1A. There was a significant main effect of day; F(3,158) = 11.93, p < .05, with consumption on Day 3 being higher than consumption on Day 1. There was a significant effect of dose on Day 5 total intake; F(3,38) = 5.51, p < .01, with the 10 mg/kg dose reducing total saccharin intake compared to the 0 mg/kg group (Fig. 1E). The dose effect was not specific to hour; ANOVAs analyzing consumption at each hour revealed significant dose effects on intake (p's < .05). Dunnett's post-hoc tests revealed that the 10 mg/kg dose reduced intake at both hours (p's < .05) (Fig. 1F).
DID in B6 mice and S(-)- baclofen: Ethanol
Acquisition of ethanol intake is shown in Fig. 2A. There was a main effect of day; F(3,186) = 3.90, p < .05, with consumption being lower on Day 4 than on Days 2 and 3. A significant effect of S(-)- baclofen dose on Day 5 total intake was revealed; F(4,62) = 2.54, p < .05. Dunnett's post-hoc tests revealed that the 10 mg/kg dose increased drinking compared to the 0 mg/kg group (p < .05) (Fig. 2B). ANOVAs analyzing the hourly intake revealed that this effect was specific to the second hour of intake; F(4,62) = 7.029, p < .001. Dunnett's post-hoc tests revealed that both the 3 and 10 mg/kg doses increased drinking in the second hour compared to the 0 mg/kg group (p's < .05) (Fig. 2C). There was no significant effect of dose on the first hour of intake (p > .05). A one-way ANOVA revealed no dose effect on BEC; F(4,39) < 1, p > .05 (Fig. 2D). There was a strong, significant correlation between total intake and BEC; r(63) = 0.763 (data not shown).
Figure 2. S(-)- baclofen increases binge-like ethanol intake.
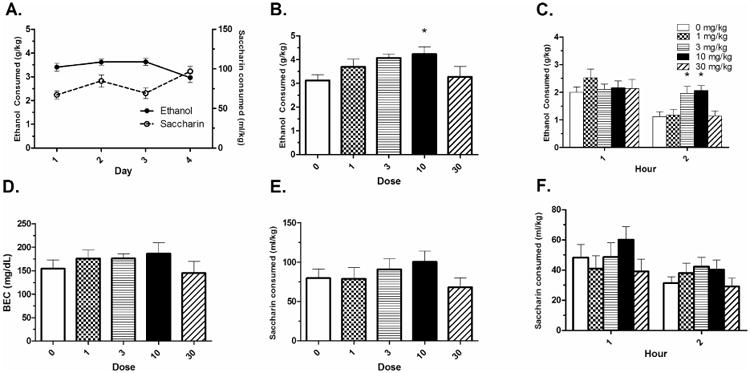
A) Acquisition of ethanol and saccharin intake. B) The S(-)- baclofen enantiomer increased total binge-like ethanol intake in B6 mice at the 10 mg/kg dose. C) The effect on intake was specific to the second hour of consumption, with the 3 mg/kg and 10 mg/kg dose increasing intake. D) Dose effects on intakes were not reflected in BECs. E) S(-)- baclofen did not alter total saccharin intake. F) S(-)-baclofen did not alter hourly saccharin intake. Mean ± SEM is depicted. Asterisk (*) indicates different than 0 mg/kg at p < .05. Group n's for panels A-C = 12-13. Group n's for panel D and E = 10.
DID in B6 mice and S(-)- baclofen: Saccharin
Acquisition of saccharin consumption is shown in Fig. 2A. There was a main effect of day; F(3,199) = 9.52, p < .05, with consumption being higher on Day 4 than on Days 1 and 3. There were no significant dose effects of S(-)- baclofen on total saccharin intake; F(4,49) = .916, p > .05 (Fig. 2E). Further, there were no significant dose effects observed during either hourly reading (p's > .05) (Fig. 2F).
Two-bottle choice in HAP1 mice and R(+)- and S(-)- baclofen: Ethanol
During acquisition, HAP1 mice reached levels of drinking previously seen by Matson & Grahame (2011) (Fig. 3A). There was a main effect of day; F(13,312) = 3.52, p < .01, and a linear contrast pattern with intake increasing over days (p < .01). Sex was initially included as a factor, but was found to be insignificant in all analyses and was therefore removed from all subsequent analyses. Day 16 dose groups were matched for total 3-hour consumption on Day 15. Mean ± Standard Error (SEM) ethanol consumption for each group on Day 15 was 5.90 ± 0.19 g/kg, 5.67 ± 0.36 g/kg and 5.04 ± 0.44 g/kg for the control, S(-)-, and R(+)- baclofen groups, respectively. For Day 16 drinking, the omnibus Dose*Time ANOVA revealed a main effect of both dose and time (p's < .05). Dunnett's post hoc test revealed that total ethanol intake was reduced in the R(+)- 10 mg/kg group, but increased in the S(-)- 10 mg/kg group compared to the 0 mg/kg group (p's < .05) (Fig. 3B). Within-subjects contrast effects showed both linear and quadratic effects of time (p's < .05), with consumption at hours 2 and 3 being lower than hour 1, and the lowest consumption occurring during hour 2 (Fig. 3C). There was no overall interaction of Time*Dose; F(4,48) = 2.16, p = .087. However, one-way ANOVAs were run at each time-point to match the analyses used for the binge-like intake. There was a significant effect of dose at each time point, with S(-)- baclofen increasing intake at hour 3 (p < .05) and trending towards increasing intake at hour 1 (p = .056) compared to the control group. R(+)- baclofen reduced ethanol intake at hours 2 and 3 compared to the control groups (p's < .05). A one-way ANOVA revealed a significant effect of dose on BEC (p < .05). Dunnett's post hoc tests revealed that the R(+)- 10 mg/kg dose significantly reduced BEC compared to the 0 mg/kg dose (p < .05), whereas the S(-)- 10 mg/kg dose trended towards increasing BEC compared to the 0 mg/kg dose (p = .057) (Fig. 3D). Total ethanol intake and BEC were strongly and significantly correlated; r(27) = 0.985, p < .01 (data not shown).
Figure 3. The separate baclofen enantiomers bidirectionally modulated free-choice ethanol intake in HAP1 mice.
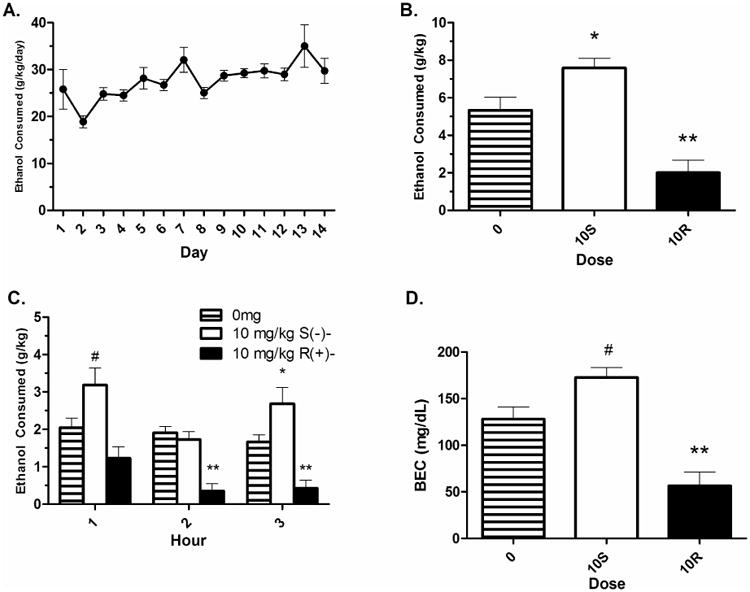
A) Acquisition of 24-hour access daily ethanol intake. B) Total 3 hour intake on the drug day indicates that R(+)- baclofen reduced ethanol intake whereas S(-)-baclofen increased drug intake compared to a saline injection. C) One-way ANOVAs at each of the three testing hours revealed dose effects at each hour of consumption. D) BEC following ethanol intake was reduced in the R(+)- baclofen group and trended towards an increase in the S(-)- baclofen group compared to control. Mean ± SEM is depicted. Asterisk (*) indicates different than 0 mg/kg at p < .05. Double asterisk (**) indicates different than 0 mg/kg p < .01. Pound sign (#) indicates a trend of p < .06 compared to 0 mg/kg. Group n's = 9-10.
The effects of R(+)- and S(-)- baclofen on water intake and ethanol preference were also examined. The Dose*Time ANOVA analyzing water consumption revealed no main effects or interaction of dose and time (p's > .05). Mean ± SEM water intakes for the groups were 0 mg/kg = 5.35 ± 2.66 ml, S(-)- 10 mg/kg = 3.60 ± 0.70 ml, R(+)- 10 mg/kg = 1.27 ± 0.56 ml. The one way ANOVA analyzing ethanol preference on the test day revealed a strong trend towards a dose effect (p = .05). However, Dunnett's post hoc tests revealed that neither drug dose was different from the 0 mg/kg control (p's > .05). The mean ± SEM ethanol preferences for each dose group were 0 mg/kg = 93.10 ± 4.18%, S(-)- 10 mg/kg = 96.94 ± 0.62%, and R(+)- 10 mg/kg = 84.92 ± 3.59%.
Two-bottle choice in HAP1 mice and R(+)- and S(-)- baclofen: Saccharin
HAP1 mice reached levels of saccharin consumption previously reported by Oberlin et al. (2011) during the acquisition phase (Fig. 4A). There was no effect of day during the acquisition phase; F(13, 351) = 1.55, p > .05. Sex was initially included as a factor, but was found to be insignificant in all analyses and was therefore removed. Total mean ± SEM saccharin intake on Day 15 was 146.49 ± 35.35 ml/kg, 155.18 ± 24.71 ml/kg, and 130. 14 ± 28.74 ml/kg for the control, S(-)-, and R(+)- baclofen groups, respectively. On Day 16, the omnibus Dose*Time ANOVA showed a main effect of dose on consumption; F(2,25) = 6.02, p < .05. However, Dunnett's post hoc test revealed that neither drug dose was significantly different from the 0 mg/kg control group (Fig. 4B). The main effect of time did not reach significance; F(2,50) = 2.92, p = .063. There was no interaction of time*dose; F(4,50) = 1.70, p > .05 (Fig. 4C). One way ANOVAs at each time point showed a significant effect of dose at hours 1 and 2 (p's < .05). However, neither R(+)- or S(-)- baclofen were significantly different from the control group at those time-points.
Figure 4. The separate baclofen enantiomers did not significantly modulate free-choice saccharin intake in HAP1 mice.
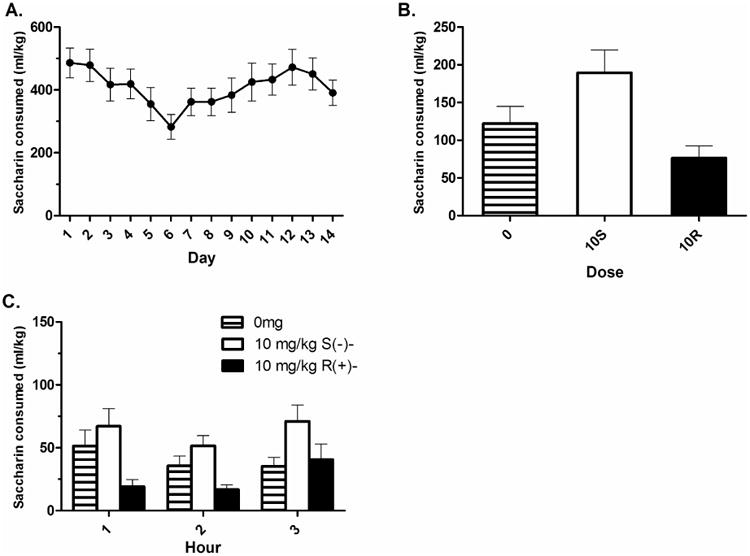
A) Acquisition of 24-hour access daily saccharin intake. B) Total 3 hour intake following the drug injection. Neither drug group was significantly different from the control group. C) Hourly intake for each of the three testing hours following the drug injection showed no significant effects. Mean ± SEM is depicted. Group n's = 9-10.
The effects of R(+)- and S(-)- baclofen on water consumption and saccharin preference were also examined. An omnibus Dose*Time ANOVA revealed no significant effects or interaction of dose and time on water intake (p's > .05). Total mean ± SEM water intakes for each group were 0 mg/kg = 12.74 ± 4.47 ml, S(-)- 10 mg/kg = 22.0 ± 12.54 ml, R(+)- 10 mg/kg = 6.84 ± 4.22 ml. A one-way ANOVA analyzing the effect of dose on saccharin preference revealed no significant effects (p > .05). Mean ± SEM saccharin preference for each group were 0 mg/kg = 91.11 ± 3.0%, S(-)- 10 mg/kg = 88.32 ± 6.25%, R(+)- 10 mg/kg = 90.55 ± 3.84%.
Home-cage locomotor activity during DID
Acquisition of ethanol intake was stable across days (p > .05). Total mean ± SEM intakes for Days 1-4 were 2.33 ± 0.30, 2.65 ± 1.22, 2.40 ± 0.35, and 2.71 ± 0.32 g/kg, respectively. The effect of R(+)- and S(-)- baclofen on 2hr ethanol intake is shown in Table 1. There was a significant effect of baclofen compound on Day 5 total 2 hour ethanol intake; F(2,22) = 11.67, p < .01. Dunnett's post-hoc tests revealed that total 2 hour drinking was lower in the group that received R(+)- baclofen compared to the saline group. There was a significant effect of compound for each hour of drinking independently (p's < .05). Dunnett's post-hoc tests revealed that at hour 1, neither baclofen group was significantly different from the saline group. At hour 2, the S(-)- baclofen group consumed more than the saline group (p < .05).
Table 1a.
| Ethanol consumed (g/kg) | Saccharin consumed (ml/kg) | |||||
|---|---|---|---|---|---|---|
| Dose | Hour 1 | Hour 2 | Total | Hour 1 | Hour 2 | Total |
| Saline | 1.63 ± 0.32 | 0.94 ± 0.20 | 2.57 ± 0.36 | 47.04 ± 4.16 | 25.98 ± 7.35 | 73.02 ± 10.43 |
| 10 mg/kg S(-)- | 1.97 ± 0.36 | 1.75 ± 0.23* | 3.71 ± 0.48 | 32.14 ± 5.72 | 22.32 ± 7.43 | 54.48 ± 8.08 |
| 10 mg/kg R(+)- | 0.69 ± 0.08 | 0.44 ± 0.08 | 1.13 ± 0.13* | 22.91 ± 5.64* | 14.43 ± 4.71 | 37.34 ± 8.45 |
Depicted are the mean ± SEM of ethanol and saccharin intake following drug injection during the locomotor activity experiment. These data accompany the locomotor activity shown in Figure 5. Asterisk (*) indicates that a value is different from the saline group for the same time period.
Acquisition of saccharin intake was stable across days (p > .05). Total mean ± SEM intakes for Days 1-4 were 70.28 ± 7.34, 82.48 ± 12.31, 74.97 ± 9.10, and 75.45 ± 8.43 ml/kg, respectively. The effect of R(+)- and S(-)- baclofen on 2hr saccharin intake is shown in Table 1. There was a strong trend towards an effect of baclofen on total 2 hour intake; F(2,22) = 3.42, p = .053. This effect was driven by decreased total drinking in the R(+)- group. There was also an effect of baclofen on the first hour of drinking; F(2,22) = 4.88, p < .05, with R(+)- baclofen reducing drinking compared to saline. There were no significant main effects of baclofen compound on intake in hour 2 (p < .05).
The corresponding locomotor activity data are shown in Figure 5. A repeated measures Day*Compound*Time Bin ANOVA analyzing the locomotor scores of animals that received access to ethanol revealed a significant main effect of Day, with total locomotion being lower on day 5 (the drug day) (p < .05). There was also a main effect of Time, but no other significant main effects or interactions (Figs. 5A, 5B). A repeated measures Day*Compound*Time Bin ANOVA revealed the same pattern of results for the animals that received access to saccharin. There was only a main effect of Day, with locomotion being lower on the drug day, and a main effect of Time Bin (Figs. 5C, 5D).
Figure 5. The separate baclofen enantiomers did not alter home-cage locomotion in B6 mice.
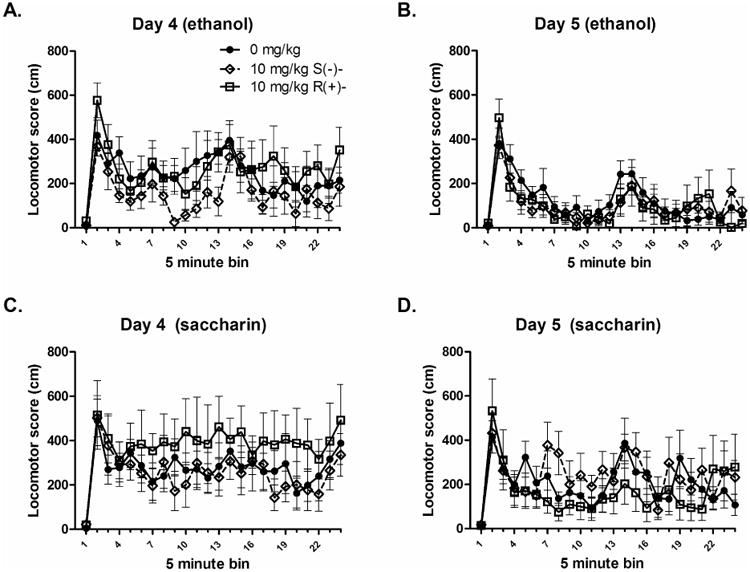
A) Home-cage locomotor scores of ethanol drinking animals on Day 4 following a saline injection. B) Home-cage locomotor scores of ethanol drinking animals on Day 5 following drug injection. C) Home-cage locomotor scores of saccharin drinking animals on Day 4 following saline injection. D) Home-cage locomotor scores of saccharin drinking animals on Day 5 following drug injections. For both reinforcers, there was a main effect of day and time. Locomotion was lower in both groups on Day 5 (panels B and D). Mean ± SEM is depicted. Group n's = 7-8.
Discussion
The current studies highlight the enantioselective effects of baclofen in a binge-like and chronic model of ethanol drinking. In both models, a 10 mg/kg dose of R(+)- baclofen reduced total ethanol intake, whereas a 10 mg/kg dose of S(-)- baclofen increased total ethanol intake. R(+)- baclofen significantly reduced binge-like saccharin intake in the DID model, but not saccharin intake in the free-choice model. As seen in Figure 4, the 10 mg/kg R(+)- baclofen dose appears to reduce saccharin consumption compared to the control group in the first and second hour of drinking, however there were no significant differences on total or hourly drinking between groups. S(-)- baclofen did not significantly alter saccharin intake in either test. Furthermore, while high levels of drinking and pharmacologically relevant BECs have been reported in HAP mice (Matson & Grahame, 2011; Oberlin et al., 2011), this study demonstrates that BECs in HAP mice correlate strongly with ethanol intake, and that corresponding intake is amenable to pharmacological intervention. The current study also elucidates some of the neurobiological underpinnings of drinking in HAP mice by confirming that chronic ethanol consumption in HAP1 mice can be modulated via administration of baclofen.
Although only male B6 mice were used in the DID studies, both male and female HAP1 mice were used in the two-bottle choice studies. Sex was initially included as a factor in all statistical analyses for the HAP1 studies, but it was not found to be a significant variable in any analysis and was therefore removed. However, sex is known to play an important role in ethanol consumption. Female HAP mice have been previously shown to consume more ethanol than their male counterparts during 24-hour free-choice drinking sessions (Matson & Grahame, 2011), and estrous cycle is known to modify the amount of ethanol consumed, the pattern of consumption, and the pharmacodynamics of ethanol (Ford, Eldridge, & Samson, 2002; Robinson, Brunner, & Gonzales, 2002). Although estrous status affects ethanol consumption, the opposite is also true. Female rats exposed to 12 days of 4 or 8 g/kg of intragastric ethanol entered a stage of disrupted estrous cycling, spending more time in diestrous (Eskay, Ryback, Goldman, & Majchrowicz, 1981). This same disruption has been observed in rats fed a liquid ethanol diet for an extended period of time (Emanuele, LaPaglia, Steiner, Kirsteins, & Emanuele, 2001; Sanchis, Esquifino, & Guerri, 1985). As female mice in the current study received 16 days total of ethanol access and consistently consumed over 20 g/kg of ethanol per day, it is likely that many of them entered a period of disrupted estrous cycling. As many of the female mice were likely in the diestrous phase at the time of drug testing, variability in the female drinking due to estrous status would have been greatly reduced. However, we cannot directly speak to estrous status or how it affected consumption in female mice, as estrous stage was not monitored in the present study.
In the B6 mice, effects on ethanol consumption were time-dependent. R(+)- baclofen reduced consumption in the first hour of access, whereas S(-)- baclofen increased consumption in the second hour of access. This late increase in consumption may explain why S(-)- baclofen failed to alter BECs following binge-like ethanol access. Our lab has previously demonstrated that S(-)- baclofen increases ethanol consumption in the second hour of drinking, but does not increase BECs following site-specific administration into the nucleus accumbens shell (Kasten & Boehm, 2014). As ethanol consumption readings were taken after a fairly large one hour intake window, it is possible that animals consumed most of that ethanol at the end of the drinking session and that it had not been fully absorbed at the point of BEC determination, which was two hours after bottles on. The differences observed following administration of S(-)- baclofen are likely not the result of leak, as bottles were tested daily for leak. Moreover, we employ the use of empty cages with bottles on them for the sole purpose of controlling for random leak. Animals were caged on the same rack within the animal room, meaning any existing leak should have occurred at a similar rate in all cages. Although there was a trend towards a significant increase in BEC in the HAP1 mice following an injection of 10 mg/kg S(-)- baclofen it did not reach significance. The HAP1 mice showed a biphasic pattern in drinking following S(-)- baclofen administration, with a trend towards an increase in the first hour of consumption and a significant increase in the 3rd hour of consumption. This late increase in drinking without significantly increased in BECs also supports the BEC data for the B6 animals.
R(+)- and S(-)- baclofen have been shown to alter a multitude of behaviors and physiological constructs, including sexual, motor and locomotor, reflexes, and spinal cord and segmental inhibition (Fromm et al., 1990; Olpe et al., 1978; Paredes & Agmo, 1989). All of these measures have shown relative resistance to the S-enantiomer. Conversely, our experiments demonstrate that S(-)- baclofen is not only significantly active in altering binge-like and free-choice ethanol intake, but that it does so in a manner opposite that of the R(+)-enantiomer. These results may explain some of the variance that has been observed in the baclofen literature on intake and self-administration using the racemic mixture.
A possible source of the differing actions of the two baclofen enantiomers may relate to where and how they bind in the brain. Binding of baclofen is dependent on the absolute configuration and the molecular rotation of the molecule. The absolute configurations of d- and l-baclofen (S- and R-, respectively), are equally efficacious at displacing GABA in low- and high-affinity membranes, but are much less efficacious at the high-affinity sites (Waddington & Cross, 1980). However, the (-) molecular rotation and GABA are equally able to displace racemic baclofen in low-affinity membranes, whereas the (+) molecular rotation displaces racemic baclofen to a much lesser extent (Bowery, Hill, & Hudson, 1983). Both (-)baclofen and R-baclofen have been shown to bind selectively in the cerebellum and hippocampus, respectively, with (-)baclofen binding to (-) selective sites (Drew, Johnston, & Weatherby, 1984; Haas, Greene, & Olpe, 1985). Our lab has begun work aimed at determining whether the enantiomers of baclofen are working in a brain region-specific manner. For example, we demonstrated that R(+)- and S(-)- baclofen bidirectionally alters ethanol, but not saccharin, intake following direct injection into the nucleus accumbens shell (Kasten & Boehm, 2014). Other brain regions of interest would include those involved in reinforcement, such as the nucleus accumbens core, ventral pallidum, ventral tegmental area, and prefrontal cortex. There may also be differences with how these molecules interact with other neurotransmitters. The (-) baclofen isomer interacts with norepinephrine whereas R-baclofen may be responsible for other non-GABAergic effects of baclofen (Karbon, Duman, & Enna, 1984; Waddington & Cross, 1980). Individual differences in populations of GABAB receptors may increase or decrease the efficacy of racemic baclofen in reducing ethanol intake in preclinical models and in treating AUDs clinically.
One concern that surrounds interpretation of baclofen studies is how the drug affects locomotion. Cryan et al. (2004) have demonstrated that open-field locomotor activity is reduced in ethanol naïve animals following one hour pretreatment with a 10 mg/kg dose of R- baclofen. To characterize how the potential sedative effects of baclofen may be interfering with the ability to consume the reinforcer, we used a home-cage locomotor activity apparatus to quantify activity during drinking. Our results provide strong evidence that sedative effects of baclofen are not responsible for the changes in drinking observed. Locomotor activity was not altered by the 10 mg/kg doses of R(+)- or S(-)- baclofen, the same doses that decrease or increase drinking, respectively. The accompanying consumption data showed the same general pattern of intake as observed in the drinking-only studies; whereas R(+)- baclofen reduced total ethanol and hour 1 saccharin intake, S(-)- baclofen increased ethanol intake in the second hour but did not significantly increase total intake. A secondary concern includes potential effects of the compounds on water consumption. While there were no significant drug effects on water consumption in the free-choice drinking studies, it is important to consider that water intake was highly variable. Although no outliers were identified, some of the animals consumed much more water than others. This range in consumption contributed to high variability which may have decreased the ability to identify an existing drug effect on water intake.
In conclusion, these studies demonstrate differential stereospecific actions of baclofen on ethanol consumption in models of excessive ethanol consumption. These studies are the first to establish that baclofen acts in a stereospecific and bidirectional manner on ethanol consumption, and that the reductions in drinking are not due to sedative effects. The effects of S(-)- baclofen are important considering the variable response observed using racemic baclofen clinically and pre-clinically. Susceptibility to one enantiomer over another in clinical and pre-clinical populations may influence response to the drug and drug effectiveness, also potentially explaining differences in the previous literature. Overall, these results show that GABAB receptors play a role in moderating excessive ethanol consumption, that modulation of ethanol consumption is stereospecific, and that GABAB receptors continue to be a viable target for development of novel therapeutic treatments for AUDs.
R(+)- baclofen reduces ethanol intake.
S(-)- baclofen increases ethanol intake.
Pharmacological characterization of High Alcohol Preferring Line 1 mice.
Acknowledgments
The authors would like to thank Dr. Nicholas Grahame for his technical assistance and advice and Ashley Frazee for contributing to data acquisition. This work was supported by IUPUI School of Science, NIAAA grant #AA016789 to Stephen L. Boehm II, and the Indiana Alcohol Research Center (AA007611).
Funding Support: NIAAA grant #AA016789 and the Indiana Alcohol Research Center (AA007611).
Footnotes
Author Contributions: CK and SLB were responsible for the study concept, design, data analysis, and interpretation of the findings. CK, SNB, and SLB contributed to interpretation of the findings and critical review and approval of the final version for publication. CK and SNB contributed to acquisition of animal data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Besheer J, Lepoutre V, Hodge C. GABAB receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6J mice. Psychopharmacology. 2004;174(3):358–366. doi: 10.1007/s00213-003-1769-3. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hill DR, Hudson AL. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. British Journal of Pharmacology. 1983;78(1):191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MAM, Lobina C, Pani M, Reali R, et al. Ability of Baclofen in Reducing Alcohol Intake and Withdrawal Severity: I—Preclinical Evidence. Alcoholism: Clinical and Experimental Research. 2000;24(1):58–66. [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Brunetti G, Carai MM, Gessa G. Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology. 2003;167(3):221–224. doi: 10.1007/s00213-003-1397-y. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, et al. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N, N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4, 6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. Journal of Pharmacology and Experimental Therapeutics. 2004;310(3):952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Stansfield KH. Ethanol and Sucrose Seeking and Consumption Following Repeated Administration of the GABAB Agonist Baclofen in Rats. Alcoholism: Clinical and Experimental Research. 2006;30(5):812–818. doi: 10.1111/j.1530-0277.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Drew CA, Johnston GAR, Weatherby RP. Bicuculline-insensitive GABA receptors: Studies on the binding of (−)-baclofen to rat cerebellar membranes. Neuroscience Letters. 1984;52(3):317–321. doi: 10.1016/0304-3940(84)90181-2. [DOI] [PubMed] [Google Scholar]
- Emanuele NV, LaPaglia N, Steiner J, Kirsteins L, Emanuele MA. Effect of Chronic Ethanol Exposure on Female Rat Reproductive Cyclicity and Hormone Secretion. Alcoholism: Clinical and Experimental Research. 2001;25(7):1025–1029. [PubMed] [Google Scholar]
- Eskay RL, Ryback RS, Goldman M, Majchrowicz E. Effect of Chronic Ethanol Administration on Plasma Levels of LH and the Estrous Cycle in the Female Rat. Alcoholism: Clinical and Experimental Research. 1981;5(2):204–206. doi: 10.1111/j.1530-0277.1981.tb04889.x. [DOI] [PubMed] [Google Scholar]
- Falch E, Hedegaard A, Nielsen L, Jensen BR, Hjeds H, Krogsgaard-Larsen P. Comparative Stereostructure-Activity Studies on GABAA and GABAB Receptor Sites and GABA Uptake Using Rat Brain Membrane Preparations. Journal of Neurochemistry. 1986;47(3):898–903. doi: 10.1111/j.1471-4159.1986.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Microanalysis of Ethanol Self-Administration: Estrous Cycle Phase-Related Changes in Consumption Patterns. Alcoholism: Clinical and Experimental Research. 2002;26(5):635–643. [PubMed] [Google Scholar]
- Fromm GH, Shibuya T, Nakata M, Terrence CF. Effects of d-baclofen and l-baclofen on the trigeminal nucleus. Neuropharmacology. 1990;29(3):249–254. doi: 10.1016/0028-3908(90)90009-g. [DOI] [PubMed] [Google Scholar]
- Grahame N, Li TK, Lumeng L. Selective Breeding for High and Low Alcohol Preference in Mice. Behavior Genetics. 1999;29(1):47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, et al. Acute Effects of Acamprosate and MPEP on Ethanol Drinking-in-the-Dark in Male C57BL/6J Mice. Alcoholism: Clinical and Experimental Research. 2008;32(11):1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Haas HL, Greene RW, Olpe HR. Stereoselectivity of l-baclofen in hippocampal slices of the rat. Neuroscience Letters. 1985;55(1):1–4. doi: 10.1016/0304-3940(85)90302-7. [DOI] [PubMed] [Google Scholar]
- Hendrickson L, Zhao-Shea R, Tapper A. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology. 2009;204(4):563–572. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali F, Mehvar R, Pasutto FM. Enantioselective aspects of drug action and disposition: Therapeutic pitfalls. Journal of Pharmaceutical Sciences. 1989;78(9):695–715. doi: 10.1002/jps.2600780902. [DOI] [PubMed] [Google Scholar]
- Janak PH, Michael Gill T. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30(1):1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of Naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology. 2007;192(2):207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Karbon EW, Duman RS, Enna SJ. GABAB receptors and norepinephrine-stimulated cAMP production in rat brain cortex. Brain Research. 1984;306(1–2):327–332. doi: 10.1016/0006-8993(84)90382-2. [DOI] [PubMed] [Google Scholar]
- Kasten CR, Boehm SLI. Intra-nucleus accumbens shell injections of R(+)- and S(-)- baclofen bidirectionally alter binge-like ethanol, but not saccharin, intake in C57Bl/6J mice. Behav Brain Res. 2014 doi: 10.1016/j.bbr.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni P, Fantini N, Froestl W, Carai MAM, Gessa GL, Colombo G. Specific Reduction of Alcohol's Motivational Properties by the Positive Allosteric Modulator of the GABAB Receptor, GS39783—Comparison With the Effect of the GABAB Receptor Direct Agonist, Baclofen. Alcoholism: Clinical and Experimental Research. 2008;32(9):1558–1564. doi: 10.1111/j.1530-0277.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Zaru A, Loi B, Lobina C, Carai MAM, Gessa GL, et al. Comparison of the Effect of the GABAB Receptor Agonist, Baclofen, and the Positive Allosteric Modulator of the GABAB Receptor, GS39783, on Alcohol Self-Administration in 3 Different Lines of Alcohol-Preferring Rats. Alcoholism: Clinical and Experimental Research. 2012;36(10):1748–1766. doi: 10.1111/j.1530-0277.2012.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addiction Biology. 2011;18(6):921–929. doi: 10.1111/j.1369-1600.2011.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., II GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacology Biochemistry and Behavior. 2007;88(1):105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tousa DS, Warnock KT, Matson LM, Namjoshi OA, Linn MV, Tiruveedhula VV, et al. Triple monoamine uptake inhibitors demonstrate a pharmacologic association between excessive drinking and impulsivity in high-alcohol-preferring (HAP) mice. Addiction Biology. 2013:n/a–n/a. doi: 10.1111/adb.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, Grahame N. Derivation and Characterization of Replicate High- and Low-Alcohol Preferring Lines of Mice and a High-Drinking Crossed HAP Line. Behavior Genetics. 2011;41(2):288–302. doi: 10.1007/s10519-010-9394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpe HR, Demiéville H, Baltzer V, Bencze WL, Koella WP, Wolf P, et al. The biological activity of d-baclofen (Lipresal®) European Journal of Pharmacology. 1978;52(1):133–136. doi: 10.1016/0014-2999(78)90032-8. [DOI] [PubMed] [Google Scholar]
- Paredes R, Agmo A. Stereospecific actions of baclofen on sociosexual behavior, locomotor activity and motor execution. Psychopharmacology. 1989;97(3):358–364. doi: 10.1007/BF00439451. [DOI] [PubMed] [Google Scholar]
- Petry NM. Benzodiazepine-GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Experimental and Clinical Psychopharmacology. 1997;5(3):183–194. [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes, Brain and Behavior. 2007;6(1):1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Brunner LJ, Gonzales RA. Effect of Gender and Estrous Cycle on the Pharmacokinetics of Ethanol in the Rat Brain. Alcoholism: Clinical and Experimental Research. 2002;26(2):165–172. [PubMed] [Google Scholar]
- Sanchis R, Esquifino A, Guerri C. Chronic ethanol intake modifies estrous cyclicity and alters prolactin and LH levels. Pharmacology Biochemistry and Behavior. 1985;23(2):221–224. doi: 10.1016/0091-3057(85)90560-x. [DOI] [PubMed] [Google Scholar]
- Smith BR, Boyle AEL, Amit Z. The Effects of the GABAB Agonist Baclofen on the Temporal and Structural Characteristics of Ethanol Intake. Alcohol. 1999;17(3):231–240. doi: 10.1016/s0741-8329(98)00053-6. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the Corticotropin Releasing Factor Type 1 Receptor Attenuates Elevated Ethanol Drinking Associated With Drinking in the Dark Procedures. Alcoholism: Clinical and Experimental Research. 2008;32(2):259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg MF. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacology Biochemistry and Behavior. 2004;78(4):743–750. doi: 10.1016/j.pbb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Cross AJ. Gaba-ergic properties of baclofen in vivo and in vitro. Brain Research Bulletin. 1980;5(Supplement 2)(0):503–505. [Google Scholar]
- Walker BM, Koob GF. The γ-Aminobutyric Acid-B Receptor Agonist Baclofen Attenuates Responding for Ethanol in Ethanol-Dependent Rats. Alcoholism: Clinical and Experimental Research. 2007;31(1):11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witczuk B, Khaunina RA, Kupryszewski G. 3-(p-Chlorophenyl)-4-aminobutanoic acid--resolution into enantiomers and pharmacological activity. Polish journal of pharmacology and pharmacy. 1980;32(2):187–196. [PubMed] [Google Scholar]


