Abstract
A set of three stable synthetic mono-substituted cationic bacteriochlorins (BC37, BC38 and BC39) were recently reported to show exceptional activity (low nanomolar) in mediating photodynamic killing of human cancer cells after a 24 h incubation upon excitation with near-infrared light (730 nm). The presence of cationic quaternary ammonium groups in each compound suggested likely activity as antimicrobial photosensitizers. Herein this hypothesis was tested against a panel of pathogenic microorganisms that have all recently drawn attention due to increased drug-resistance (Gram-positive bacteria, Staphylococcus aureus and Enterococcus faecalis; Gram-negative bacteria, Escherichia coli and Acinetobacter baumannii; and fungal yeasts Candida albicans and Cryptococcus neoformans). All three bacteriochlorins were highly effective against both Gram-positive species (> 6 logs of eradication at ≤ 200 nM and 10 J/cm2). The dicationic bacteriochlorin (BC38) was best against the Gram-negative species (> 6 logs at 1–2 μM) and the lipophilic monocationic bacteriochlorin (BC39) was best against the fungi (> 6 logs at 1 μM). The bacteriochlorins produced substantial singlet oxygen (and apparently less Type-1 reactive-oxygen species such as hydroxyl radical) as judged by activation of fluorescent probes and comparison with 1H-phenalen-1-one-2-sulfonic acid; the order of activity was BC37 > BC38 > BC39. A short incubation time (30 min) resulted in selectivity for microbial cells over HeLa human cells. The highly active photodynamic inactivation of microbial cells may stem from the amphiphilic and cationic features of the bacteriochlorins.
Keywords: Antimicrobial photoinactivation, Drug-resistance, Stable synthetic bacteriochlorins, Monosubstituted cationic, Near infrared light, Broad-spectrum activity, Selectivity for microbial cells
1. Introduction
Bacteriochlorins (and related bacteriopheophorbides) are highly attractive as photosensitizers to mediate photodynamic therapy (PDT) of cancer, and/or photodynamic inactivation (PDI) of microbial cells due to the characteristic strong long wavelength absorption in the near-infrared spectral region (700–900 nm) [1–4]. We have reported a synthetic route to bacteriochlorins that are stabilized against adventitious oxidation by introduction of a geminal dimethyl group in each reduced pyrrole ring [5, 6]. The synthetic route also allows peripheral substituents to be tailored to vary the lipophilicity, polarity and overall charge (cationic, anionic or neutral) borne by the molecule [7]. The synthetic route to such bacteriochlorins as well as semisynthetic methods enable the synthesis of amphiphilic bacteriochlorins [8]. This synthetic versatility has enabled the design of photosensitizers that are able to kill cancer cells at extremely low concentrations upon activation by near-infrared light [9].
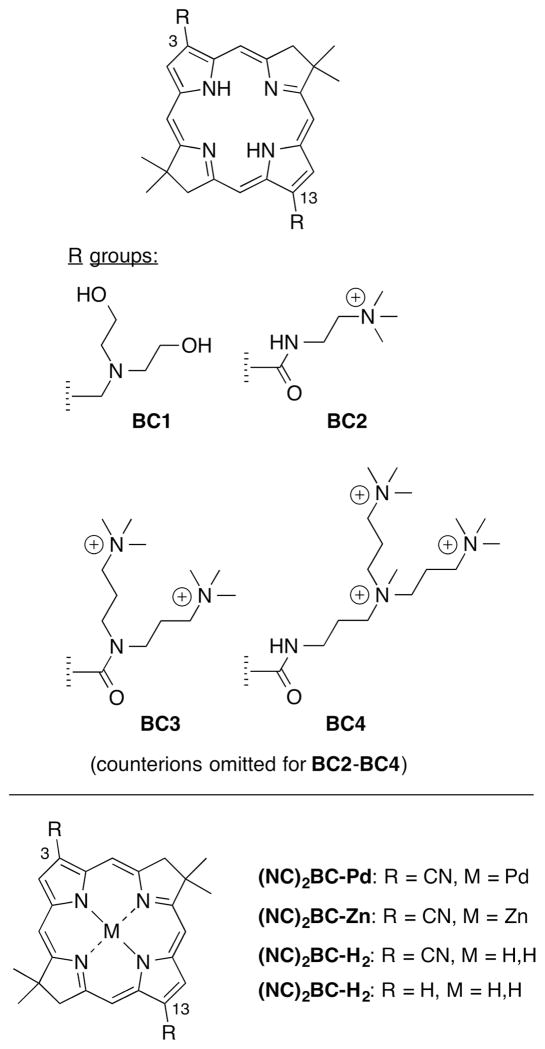
A bacteriochlorin photosensitizer was able to effectively reverse the resistance to PDT exhibited by pigmented melanoma both in vitro and in a mouse model in vivo [10]. The chelation of a palladium atom in the bacteriochlorin macrocycle increased the PDT potency, possibly due to increased probability of electron-transfer to produce Type 1 reactive-oxygen species such as hydroxyl radicals [11, 12]. Type 1 activity was likely further enhanced by the addition of two electron-withdrawing cyano groups symmetrically substituted at the 3- and 13-positions, which also improved the photostability [11, 12]. Four members of a set of symmetrically di-substituted bacteriochlorins (bearing two tertiary amines, or two, four, or six quaternary cationic groups; structures given below) were investigated as broad-spectrum antimicrobial photosensitizers [13]. The optimum photosensitizer structure was found to be different for each class of microbial cells. The di-tertiary amine substituted bacteriochlorin was best for killing fungal cells, the di-quaternized analogue was best for killing Gram-positive bacteria, and the hexa-quaternized analogue was best for killing Gram-negative bacteria [13].
Very recently, three mono-substituted cationic bacteriochlorins (BC37, BC38, BC39; Fig. 1) were synthesized and tested for PDT-mediated killing of human cancer cells [14]. These compounds exhibited exceptional activity, with substantial cancer cell killing at concentrations as low as 6 nM excited with modest fluences of light (5 J/cm2) after 24-h incubation. The fact that these three compounds possessed cationic (quaternary ammonium) groups suggested the likelihood of good activity as antimicrobial photosensitizers. Antimicrobial PDI is attracting increasing interest as an alternative antimicrobial technique as the inexorable rise of drug-resistance in pathogens continues unabated [15–18].
Figure 1.
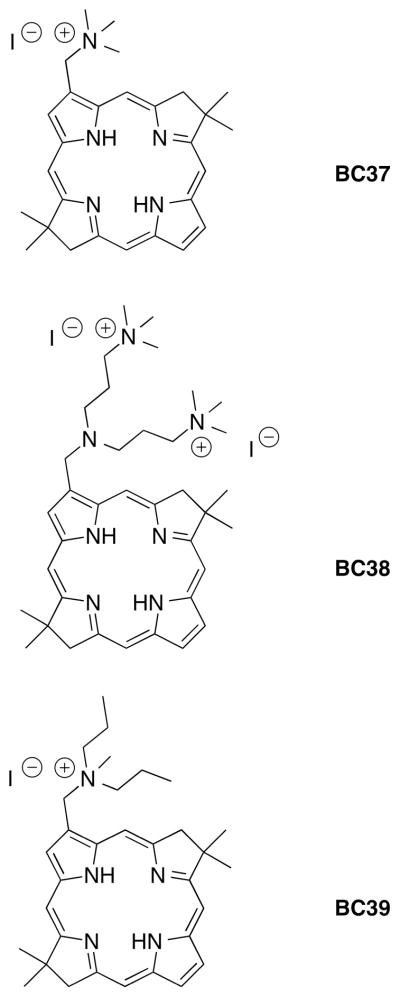
Structures of the three bacteriochlorin photosensitizers.
Herein we tested this hypothesis against a panel of pathogenic microorganisms that have all recently drawn attention due to their increased drug-resistance. The microorganisms included Gram-positive bacteria, Staphylococcus aureus [19] and Enterococcus faecalis [20]; Gram-negative bacteria, Escherichia coli [21] and Acinetobacter baumannii [22]; and fungal yeasts Candida albicans [23] and Cryptococcus neoformans [24]. PDI is postulated to have the greatest clinical impact against drug-resistant organisms that cause localized infections in wounds, burns and surgical sites [25] For this anti-infective approach to advance to clinical trials, it is necessary to demonstrate that the new bacteriochlorin photosensitizers do not mediate PDT damage to host human cells at the short (<30 min) incubation times generally used in PDI of microbial cells.
2. Materials and methods
2.1. Bacteriochlorins
The bacteriochlorins were designed and synthesized as described by Sharma et al. [14]. BC37 and BC39 each bears a single quaternized ammonium group and hence is monocationic. BC38 bears two quaternized ammonium groups and one trialkylamine. The latter is basic and is expected to be predominantly in the protonated state at neutral pH in aqueous solution. Nonetheless, given the presence of two permanent charges, BC38 is referred to as dicationic. All three bacteriochlorins are mono-substituted at the 3-position. The presence of a geminal dimethyl group at position 8 and at position 18 blocks the reduced (pyrroline) rings from undergoing adventitious dehydrogenation and thereby afford a robust bacteriochlorin chromophore.
2.2. Studies with fluorescence probes
Black-sided 96-well plates were used for experiments that employ fluorescence probes. Singlet oxygen sensor green reagent (SOSG), 3′-(p-aminophenyl)fluorescein (APF) or 3′-(p-hydroxyphenyl)fluorescein (HPF) (all from Molecular Probes, Life Technologies, Grand Island, NY) at a final concentration of 10 μM was added to a 10 μM solution of bacteriochlorin in 200 μL of phosphate-buffered saline per well. 1H-Phenalen-1-one-2-sulfonic acid (PN, a kind gift from Dr Santi Nonell at IQS Barcelona, Spain) was dissolved in water to give a 2 mM stock solution and was also used at 10 μM concentration. Fluorescence spectrometry (SpectraMax M5 plate reader, Molecular Devices, Sunnyvale, CA) used excitation/emission at 504/525 nm for SOSG and 490/515 nm for APF and HPF. Increasing fluences (J/cm2) were delivered using near-infrared light (700–850 nm band pass filter, Lumacare, Newport Beach, CA) for bacteriochlorins, and violet light from a 415±15nm LED array (Omnilux Clear-U, Photomedex, Horsham, PA) for PN. Light was delivered an irradiance of 100 mW/cm2 as measured with a power meter (model DMM 199 with 201 standard head; Coherent, Santa Clara, CA). The plate fluorescence was measured after each increment in the fluence was delivered.
2.3. Bacterial strain and culture conditions
The bacteria used in this study were as follows: Gram-positive bacteria S. aureus 8325-4 and E. fecalis ATCC29212 (ATCC, Manassas, VA; Gram-negative bacteria E. coli K12 and A. baumannii ATCC BAA747; fungal yeasts C. albicans DAY286 and C. neoformans KN99α. Planktonic bacterial cells were cultured in brain heart infusion broth (Fischer Scientific) with overnight aeration in an orbital shaking incubator at 130 rpm at 37 °C to stationary phase. An aliquot of this suspension was then refreshed in fresh brain heart infusion broth to mid-log phase (unless otherwise stated). Cell numbers were estimated by measuring the optical density [10] at 600 nm; in this case an optical density = 0.5 corresponds to 108 colony forming units (CFU) per mL. Fungal yeasts were cultured in yeast-peptone-dextrose broth with overnight aeration at 30 °C. The fungal yeast cell number was assessed with a hemacytometer.
2.4. Photodynamic inactivation studies
A cell suspension consisting of 108 cells/mL for bacteria (107 cells/mL for fungal yeasts: C. albicans and C. neoformans) was incubated with various concentrations of the bacteriochlorins in pH 7.4 phosphate-buffered saline for 30 min in the dark at room temperature. Aliquots (1 mL) were transferred to a 24-well plate and illuminated from the top of the plates in the dark at room temperature with a near infrared light source (700–850 nm band pass filter) to deliver a fluence of 10 J/cm2 or different fluences at an irradiance of 100 mW/cm2 as measured with a power meter. Bacteriochlorin stock solutions were prepared at 2 mM in dimethyl sulfoxide and stored in dark at 4 °C. The concentrations of diluted samples were checked by absorption spectroscopy assuming the molar absorption coefficient at the long wavelength band was 100,000 M−1cm−1. Cells treated with bacteriochlorins in the dark were incubated covered with aluminum foil for the same time as the PDI groups (30 min).
At the completion of illumination (or dark incubation), the contents of the wells were mixed before sampling. Aliquots (100 μL) were taken from each well to determine the CFU value. Care was taken to ensure that the contents of the wells were mixed thoroughly before sampling, as bacteria can settle at the bottom. The aliquots were serially diluted 10-fold in phosphate-buffered saline to give dilutions of 10−1 to 10−5 times the original concentration; then, 10-μL aliquots of each of the dilutions were streaked horizontally on square brain heart infusion broth (for bacteria) or yeast-peptone-dextrose broth (for C. albicans and C. neoformans) plates by the method of Jett et al [26]. Plates were streaked in triplicate and incubated for 12–36 h at 30 °C (for fungal yeasts) or 37 °C (for bacteria) in the dark to allow colony formation. A control group of cells treated with light alone (no bacteriochlorin added) showed the same number of CFU as the absolute control (data not shown). Survival fractions were routinely expressed as ratios of CFU of microbial cells treated with light and bacteriochlorin (or bacteriochlorin in the absence of light) to CFU of microbes treated with neither. Each experiment performed at least three independent times.
2.5. Photodynamic killing of mammalian cells
A human cervical cancer cell line, HeLa, was obtained from ATCC (Manassas, VA). The cells were cultured in RPMI-1640 medium with L-glutamine and NaHCO3 (Gibco-Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum and penicillin (100 U/mL) (Sigma, St. Louis, MO) at 37 °C in 5% CO2-humidified atmosphere in 75-cm2 flasks (Falcon-Invitrogen, Carlsbad, CA). When the cells reached 80% confluence, they were washed with phosphate-buffered saline and harvested with 2 mL of 0.25% trypsin-EDTA solution (Sigma). Cells were then centrifuged and counted in Trypan Blue to ensure viability and plated at a density of 5,000/well in flat-bottom 96-well plates (Fisher Scientific, Pittsburgh, PA). On the following day, dilute bacteriochlorin solutions were prepared (from the 2 mM stock) in two different kinds of medium: (i) complete growth medium with 10% serum and (ii) serum-free medium. These dilute bacteriochlorin solutions (0.1 to 5 μM) were added to the cells (to achieve the target bacteriochlorin concentration) for 30-min incubation. The dimethyl sulfoxide concentration in the medium did not exceed 0.2%. The medium was replaced with fresh medium without bacteriochlorin and 10 J/cm2 of illumination was delivered. The light spot was larger than four wells but light exposure was limited to four wells by a square template cut out of black card to ensure equal power density to each well. These wells were considered one experimental group illuminated at the same time. Control groups were as follows: no treatment, light alone, and medium with the same bacteriochlorin dilutions as described above. Following illumination the cells were returned to the incubator. After overnight incubation the media containing the cells was removed and replaced with 500 μg/mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide diluted in serum-free media [27]. The cells were incubated for 4 h. At the end of incubation the media was removed, and the cells were dissolved in dimethyl sulfoxide. The plate absorbance was measured at 570 nm using a microplate spectrophotometer (Spectra Max 340 PC, Molecular Devices, Sunnyvale, CA). Each experiment was repeated three times.
2.6. Statistics
Reported values are the mean of three separate experiments, and bars presented in the graphs are standard errors of the means (SEM). Differences between means were tested for significance by one-way ANOVA. P values of <0.05 were considered significant.
3. Results
3.1. Photochemical mechanism study using fluorescence probes
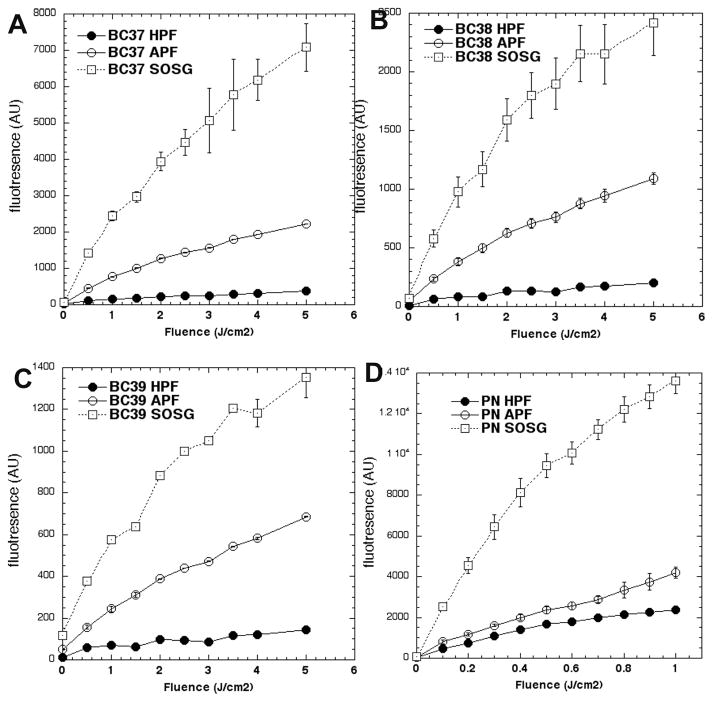
Fluorescence probes can be used to report (relative) specificity for the main different reactive-oxygen species involved in PDT, including singlet oxygen (1O2) and hydroxyl radical (HO·). The results can give information on the photochemical mechanism (Type 1 or Type 2) involved in the photodynamic effect [28, 29]. SOSG is mainly sensitive to 1O2. APF is ~130-fold more sensitive than HPF to HO•. APF is also sensitive to 1O2 but apparently HPF is not [29]. To aid in assessing the ability of the three bacteriochlorins to produce these two reactive-oxygen species, we carried out parallel studies with a photosensitizer (PN) that has been reported to operate exclusively by a Type 2 (1O2) mechanism [30]. Figure 2 shows that the order of activation for all three bacteriochlorins and for PN is SOSG ≫ APF > HPF. The SOSG/APF ratio is about 3:1 for all four sensitizers. When compared to PN, the APF/HPF ratio is roughly two-fold greater for BC38 and BC39 and four-fold greater for BC37. These combined results suggest that the bacteriochlorins produce significant 1O2 as well as a certain amount of Type 1 (HO•) photochemistry, and therefore do not function completely via a Type 2 process as has been reported for PN.
Figure 2. Activation by photoexcited bacteriochlorins and PN of fluorescent probes specific for certain reactive-oxygen species.
Bacteriochlorins, 1H-phenalen-1-one-2-sulfonic acid (PN) and probes were all used at 10 μM. Bacteriochlorins were excited by 700–850 nm light and PN by 400–430 nm light. Probes were singlet oxygen sensor green (SOSG) aminophenylfluorescein (APF) and hydroxyphenylfluorescein (HPF). (A) BC37; (B) BC38; (C) BC39; (D) PN.
3.2. Effect of bacteriochlorin concentration in PDI of microbial cells
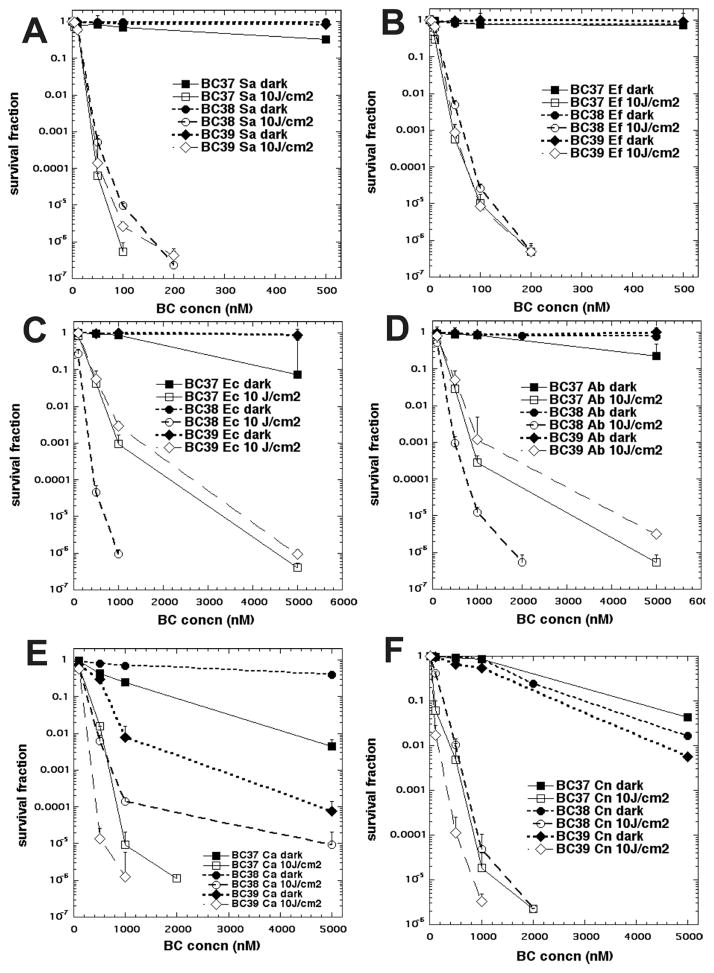
The best way to compare the phototoxicity of a group of photosensitizers with very different potencies is to vary the concentration over a wide range and determine the cell survival fraction with a single light dose (PDI) or without a single light dose (dark toxicity). The results for the six microbial species and the three bacteriochlorins are shown in Fig. 3.
Figure 3.
Concentration-dependent PDI of six species of microbial cells mediated by the three bacteriochlorins. Microbial cells were incubated with bacteriochlorin for 30 min and exposed (or not) to 10 J/cm2 of 700–850 nm light. (A) Gram-positive S. aureus; (B) Gram-positive E. faecalis; (C) Gram-negative E. coli; (D) Gram-negative A. baumannii; (E) Fungal yeast C. albicans; (F) Fungal yeast C. neoformans.
All three compounds were highly effective against the two Gram-positive species S. aureus (Fig. 3A) and E. faecalis (Fig. 3B). Both species show >6 logs of killing (eradication) at 200 nM and 10 J/cm2 light, and multiple logs of killing at 50 nM and 100 nM. Bacteriochlorin BC37 was better than BC38 and BC39 against S. aureus but not against E. faecalis. There was negligible dark toxicity (<1 log) in all cases.
There was a marked difference between the activities of the three bacteriochlorins against the two Gram-negative species E. coli (Fig. 3C) and A. baumannii (Fig. 3D). The dicationic BC38 gave eradication of both species at 1 μM, while the monocationic BC37 required 5 μM to give eradication, and the monocationic BC39 left a few remaining CFU (5 logs of killing) at 5 μM. There was minor dark toxicity (around 1 log) with BC37.
The bacteriochlorins also show different behavior toward the two fungal yeast species C. albicans (Fig. 3E) and C. neoformans (Fig. 3F). The results show that C. albicans was more resistant to PDI compared to C. neoformans. C. albicans also showed a larger difference between the activities of the three bacteriochlorins. In particular, C. albicans (Fig. 3E) was eradicated by BC39 at 1 μM, while BC37 required 2 μM to give the same eradication, and BC38 only gave 5 logs of cell killing at 5 μM. Dark toxicity was observed with both BC39 and BC37, with 4 and 2 logs, respectively, of dark killing seen at 5 μM. C. neoformans (Fig. 3F) was eradicated by BC39 at 1 μM and by both BC38 and BC37 at 2 μM. Dark toxicity was less pronounced with this species, showing 1–2 logs of dark killing at 5 μM.
3.3. Effect of light dose and incubation time in PDI of microbial cells
A fundamental concept in PDT/PDI is that cell killing should depend not only on the photosensitizer concentration but also on the delivered light dose (fluence). Although it is well established that a relatively short incubation time is adequate for PDI of microbial cells, the effect of varying this time from very short (7 min) to somewhat longer (30 min and also 2 h for fungi) has not been explored extensively. We used the best performing bacteriochlorin at concentrations determined from Fig. 3 to explore these effects in one species of each class of microorganisms. The selected compounds and concentrations were 100 nM BC37 for Gram-positive S. aureus; 1 μM BC38 for Gram-negative E. coli; and 500 nM BC39 for fungal yeast C. albicans. The light dose-response curves at different incubation times are shown in Fig. 4. All three microorganisms displayed a pronounced dependence of the cell killing on fluence (total amount of light energy delivered) as expected for a PDI process.
Figure 4.
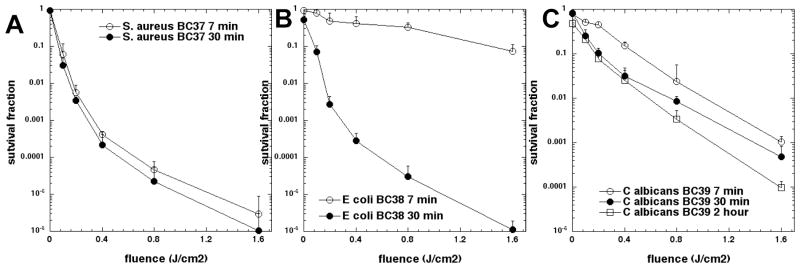
Light (700–850 nm) fluence-dependent PDI of three species of microbial cells mediated by selected bacteriochlorins at different incubation times. (A) S. aureus incubated with 200 nM of BC37; (B) E. coli incubated with 1 μM of BC38; (C) C. albicans incubated with 500 nM of BC39.
For the Gram-positive S. aureus (Fig. 4A) there was hardly any difference between the killing obtained using a 7-min versus 30-min incubation of cells with 200 μM BC37. By contrast, for the Gram-negative E. coli incubated with 1 μM BC38 there is a very large difference in the two killing curves (Fig. 4B). The short incubation time of 7 min gave less than 1 log of killing but the longer 30-min incubation gave 6 logs of killing at the highest fluence. The fungal Candida cells (Fig. 4C) showed an intermediate dependence on the incubation time. The observation that 30-min incubation gave an increase in killing (0.5–1 log) compared to 7 min prompted an even longer incubation of 2 h. The extra increase in killing of Candida observed after 2 h confirmed that for fungal cells the incubation time plays a definite role in susceptibility to PDI, but not to as great an extent as for Gram-negative bacteria.
3.4. Investigation of human cells killing by bacteriochlorins at short incubation times
The selectivity of the three mono-substituted cationic bacteriochlorins for microbial cells over host mammalian cells was demonstrated using the human cervical cancer cell line HeLa. We had previously shown that these same three compounds had high activity against HeLa cells after a 24-h incubation [14]. The PDT killing of HeLa cells (in complete medium) after short 30-min incubation is shown in Fig. 5. The data show that there was only minor toxicity with all three bacteriochlorins. The majority of cell killing was dark toxicity and there was only a small additional contribution of phototoxicity by light-mediated activation of each bacteriochlorin even at the highest concentration tested (5 μM).
Figure 5.
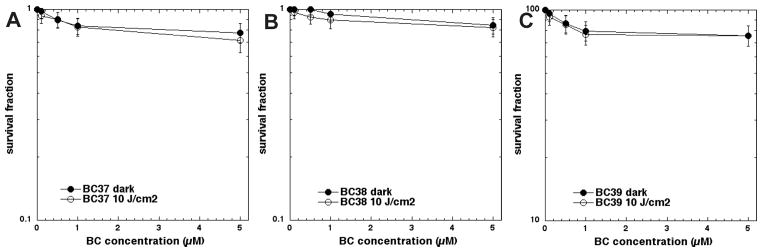
Concentration-dependent PDT of human HeLa cells mediated by the three bacteriochlorins after 30-min incubation excited (or not) with 10 J/cm2 of 700–850 nm light. (A) BC37; (B) BC38; (C) BC39.
4. Discussion
The results obtained herein show that mono-substituted cationic bacteriochlorins (Fig. 1) have high activity as broad-spectrum antimicrobial photosensitizers. Studies with relatively specific fluorescence probes for distinct reactive-oxygen species and comparison with a photosensitizer known to operate exclusively via Type 2 photochemistry revealed that the bacteriochlorins BC37-BC39 produce substantial 1O2 (as assayed via the SOSG probe) as well as some HO• (as assayed via APF and HPF probes). The mechanism of PDT activity in the cellular milieu would depend on such capabilities to produce these reactive-oxygen species. The production of HO• also may be altered if the (dielectric) properties of the specific cellular locale affect the redox potentials of the cationic bacteriochlorins.
Our previous studies for cancer cell killing both with bacteriochlorins [10–12], and also with imidazole porphyrins [31] have shown that the tendency to produce more hydroxyl radicals correlates with higher PDT cell killing. This correlation has not yet been shown for microbial cell killing; however, some comparisons are useful. In the recent study of cancer cell killing [11], palladium(II) dicyanobacteriochlorin (NC)2BC-Pd and free base analogue (NC)2BC-H2 showed activation of HO• probe HPF 5–10-fold greater than zinc chelate (NC)2BC-Zn and bacteriochlorin BC-H2 that lacks the cyano groups. The HO• activation level of (NC)2BC-Zn and BC-H2 was comparable to that found here and noted above for bacteriochlorins BC37-BC39 under comparable conditions (e.g., at 5 J/cm2 light fluence). Furthermore, the SOSG/HPF activity ratio for (NC)2BC-Zn and BC-H2 was comparable to that found here for BC37-BC39 but was 3–4-fold lower for (NC)2BC-Pd and (NC)2BC-H2. Collectively, these results are consistent with a relatively lower contribution of Type 1 HO• photochemistry and a greater contribution of Type 2 (1O2) photochemistry for BC37-BC39 in antimicrobial cell killing.
It is similarly plausible that the mono-substituted BC37-BC39 (Figure 1) have a lower propensity for producing Type 1 photochemistry than di-substituted analogues such as those shown in Figure 6 that we recently studied as antimicrobial photosensitizers [13] and for cancer cell killing [11]. In particular the bacteriochlorins bearing fewer cationic groups are expected to be harder to reduce (and easier to oxidize) than analogues with more positively charged substituents, and excited-state reduction (as opposed to photooxidation) appears to contribute substantially to the activity of the tetrapyrrole photosensitizers [12]. The relative contributions of Type 1 and Type 2 mechanisms of bacteriochlorins bearing various numbers of cationic substituents also may differ depending on the ability to reach target sites, and the relative sensitivity of those locales to singlet oxygen and hydroxyl radicals, as discussed further below.
Figure 6.
Four 3,13-disubstituted bacteriochlorins (described in [13]) bearing amines for comparison with the 3-monosubstituted BC37–BC39 (top) and four prior bacteriochlorins [12] lacking charged or ionizable substituents (bottom).
The molecular rationale for the observed differences in 1O2 production (BC37 > BC38 > BC39) is unclear at present. It is particularly noteworthy that these compounds (especially BC39) show exceptionally high activity against two different species of fungal cells (C. albicans and C. neoformans). The multiple logs of killing at nanomolar concentrations with a modest fluence of light in two different fungal species is remarkable. Previously, even highly active antifungal photosensitizers have required concentrations of at least a few micromolar to show good activity [13].
It is worthwhile to point out that the number of bacteriochlorin molecules in each well examined herein far exceeds the number of microorganisms. For bacteria, a bacteriochlorin concentration of 100 nM or 1 μM corresponds to 600,000 or 6,000,000 molecules/cell, respectively. For fungi, the same bacteriochlorin concentrations correspond to 6,000,000 or 60,000,000 molecules/cell.
In some cases the use of 10 J/cm2 NIR light in experiments (Figure 3) did not provide more microbial killing than much smaller fluences (Figure 4). This apparent contradiction is readily attributed to photobleaching. Although we did not specifically measure photobleaching in the present study, a previous study [12] showed that certain comparable bacteriochlorin structures, underwent significant photobleaching (>75%) after delivery of 10 J/cm2 NIR light, whereas others showed high photostability up to 100 J/cm2. The extent of photobleaching involves factors such as solubility (light-induced aggregation) and redox properties (e.g., photooxidation) that in turn depend on the host milieu and bacteriochlorin molecular and electronic structure [12]. The introduction of two cyano groups into the pyrrole rings did substantially reduce the photobleaching, but this structural modification was not available in the present series of compounds.
The structure–function relationships found with regard to the three classes of microbial cells show some aspects in common with the findings in our previous study of symmetrically 3,13-disubstituted bacteriochlorins (Fig. 6) as antimicrobial photosensitizers [13]. In the present study all three mono-substituted compounds are highly active in mediating the photokilling of Gram-positive bacteria, with the monocationic BC37 being slightly better against S. aureus. For killing Gram-negative bacteria, the dicationic BC38 is substantially better against both species, followed by BC37 and then BC39. For the fungal species the monocationic but more lipophilic BC39 is most active followed by BC37 and then BC38. In the previous study [13], the best bacteriochlorin photosensitizer against Gram-positive bacteria was BC2, a symmetric dicationic compound; the best bacteriochlorin against Gram-negative was BC4, the symmetric hexacationic analogue; and the best compound against fungal cells was BC1, the symmetric diamino analogue (which may be protonated in aqueous solution at neutral pH) (Fig. 6).
These two studies taken together, lead to the deduction that Gram-positive bacteria are photoinactivated preferentially by compounds with only a moderate number of cationic charges accompanied by low hydrophobicity. On the other hand, targeting Gram-negative bacteria requires as many cationic charges as possible on the nominally hydrophobic tetrapyrrole photosensitizer. In turn, fungal cells are best targeted with photosensitizers that bear a low number of (or zero) cationic charges but a higher degree of hydrophobicity. It warrants emphasis that in the above comparisons, the chromophore is constant among mono-substituted BC37–39 and disubstituted BC1 (each bearing a methylene unit between the bacteriochlorin π-system and the amino group) where only the nature of the peripheral substituents are varied. In disubstituted bacteriochlorins BC2–BC4, the connection motif is an amide (i.e., a carbonyl between the bacteriochlorin π-system and the amino group), which imparts a slight degree of polarity and a slight bathochromic shift of the long-wavelength absorption band.
Comparison of the two studies clearly shows that unsymmetrically 3-mono-substituted bacteriochlorins are significantly more effective than symmetrically 3,13-disubstituted analogues. Upon comparison of compounds with similar numbers of cationic charges – either mono-substituted in the present study versus di-substituted compounds in the previous study – it becomes apparent that the mono-substituted bacteriochlorins are much more active (about ten-fold) than the di-substituted analogues. The reason for this difference presumably rests in the amphiphilic nature of the mono-substituted molecule (BC37–BC39), that is less pronounced (or absent) in the di-substituted analogue. The amphiphilic design of the bacteriochlorins BC37-BC39 stems from the presence of a cationic moiety at one site on the otherwise hydrophobic and electron-rich bacteriochlorin chromophore. The resulting molecular asymmetry likely enables localization at a certain position in a lipid bilayer and therefore can generate reactive-oxygen species at particularly sensitive sites. In addition to the cationic bacteriochlorins described herein and in our previous report [13], similar structures such as the di-substituted cationic porphyrins (XF70 and XF73) have been reported [32] to have very high activity (nM concentrations) in mediating PDI of drug-resistant bacteria. While it is tempting to ascribe the observed greater activity of the mono-substituted bacteriochlorin versus the disubstituted to the amphiphilic character of the former, an alternative explanation may be that the overall architecture of the mono-substituted bacteriochlorin is simply smaller than that of the di-substituted bacteriochlorin. This alternative explanation is potentially subject to examination through use of amphiphilic bacteriochlorins with non-polar tails of various lengths. The preparation and testing of such compounds may form the basis for studies beyond the scope of the present manuscript.
The observed differences in PDI effectiveness with variation in incubation time (Fig. 4) are quite interesting. The largest difference in cell killing versus incubation time was observed in Gram-negative E. coli, with a much smaller difference against fungi (C. albicans) and almost no difference was seen with Gram-positive S. aureus. Since Gram-positive bacteria are considered to be porous to tetrapyrrole molecules, it is entirely possible that molecules diffuse through the cell wall relatively rapidly. This diffusion process may be related to the uptake of heme (i.e., iron-chelated protoporphyrin IX) into Gram-positive bacteria [33]. The same consideration also applies to the fungal cell wall, which is also considered to be porous to tetrapyrrole photosensitizers [34] although somewhat less so than Gram-positive bacteria, which accounts for the more pronounced incubation-time dependence.
The mechanism of penetration of cationic photosensitizers into Gram-negative bacteria is entirely different than those that rely on diffusion. Here the polycationic photosensitizer molecules displace the divalent cations (Ca2+ and Mg2+) that hold the anionic lipopolysaccharide molecules in place in the outer membrane, and thereby disrupt the permeability barrier that is characteristic of this class of microorganisms [35]. This disruption process is likely to be significantly slower than the process of diffusion into Gram-positive bacteria and fungal cells.
In conclusion the mono-substituted cationic bacteriochlorins studied herein are highly active, broad-spectrum and selective antimicrobial photosensitizers with particular activity against fungal cells and minimal toxicity (light or dark) for (host) mammalian cells. As such, this new class of photosensitizers may have future clinical application.
Highlights.
Three stable synthetic monosubstituted cationic bacteriochlorins have been tested as antimicrobial photosensitizers.
Gram-positive bacteria, Gram-negative bacteria and fungal yeasts were, all killed by nanomolar concentrations and 10J/cm2 NIR light.
The bacteriochlorins produced both singlet oxygen and hydroxyl radicals.
For Gram-negative bacteria (and to a lesser extent fungi) longer incubation times increased killing.
Mammalian HeLa cells were not killed under equivalent conditions.
Acknowledgments
This work was supported by US NIH grants R41AI072854 (to NIRvana Pharmaceuticals Inc.) and R01AI050875 (to M. R. Hamblin). Liyi Huang was supported by National Natural Science Foundation of China (81260239), Guangxi Scientific and Technological Projects (2013BC26041, 1355005-1-2), and Guangxi Provincial Department of Education (200610MS117).
Abbreviations
- APF
Aminophenylfluorescein
- CFU
Colony-forming units
- HPF
Hydroxyphenylfluorescein
- PDI
Photodynamic inactivation
- PDT
Photodynamic therapy
- PN
Phenalen-1-one-2-sulfonic acid
- SOSG
Singlet oxygen sensor green
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dabrowski JM, Arnaut LG, Pereira MM, Monteiro CJ, Urbanska K, Simoes S, Stochel G. New halogenated water-soluble chlorin and bacteriochlorin as photostable PDT sensitizers: synthesis, spectroscopy, photophysics, and in vitro photosensitizing efficacy. ChemMedChem. 2010;5:1770–1780. doi: 10.1002/cmdc.201000223. [DOI] [PubMed] [Google Scholar]
- 2.Ashur I, Goldschmidt R, Pinkas I, Salomon Y, Szewczyk G, Sarna T, Scherz A. Photocatalytic generation of oxygen radicals by the water-soluble bacteriochlorophyll derivative WST11, noncovalently bound to serum albumin. The journal of physical chemistry A. 2009;113:8027–8037. doi: 10.1021/jp900580e. [DOI] [PubMed] [Google Scholar]
- 3.Yakubovskaya RI, Plotnikova C, Plyutinskaya AD, Morozova NB, Chissov VI, Makarova EA, Dudkin SV, Lukyanets EA, Vorozhtsov GN. Photophysical properties and in vitro and in vivo photoinduced antitumor activity of cationic salts of meso-tetrakis(N-alkyl-3-pyridyl)bacteriochlorins. Journal of photochemistry and photobiology. B Biology. 2014;130:109–114. doi: 10.1016/j.jphotobiol.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal A, Thompson S, Singh S, Newton B, Moore A, Gao R, Gu X, Mukherjee S, Drain CM. Photophysics of glycosylated derivatives of a chlorin, isobacteriochlorin and bacteriochlorin for photodynamic theragnostics: discovery of a two-photon-absorbing photosensitizer. Photochemistry and photobiology. 2014;90:419–430. doi: 10.1111/php.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniguchi M, Cramer DL, Bhise AD, Kee HL, Bocian DF, Holten D, Lindsey JS. Accessing the near-infrared spectral region with stable, synthetic, wavelength-tunable bacteriochlorins. New J Chem. 2008;32:947–958. [Google Scholar]
- 6.Yang E, Kirmaier C, Krayer M, Taniguchi M, Kim HJ, Diers JR, Bocian DF, Lindsey JS, Holten D. Photophysical properties and electronic structure of stable, tunable synthetic bacteriochlorins: extending the features of native photosynthetic pigments. J Phys Chem B. 2011;115:10801–10816. doi: 10.1021/jp205258s. [DOI] [PubMed] [Google Scholar]
- 7.Ruzie C, Krayer M, Balasubramanian T, Lindsey JS. Tailoring a bacteriochlorin building block with cationic, amphipathic, or lipophilic substituents. J Org Chem. 2008;73:5806–5820. doi: 10.1021/jo800736c. [DOI] [PubMed] [Google Scholar]
- 8.Aravindu K, Mass O, Vairaprakash P, Springer JW, Yang E, Niedzwiedzki DM, Bocian DF, Holten D, Lindsey JS. Amphiphilic chlorins and bacteriochlorins in micellar environments. Molecular design, de novo synthesis, and photophysical properties. Chem Sci. 2013;4:3459–3477. [Google Scholar]
- 9.Huang YY, Mroz P, Zhiyentayev T, Sharma SK, Balasubramanian T, Ruzie C, Krayer M, Fan D, Borbas KE, Yang E, Kee HL, Kirmaier C, Diers JR, Bocian DF, Holten D, Lindsey JS, Hamblin MR. In vitro photodynamic therapy and quantitative structure-activity relationship studies with stable synthetic near-infrared-absorbing bacteriochlorin photosensitizers. J Med Chem. 2010;53:4018–4027. doi: 10.1021/jm901908s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mroz P, Huang YY, Szokalska A, Zhiyentayev T, Janjua S, Nifli AP, Sherwood ME, Ruzie C, Borbas KE, Fan D, Krayer M, Balasubramanian T, Yang E, Kee HL, Kirmaier C, Diers JR, Bocian DF, Holten D, Lindsey JS, Hamblin MR. Stable synthetic bacteriochlorins overcome the resistance of melanoma to photodynamic therapy. Faseb J. 2010;24:3160–3170. doi: 10.1096/fj.09-152587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YY, Balasubramanian T, Yang E, Luo D, Diers JR, Bocian DF, Lindsey JS, Holten D, Hamblin MR. Stable synthetic bacteriochlorins for photodynamic therapy: role of dicyano peripheral groups, central metal substitution (2H, Zn, Pd), and Cremophor EL delivery. ChemMedChem. 2012;7:2155–2167. doi: 10.1002/cmdc.201200351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang E, Diers JR, Huang YY, Hamblin MR, Lindsey JS, Bocian DF, Holten D. Molecular electronic tuning of photosensitizers to enhance photodynamic therapy: synthetic dicyanobacteriochlorins as a case study. Photochemistry and photobiology. 2013;89:605–618. doi: 10.1111/php.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang L, Huang YY, Mroz P, Tegos GP, Zhiyentayev T, Sharma SK, Lu Z, Balasubramanian T, Krayer M, Ruzie C, Yang E, Kee HL, Kirmaier C, Diers JR, Bocian DF, Holten D, Lindsey JS, Hamblin MR. Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers. Antimicrob Agents Chemother. 2010;54:3834–3841. doi: 10.1128/AAC.00125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma SK, Krayer M, Sperandio FF, Huang L, Huang YY, Holten D, Lindsey JS, Hamblin MR. Synthesis and evaluation of cationic bacteriochlorin amphiphiles with effective photodynamic activity against cancer cells at low nanomolar concentration. J Porphyrins Phthalocyanines. 2013;17:73–85. doi: 10.1142/S108842461250126X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers in surgery and medicine. 2006;38:468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 17.Maisch T. A new strategy to destroy antibiotic resistant microorganisms: antimicrobial photodynamic treatment. Mini reviews in medicinal chemistry. 2009;9:974–983. doi: 10.2174/138955709788681582. [DOI] [PubMed] [Google Scholar]
- 18.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) The Journal of antimicrobial chemotherapy. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto M, Mu Y, Lynfield R, Bulens SN, Nadle J, Aragon D, Petit S, Ray SM, Harrison LH, Dumyati G, Townes JM, Schaffner W, Gorwitz RJ, Lessa FC. Trends in invasive methicillin-resistant Staphylococcus aureus infections. Pediatrics. 2013;132:e817–824. doi: 10.1542/peds.2013-1112. [DOI] [PubMed] [Google Scholar]
- 20.Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30:1136–1149. doi: 10.1592/phco.30.11.1136. [DOI] [PubMed] [Google Scholar]
- 21.Szmolka A, Nagy B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Frontiers in microbiology. 2013;4:258. doi: 10.3389/fmicb.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnin RA, Nordmann P, Poirel L. Screening and deciphering antibiotic resistance in Acinetobacter baumannii: a state of the art. Expert Rev Anti-Infect Ther. 2013;11:571–583. doi: 10.1586/eri.13.38. [DOI] [PubMed] [Google Scholar]
- 23.Huang M, Kao KC. Population dynamics and the evolution of antifungal drug resistance in Candida albicans. FEMS Microbiol Lett. 2012;333:85–93. doi: 10.1111/j.1574-6968.2012.02587.x. [DOI] [PubMed] [Google Scholar]
- 24.Cheong JW, McCormack J. Fluconazole resistance in cryptococcal disease: emerging or intrinsic? Med Mycol. 2013;51:261–269. doi: 10.3109/13693786.2012.715763. [DOI] [PubMed] [Google Scholar]
- 25.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections-State of the art. Photodiagnosis Photodyn Ther. 2009;6:170–188. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. BioTechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 27.Merlin JL, Azzi S, Lignon D, Ramacci C, Zeghari N, Guillemin F. MTT assays allow quick and reliable measurement of the response of human tumour cells to photodynamic therapy. European journal of cancer. 1992;28A:1452–1458. doi: 10.1016/0959-8049(92)90542-a. [DOI] [PubMed] [Google Scholar]
- 28.Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. The Journal of biological chemistry. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 29.Price M, Reiners JJ, Santiago AM, Kessel D. Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy. Photochemistry and photobiology. 2009;85:1177–1181. doi: 10.1111/j.1751-1097.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nonell S, Gonzalez M, Trull FR. 1H-phenalen-1-one-2-sulfonic acid - An extremely efficient singlet molecular-oxygen sensitizer for aqueous-media. Afinidad. 1993;50:445–450. [Google Scholar]
- 31.Mroz P, Bhaumik J, Dogutan DK, Aly Z, Kamal Z, Khalid L, Kee HL, Bocian DF, Holten D, Lindsey JS, Hamblin MR. Imidazole metalloporphyrins as photosensitizers for photodynamic therapy: role of molecular charge, central metal and hydroxyl radical production. Cancer letters. 2009;282:63–76. doi: 10.1016/j.canlet.2009.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maisch T, Bosl C, Szeimies RM, Lehn N, Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob Agents Chemother. 2005;49:1542–1552. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. Passage of heme-iron across the envelope of Staphylococcus aureus. Science. 2003;299:906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 34.Di Palma MA, Alvarez MG, Ochoa AL, Milanesio ME, Durantini EN. Optimization of cellular uptake of zinc(II) 2,9,16,23-tetrakis[4-(N-methylpyridyloxy)]phthalocyanine for maximal photoinactivation of Candida albicans. Fungal biology. 2013;117:744–751. doi: 10.1016/j.funbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44:522–527. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]