Abstract
The PIM1 oncogene is over-expressed in human prostate cancer epithelial cells. Importantly, we observe that in human hyperplastic and cancerous prostate glands PIM1 is also markedly elevated in prostate fibroblasts, suggesting an important role for this kinase in epithelial/stromal crosstalk. The ability of PIM1 to regulate the biologic activity of stromal cells is demonstrated by the observation that expression of PIM1 kinase in human prostate fibroblasts increases the level and secretion of the extracellular matrix molecule, collagen 1A1 (COL1A1), the pro-inflammatory chemokine CCL5, and the platelet-derived growth factor receptors (PDGFR). PIM1 is found to regulate the transcription of CCL5. In co-cultivation assays where PIM1 overexpressing fibroblasts are grown with BPH1 prostate epithelial cells, PIM1 activity markedly enhances the ability of these fibroblasts to differentiate into myofibroblasts and express known markers of cancer-associated fibroblasts (CAFs). This differentiation can be reversed by the addition of small molecule PIM kinase inhibitors. Western blots demonstrate that PIM1 expression in prostate fibroblasts stimulates the phosphorylation of molecules that regulate 5’Cap driven protein translation, including 4EBP1 and eIF4B. Consistent with the hypothesis that the kinase controls translation of specific mRNAs in prostate fibroblasts, we demonstrate that PIM1 expression markedly increases the level of COL1A1 and PDGFRβ mRNA bound to polysomes. Together these results point on PIM1 as a novel factor in regulation of the phenotype and differentiation of fibroblasts in prostate cancer by controlling both the transcription and translation of specific mRNAs.
Keywords: PIM1 kinase, prostate cancer, myofibroblasts, COL1A1, CCL5, PDGDRβ
1. Introduction
Prostate cancer (PCa) tumor growth and metastasis are stimulated by “activated” fibroblasts, also known as cancer associated fibroblasts (CAFs) located in the tumor stroma. Phenotypically, CAFs closely resemble myofibroblasts exhibiting different levels of specific marker expression relative to benign fibroblasts (1–4). Myofibroblasts secrete multiple growth factors (e.g., TGFα, TGFβ, CXCL12/SDF1, IL-6) and extracellular matrix proteins (e.g., collagens, periostin, integrins, cadherin 11) involved in regulation of angiogenesis and epithelial cell growth and migration. These in turn enable tumor growth and dissemination (2, 5–8). Specific intracellular signal transduction pathways are needed to induce the myofibroblast/CAF differentiation of normal fibroblasts and to regulate the secretion of the factors that stimulate tumor growth.
The pim-1 gene was identified as a proviral insertion site of the Moloney murine leukemia virus in experiments designed to find new genes, which are involved in tumorigenesis (9). Signal transduction pathways regulated by PIM serine/threonine kinases are strongly implicated in the human prostate cancer progression and growth (10). PIM1 activity regulates the growth of the epithelial component of human PCa (11–13). Overexpression of PIM1 has been detected in human PCa both at the mRNA and protein levels, although there is no clear definition of which cell types within the tumor were involved (12). Moderate to strong staining of PIM1 was seen in tumors of 68% of patients with a Gleason score of seven or higher (14), and PIM staining may be helpful in differentiating benign glands from intraepithelial neoplasia (15). Human PCa epithelial cells secrete a large number of factors, including IL-6 and GM-CSF, that control PIM kinase levels, and hypoxia is another stimulant known to elevate PIM levels in multiple cell types (16–18).
Using human tissue microarrays, our experiments demonstrate that PIM1 protein kinase expression levels are significantly elevated not only in the epithelial compartment, but also importantly in the prostate stromal compartment. Evaluation of prostate biopsies of human tumours demonstrates elevated levels of PIM1 mRNA in myofibroblasts/CAFs versus normal fibroblasts. This data suggests a potential role for the PIM1 protein kinase in regulation of both the differentiation state and the biologic activity of prostate fibroblasts.
2. Materials and Methods
2.1 Antibodies
reagents and all lentiviral and retroviral expression vectors used in this study are described in the supplementary Materials and Methods.
2.2 Cell culture and generation stable cell lines
Immortalized stromal fibroblasts BHPrS1 and epithelial BPH1 cells were kindly provided by Dr. Simon W. Hayward (Vanderbilt University). Both cell lines were isolated from non-tumorigenic prostate surgical samples (19–21). The primary normal human prostate fibroblasts hPrF at passage 2 were purchased from ScienCell and grown in the recommended media (Fibroblast medium FM #2301).
Primary cultures of human prostate stromal cells were obtained from explants of needle biopsies from the peripheral or transition zones of prostate from patients with advanced prostate cancer and from patients without evidence of neoplasia. This work was performed by J. Cerda-Infante and V.P. Montecinos, using the pathology resource at the Hospital Clínico, Pontificia Universidad Católica de Chile under IRB-approved protocols. Stromal fibroblasts were cultivated as described (22) in the DMEM supplemented with 5% fetal bovine serum (FBS) for two passages. Cells were then assayed for expression of CAFs-associated markers αSMA, vimentin, pro-collagen and calponin by qRT-PCR, immunocytochemistry and western blot analysis (data not shown) and used for measurement of PIM1 levels.
All other human cell lines, including prostate fibroblasts WPMY1, bone marrow mesenchymal cells HS-5, PC3, were supplied by American Type Culture Collection. Wild type (WT) and PIM triple knockout (TKO) mouse embryonic fibroblasts (MEFs) were established as described (23).
2.3 Cell growth
lentiviral transduction and selection of the stable cell pools expressing inducible PIM1 kinase were performed as described in the supplementary Materials and Methods.
2.4 Co-cultivation assays
Co-cultivation of stromal fibroblasts expressing inducible PIM1 and BPH1/GFP were performed either in the absence or presence of Doxycycline (Dox) (20ng/ml). 2×105 of BHPrS1 and BPH1/GFP cells were plated in 10 cm dishes, grown until confluent, trypsinized, diluted to 4×105 cells/plate and cultivated in a fresh media. After the third passage, 15 total days of co-cultivation, the stromal fibroblasts were separated from the epithelial cells by differential trypsinization and plated in media containing puromycin (2µg/ml) for 48 hours to remove puromycin-sensitive BPH1/GFP cells. After 48 hours growth in puromycin containing media, cells were re-plated in antibiotic-free complete media and used for analysis. To isolate BPH1/GFP cells, epithelial cells attached to the plates after differential trypsinization were collected and subjected to flow cell sorting. The resultant GFP-positive epithelial cells were then assayed for cell growth and migration. To study the activity of kinase inhibitors, after the second passage of co-cultivation, cells were plated in a fresh media and after attachment overnight, PIM1 or PDGFR inhibitors were added for 5 days until cells reached confluence followed by further analysis.
2.5 CM preparation
To prepare CM, 1×106 prostate stromal fibroblasts grown in monoculture or after co-cultivation were first plated in complete media with or without Dox, and grown for 48 hours. Cells were then washed twice with PBS and placed in serum free media. CM was collected after 48 hours, centrifuged at 4,000 rpm to pellet debris, and used either for the BPH1 cell migration assay (Supplementary Fig. S2C) or concentrated with an Amicon ultra 15 centrifugal unit (Millipore). The protein concentrations in each sample were determined by Bradford assay (Bio-Rad) and equal amounts of protein were used for Western blot or ELISA. Quantitation of CCL5 in the CM was measured by the Human Cytokine Array Kit (R&D Systems) or ELISA (Ray Biotech), according to the manufacturing protocols.
2.6 Tissue microarray and immunocytochemistry (IHC), cell growth and migration assays
qRT-PCR analysis, polysome profiling were done as described in the supplementary Materials and Methods.
2.7 Statistical analysis
All experiments were repeated a minimum of two times and the results of quantitative studies are reported as the mean +/−SD. Differences were analyzed by Student’s t test. P values of < 0.05 were regarded as significant.
3. Results
3.1. PIM1 protein levels are elevated in PCa fibroblasts
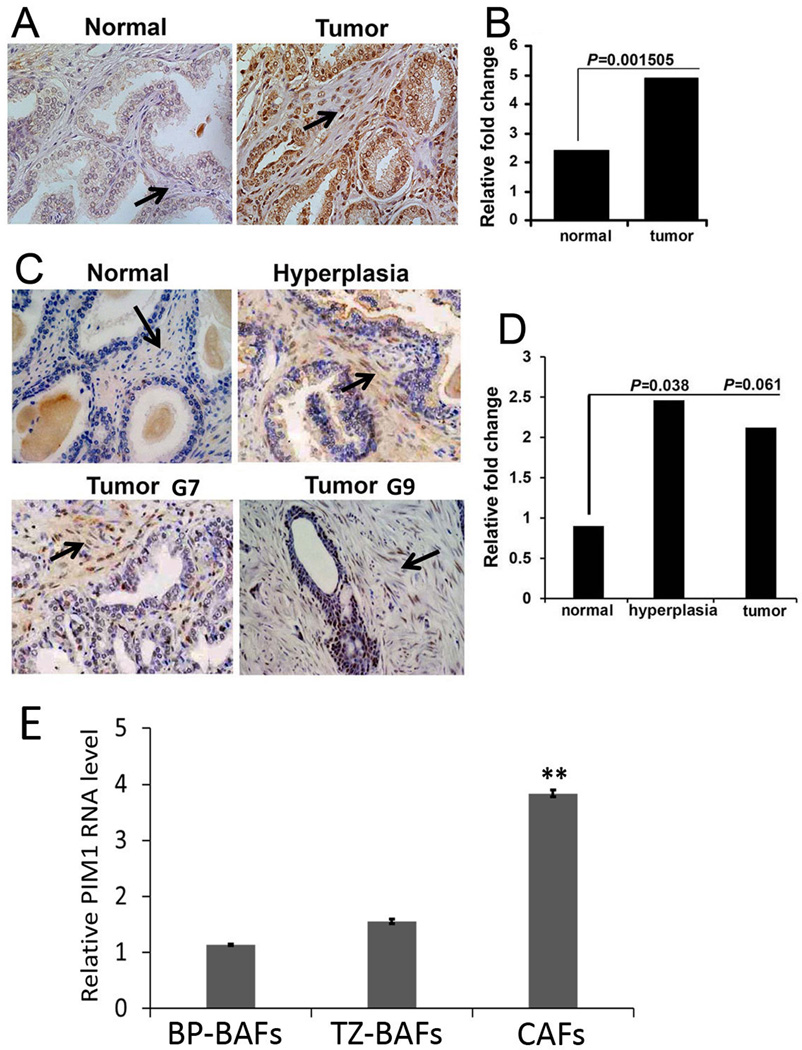
To evaluate the levels of the PIM1 protein kinase in PCa stromal cells, 28 cancer and adjacent normal samples were evaluated by IHC using the 19F7 PIM1 mouse monoclonal antibody and samples were scored based on staining intensity (Fig. 1A and B). This antibody specifically recognizes PIM1 but not the other PIM family members and had been used by others (24). This analysis demonstrated that PIM1 is highly and significantly over expressed in human PCa stroma when compared to those from normal prostate tissues. This observation was confirmed using an alternate prostate tissue microarray containing 32 adenocarcinomas, 26 hyperplasia and 20 normal tissues (Fig.1 C and D) with staining performed with rabbit monoclonal PIM1 antibodies (Epigenetic). Although the direct correlation between PIM1 immunostaining in PCa stroma and the Gleason grades was not found, the up-regulation of PIM1 kinase can shed light onto stromagenic response in prostate cancer.
Figure 1. Expression of PIM1 in human prostate stroma.
(A) IHC staining of PIM1 using a prostate tissue microarray (TMA, MUSC). PIM1 expression is indicated in prostate stroma (arrows). (B) Semi-quantitative analysis of IHC. In blinded quantitative analysis of PIM1 immunostaining the percentage of PIM1 positive stromal cells was scored on a scale of 0–4, where 0=no positive cells, 1=1–25% positive cells, 2=26–50% positive cells, 3=51–75% positive cells, and 4=76–100% positive cells. The intensity of staining was scored on a scale of 0–3, where 0=negative, 1=mild, 2=moderate, and 3=intensive staining. A final score was calculated by multiplying the percentage and intensity scores. P values were calculated by t test and represent the probability of no difference between PIM1 positive cells in the stroma of normal and cancer tissues. (C) IHC staining of PIM1 using a TMA (Folio Bioscience). PIM1 expression is indicated in both the hyperplastic and cancer stroma (arrows; G7; G9- Gleason score 7 and 9 accordingly). (D) Semi-quantitative analysis of IHC. Percentage of PIM1 positive stromal cells was scored as described above. (E) qRT-PCR analysis of pooled RNA samples obtained from primary CAFs and BAFs. Each value represents the mean ± SD of six pooled measurements produced from two independent experiments. **, P < 0.001; P values were calculated by t tests and represent the probability of no difference between PIM1 mRNA levels in BAFs and CAFs.
To confirm whether the PIM1 kinase level is up-regulated in the PCa stromal fibroblasts we analyzed pooled RNA samples obtained from primary CAFs cultures isolated from the peripheral zone of primary tumor of advanced/metastatic PCa patients (CAFs, n=7), from the benign peripheral zone (BP-BAFs, n=9) and from the benign transition zone (TZ-BAFs, n=6) of the prostate. As shown in Fig. 1E the level of PIM1 transcript is significantly elevated in CAFs compared to benign prostate fibroblasts. Together these results suggest a potential role of PIM1 in the activity of fibroblasts found adjacent to transformed or abnormal epithelial cells.
3.2. Overexpression of PIM1 kinase in prostate stromal cells results in increased COL1A1 expression
To investigate the role of elevated PIM1 in prostate fibroblasts, a doxycycline (Dox)-inducible lentiviral vector encoding PIM1 kinase was used for transient and stable expression of PIM1 in human prostate fibroblasts, including primary fibroblasts hPRF (ScienCell), the immortalized prostate fibroblast cell line WPMY1 (ATCC), and BHPrS1. The application of 20 ng/ml of Dox to these cells induced moderate increases in PIM1 (Supplementary Fig. S1) similar to levels commonly seen in both PCa and BPH, suggesting that these cells can be used as a model to study the result of PIM1 activity in prostate fibroblasts.
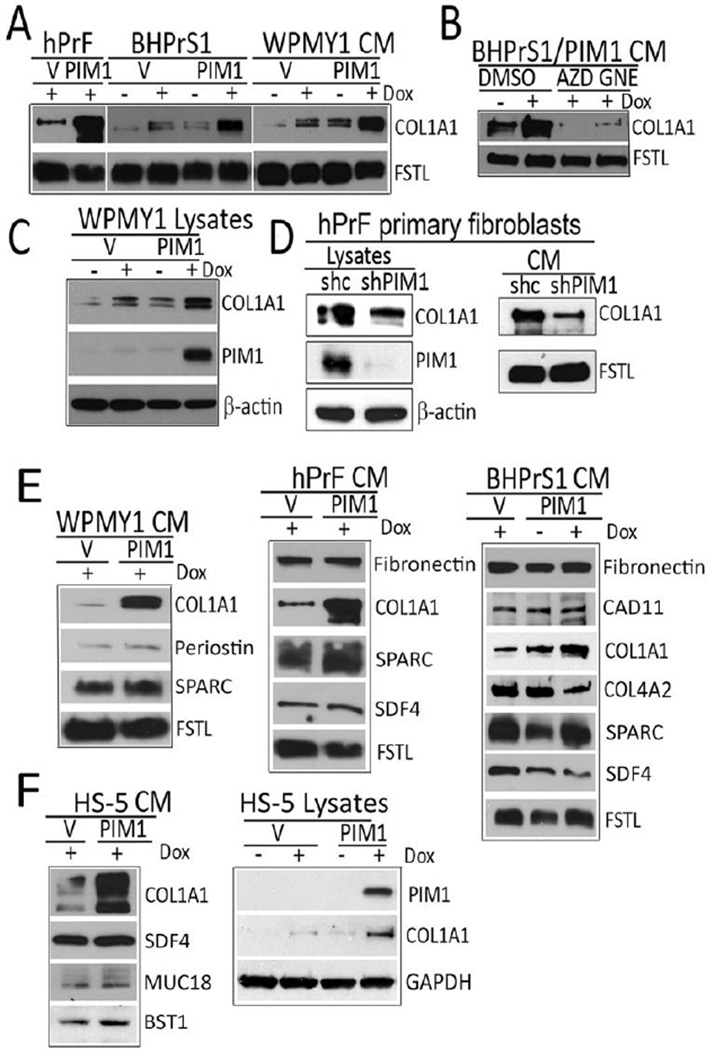
Cancer associated fibroblasts (CAFs) exhibit extracellular matrix (ECM) remodeling associated with the increased levels of collagens (COL1A1, COL4A), fibronectin, periostin, SPARC, cadherin 11 (CAD11), that function to magnify the matrix stiffness and enhance the metastatic capacity of epithelial cancer cells (2, 5, 7, 25). To validate whether increases in PIM1 levels in normal prostate fibroblasts can change the production of ECM, human primary prostate fibroblasts (hPrF) and immortalized prostate fibroblasts (BHPrS1, WPMY1), expressing vector control or inducible PIM1 kinase were evaluated. Cells were treated with or without Dox for 96 hours and then lysates and conditioning media (CM) were analyzed for expression of the ECM and secreted factors that have been previously identified as markers of the CAF phenotype. Increasing PIM1 expression in the prostate fibroblasts markedly increased intracellular and secreted levels of collagen 1A1 (COL1A1) (Fig. 2A–C). This induction was blocked by the addition to the media of either of two pan-PIM1 inhibitors, AZD1208 (AstraZeneca) (26) or GNE-652 (Genentech) (27)(Fig. 2B). Additionally, in hPrF the knockdown of endogenous PIM1 decreased baseline intracellular and secreted levels of COL1A1 (Fig. 2D). In contrast to COL1A1, short-term induction of PIM1 did not change the levels of periostin, SPARC, SDF4, CAD11 and COL4A2 (Fig. 2E). The correlation between PIM1 levels and an elevation in the intracellular or secreted COL1A1 was also observed in a human mesenchymal bone marrow cell line HS-5, suggesting that the ability of short term expression of PIM1 to elevate COL1A1(Fig. 2F) was not limited to prostate fibroblasts.
Figure 2. Overexpression of PIM1 kinase in prostate stromal cells increases COL1A1 levels.
(A) Primary (hPrF1) and immortalized (BHPrS1 and WPMY1) prostate stromal cell lines were transduced with lentiviruses encoding inducible PIM1 kinase. PIM1 expression was induced (20ng/ml Dox) and CM collected and analyzed for collagen 1 (COL1A1) production by Western blotting. (B) DMSO (vehicle control) or PIM1 inhibitors, 3µM AZD1208 (AZD) or 1 µM GNE- 652 (GNE), were added to the PIM1 overexpressing cells for 48 hours. The CM was collected and analyzed by western blotting with the indicated antibodies. (C) Analysis of WPMY1 cell lysates after induction of PIM1 kinase expression by Dox. (D) hPrF cells were transduced with lentiviruses encoding PIM1 shRNA (Open Biosystem), and cultivated for 72 h. Lysates and CM were then analyzed for COL1A1 production by Western blotting. (E and F) Over-expression of PIM1 kinase in stromal cells increases COL1A1 levels whereas other secreted protein levels are not changed. Human prostate fibroblasts (E) or bone marrow mesenchymal cells (F) were transduced with lentiviruses encoded inducible PIM1 kinase or vector control, and then incubated in the complete media with or without Dox (20ng/ml) for 48 hours following by additional 48 hours incubation in serum free media. The CM and cell lysates (F, right panel) were collected and assayed for indicated proteins by Western blotting. Secreted follistatin like protein1 (FSTL) and β-actin serve as loading controls for the CM and lysates respectively. All experiments were done in triplicate.
3.3. PIM1 increases secreted and mRNA levels of the pro-inflammatory cytokine CCL5
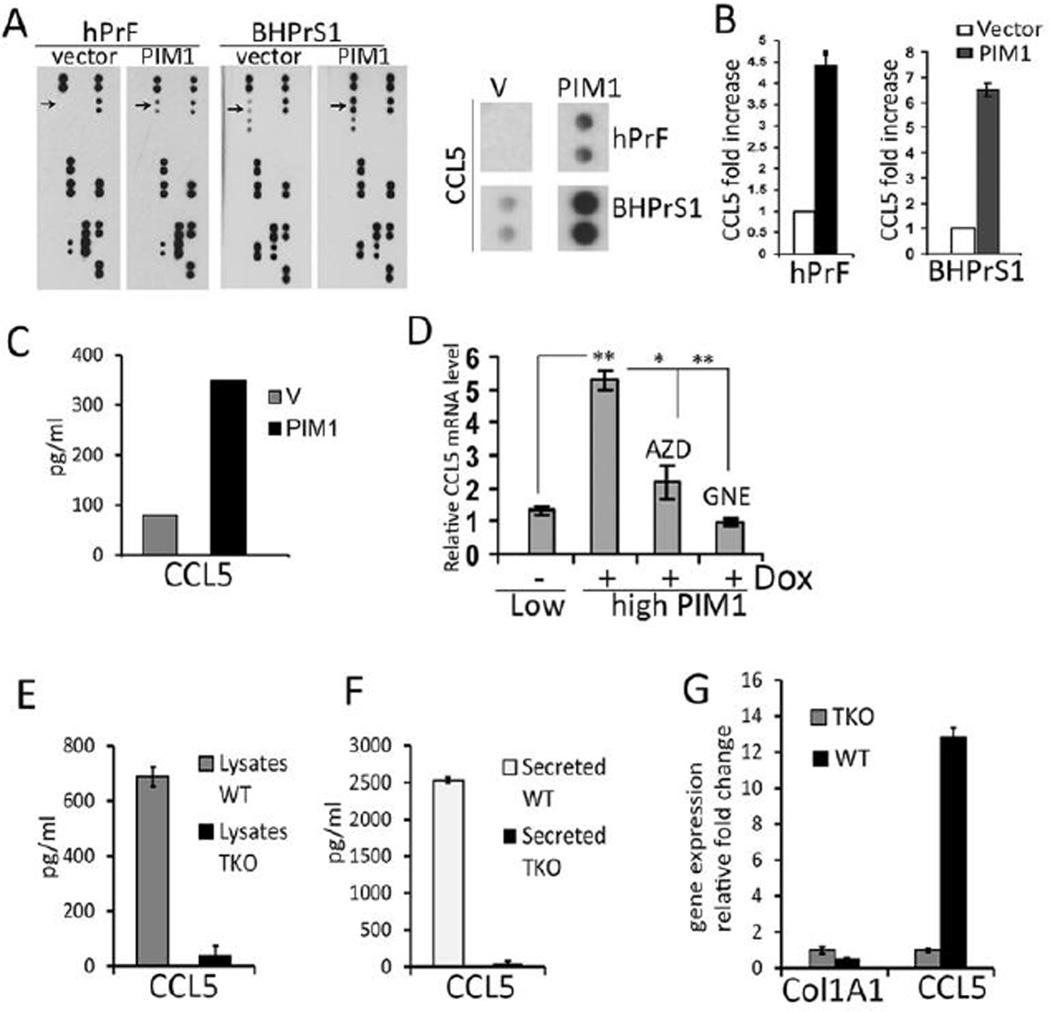
In addition to ECM remodeling, CAFs can provide paracrine stimulation of PCa growth by secretion of specific growth factors. To investigate whether increased expression of the PIM1 protein kinase change the secretion of growth factors or cytokines from prostate fibroblasts, the CM produced by hPrF and BHPrS1 fibroblasts expressing vector or the PIM1 protein kinase (Fig. 3A) were evaluated by proteome profiling (R&D Systems). This analysis demonstrated that CCL5, a chemokine that stimulates PCa cell growth and migration (28), was elevated by PIM1 expression, while no significant changes were seen in other 36 hormones and growth factors (Fig. 3A and B). CCL5 belongs to the C-C chemokine family and plays an active role in recruiting a variety of leukocytes into inflammatory sites including T cells, macrophages, eosinophils and basophils (29). Using ELISA assays, CCL5 levels in the CM were shown to increase 4.5 – 6-fold after PIM1 expression (Fig. 3C). qRT-PCR analysis demonstrated that PIM1 expression in BHPrS1 fibroblasts induced a 5-fold increase in CCL5 transcripts, and this induction was blocked by two structurally different PIM1 inhibitors, suggesting that the levels of CCL5 protein were being controlled transcriptionally (Fig. 3D). To further confirm that PIM1 kinase regulated CCL5 expression we employed MEFs deficient in PIM1, −2, and −3 (TKO) (23), and WT littermate controls. Consistent with the observation that PIM1 expression increased CCL5 transcription in human prostate fibroblast cells, TKO MEFs had significantly lower levels of intracellular and secreted CCL5 compared to WT MEFs (16.9 fold decrease in lysates, 54.7 fold decrease in the CM (Fig. 3E and F). TKO MEFs contained significantly less CCL5 mRNA when compared to WT, while the level of COL1A1 transcript was not changed (Fig. 3G). Taken together these results suggest that PIM1 kinase can control the expression of the chemokine CCL5.
Figure 3. PIM1 increases secreted CCL5 level and CCL5 transcription.
(A) Primary hPrF and immortalized BHPrS1 stromal fibroblasts were transduced with lentiviruses expressing inducible PIM1 kinase or vector control. Cells were incubated with Dox (20ng/ml) for 72 h in serum free media, CM was collected, concentrated and analyzed for cytokine levels using a human cytokine array (R&D system). Changes in CCL5 level are indicated by arrows (left panel) and by the enlarged image of cytokine array section (right panel). (B) Quantitation of CCL5 levels by spot densitometry analysis. (C) BHPrS1 stromal fibroblasts were transduced with lentiviruses expressing inducible PIM1 kinase or vector control. Cells were incubated with Dox (20ng/ml) for 48 hours in a serum free media then the CM was collected, concentrated and analyzed for CCL5 levels using human CCL5 ELISA (RayBiotech) accordingly with the manufacturing protocol. (D) BHPrS1 cells expressing inducible PIM1 kinase were incubated with PIM1 inhibitors 3µM AZD 1208 (AZD), 1 µM GNE- 652 (GNE) for 48 h. mRNA levels were analyzed by qRT-PCR and are expressed relative to those found in PIM1-non-induced control cells. Each value represents the mean ± SD of 6 pooled measurements produced from two independent experiments. *, P< 0.01, **, P < 0.001; P values were calculated by t tests and represent the probability of no difference between mRNA levels in non-induced (low PIM1) and PIM1 over-expressing cells (high PIM1). Detection of CCL5 levels in the lysates (E) or CM (F) obtained from WT and TKO MEFs. MEF cells were seeded in 10 cm culture dishes at the density of 1 ×106 cells and cultivated in completed growth media for 48 hours. Cells were lysed in cold NP40 lysis buffer (Life Technologies) and subjected to centrifugation at 16,000 × g for 10 min at 4°C. Resulting lysates (200 µg protein) were used for Immunoassay (E). Cultured medium (15ml) was collected and 250µl was used for Immunoassay (F). Duplicate samples were assayed for 23 mouse cytokines/chemokines including CCL5 using a multiplex mouse assay (Bio-Plex Pro Mouse Cytokine Standard 23-Plex;Bio-Rad Laboratories), according to the protocol of the manufacturer. Samples were analyzed using the Bio-Plex Protein Array System and the related Bio-Plex Manager (Bio-Rad). (G) COL1A1 and CCL5 mRNA levels in WT and TKO MEFs as determined by qRT-PCR analysis. Values are the average of two independent experiments and the standard deviation from the mean is shown.
3.4. PIM1 kinase regulates PDGF receptors in prostate fibroblast cells
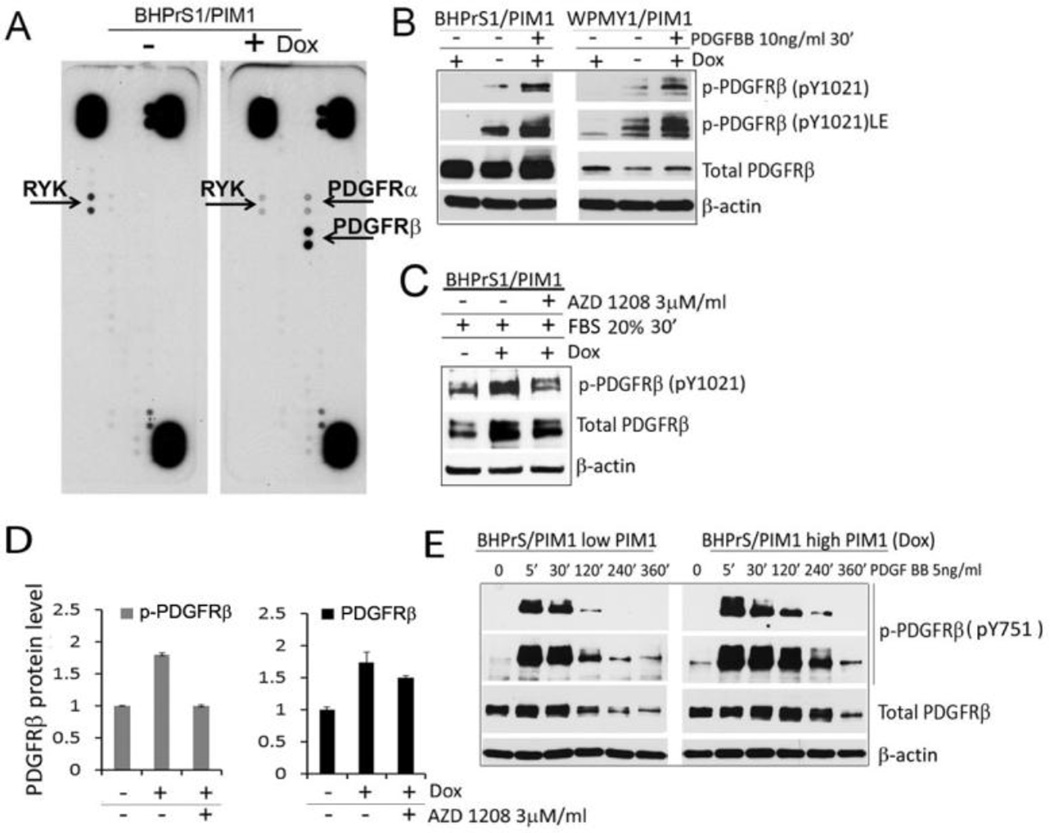
Because we had previously established that PIM1 expression in PCa epithelial cells can control the levels of receptor tyrosine kinases (27, 30), we studied whether elevated PIM1 levels also mediated changes in tyrosine receptors (RTKs) in prostate fibroblasts. BHPrS1/PIM1 cells with and without PIM1 induction by Dox were examined by phosphoreceptor tyrosine kinases arrays (R&D Systems) (Fig. 4A). Analysis of these arrays demonstrated that PIM1 elevated the phosphorylation of the PDGFRα/β receptors and decreased the phosphorylation of RYK (Fig. 4A). Since increases in PDGFRβ level have been documented in CAFs and implicated in CAFs/myofibroblast phenotype (31, 32), we focused further studies on this receptor. Western blots demonstrated that Dox induction of PIM1 expression caused an increase in phosphorylated and total PDGFRβ levels in both WPMY1 and BHPrS1 prostate fibroblasts and this PIM1-induced PDGFRβ up-regulation was abolished in cells treated with the PIM kinase inhibitor AZD 1208 (Fig.4 B–D). The addition of PDGF ligand (PDGFBB) to starved cells caused internalization of this receptor, suggesting that it was active and functional on the cell surface (Fig 4E). Similar to prostate epithelial tumor cells in stromal fibroblasts, PIM1 regulated the levels and the activity of specific RTKs.
Figure 4. PIM1 kinase expression leads to increases PDGFR level and phosphorylation.
(A) BHPrS1stable pools expressing inducible PIM1 kinase were incubated with or without Dox (20 ng/ml) for 48 hours, then lysed and 300µg of total proteins were assayed by the human phospho-RTK array (R&D Biosystem). Arrows indicate RYK and PDGFR kinases. (B) BHPrS1/PIM1 and WPMY1/PIM1 cells with or without PIM1 induction were incubated in a serum free media overnight, and then treated with PDGFRβ ligand (PDGFBB) for 30 min. Cell lysates were analyzed by Western blotting for the indicated proteins. (C) BHPrS1/PIM1 cells with and without PIM1 induction by Dox were incubated in serum free media overnight in the presence of DMSO (vehicle control) or 3µM AZD1208, the cells were then stimulated with 20% FBS for 30 min and assayed by Western blotting with the indicated antibodies. (D) Spot densitometry analysis was performed for PDGFRβ protein levels seen in C and intensity was normalized to the β-actin loading control. (E) BHPrS1/PIM1 cells with and without PIM1 induction by Dox were incubated in serum free media overnight and PDGFRβ activation was induced by PDGFBB ligand for indicated time period.
3.5. PIM1 enhances the induction of myofibroblast/CAF markers when prostate fibroblasts are co-cultivated with epithelial cells
An elevated level of alpha smooth muscle actin (αSMA) has been described in prostate myofibroblastic CAFs, and this elevation has been correlated with increases in COL1A1 and PDGFRβ levels (2, 31, 32). However, short term over- expression of PIM1 either in primary hPrF or the WPMY1 or BHPrS1 cells did not raise αSMA levels, suggesting that increases in the PIM1 protein kinase were not sufficient to induce the transition to myofibroblast phenotype (Supplementary Fig. S2A). To investigate whether PIM1 induced COL1A1 and CCL5 secretion in fibroblasts could stimulate prostate epithelial cell growth/migration, prostate non-tumorigenic epithelial BPH1 cells were incubated with the CM produced by BHPrS1 fibroblasts with or without PIM1 over-expression. BPH1 cell line was chosen as a target of paracrine activity because prostatic CAFs have been shown to irreversibly convert BPH1 cells from benign to malignant phenotype (20). However, the incubation of the CM produced by PIM1 overexpressing fibroblasts with BPH1 cells did not stimulate the growth or migration rates of BPH1 (Supplementary Fig. S2B and C).
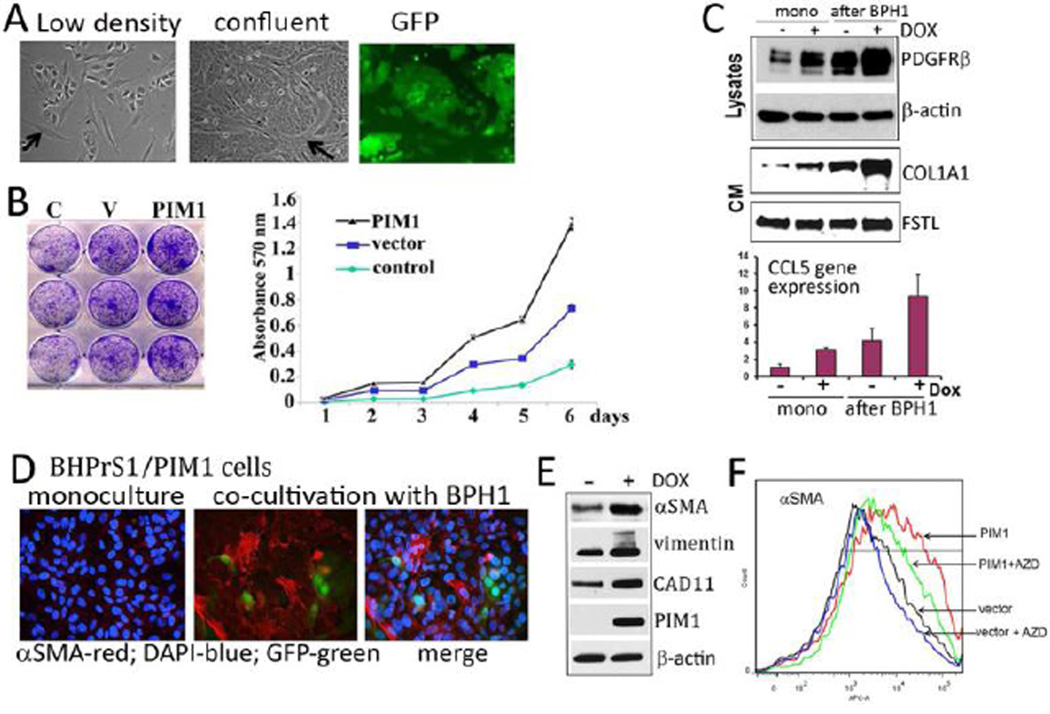
To further evaluate the interaction between prostate epithelial cells and stromal fibroblasts, a co-cultivation assay was employed. BPH1 cells were labeled with GFP to allow separation of epithelial and stromal populations by cell sorting. BHPrS1 with or without PIM1 expression and BPH1/GFP cells were grown together for three passages then separated and evaluated as described in the 2.4. Materials and Methods. Figure 5A demonstrates that when grown together the fibroblasts cells surround the nests of epithelial cells in a similar fashion to that seen in the prostate gland. We found that after co-cultivation with BHPrS1/PIM1 fibroblasts, prostate epithelial BPH1 cells displayed an enhanced growth rate compared to BPH1 cells grown alone (control) or to BPH1 cells co-cultivated with BHPrS1 fibroblasts expressing vector control (Fig. 5B). Results also demonstrated that the PIM1 mediated induction of PDGFRβ, COL1A1 and CCL5 in BHPrS1 fibroblasts was greatly enhanced after co-cultivation with epithelial cells compared to fibroblasts grown alone (Fig. 5C).
Figure 5. PIM1 primes stromal fibroblasts for myofibroblast differentiation in a co-cultivation system.
(A) 2 ×105 BPH1/GFP cells were mixed with 2×105 BHPrS1 cells and Dox (20ng/ml) was added to the culture to induce PIM1. The low density cultures are shown in the left panel, and confluent cultures (middle and right panel; phase-contrast and GFP image respectively). Arrows identify stromal cells that are surrounding the epithelial cells. (B) After 3 passages with stromal-epithelial cell co-cultivation, GFP-positive epithelial BPH1 cells were isolated from BHPrS1 fibroblasts as described in 2.4 Materials and Methods and plated in triplicates for growth assays. The left panel demonstrates cell density using crystal violet staining and the right panel indicates dye absorbance as a measure of cell growth. The control (C) is BPH1/GFP cells grown without co-cultivation with fibroblasts. Vector (V) represents BPH1/GFP cells grown after co-cultivation with BHPrS1 cells expressing normal level of PIM1, and “PIM1- BPH1/GFP” indicates cell growth rate after co-cultivation with BHPrS1 fibroblasts over-expressing PIM1 kinase. (C) BHPrS1/PIM1 fibroblasts cultured alone (mono) or after co-cultivation with BPH1 epithelial cells were analyzed by Western blotting for the changes in PDGFR, COLA1A and actin. The mRNA expression of CCL5 was quantitated by qRT-PCR. PIM1 expression was induced by Dox (20ng/ml). (D) Immunofluorescent images of prostate fibroblast and epithelial cell mixtures performed with CY3 conjugated anti αSMA antibodies to visualize αSMA expression (Red) and followed by DAPI staining to visualize nuclei (blue). Epithelial BPH1/GFP cells are green. (E) The level of proteins in low PIM1 (−DOX) and PIM1 over-expressing (+DOX) fibroblast cells after co-cultivation with BPH1 epithelial cells measured by Western blotting with the indicated antibodies. (F) BHPrS1 cells expressing vector control (vector) or PIM1 kinase (PIM1) were co-cultivated for 2 passages in the presence of Dox (20ng/ml). Then, AZD1208 (3 µM) was added at passage 3 for 5 days. Fibroblasts were separated from epithelial cells, cultivated as described in 2.4. Materials and Methods, and then assayed by flow cytometry for αSMA expression using anti-αSMA APC-conjugated antibodies.
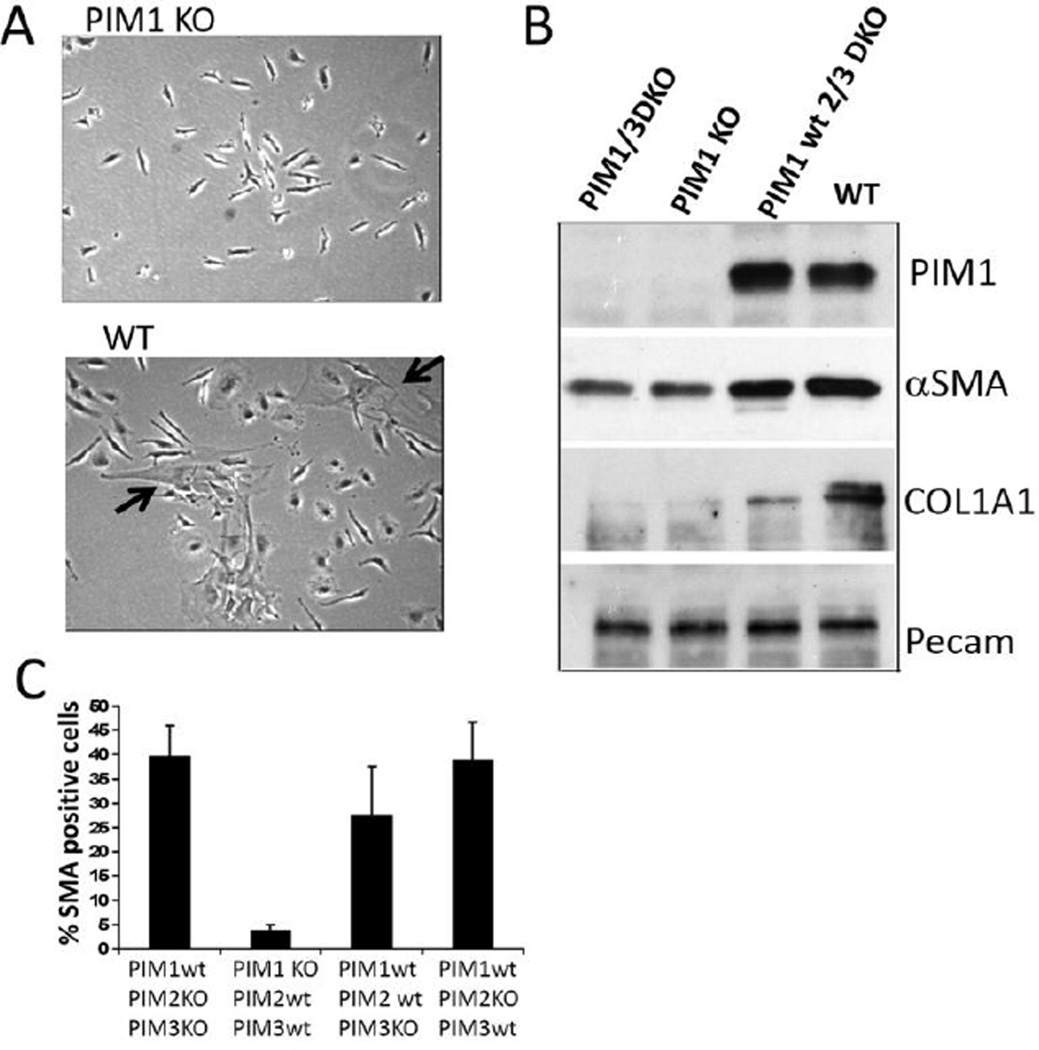
Although BHPrS1 cells with or without induced PIM1 over-expression did not produce elevated levels of αSMA when grown alone, BHPrS1 fibroblasts formed αSMA-positive contractile bundles and were positive for markers associated with the myofibroblastic/CAF phenotype such as CAD11 and vimentin, when co-cultivated with BPH1 epithelial cells (Fig. 5D). Induction of PIM1 expression in the co-cultivated fibroblasts markedly increased the level of these myofibroblast-specific markers (αSMA, CAD11, and vimentin) when compared to co-cultivated cells with low PIM1 level (Fig. 5E and F). Consistent with this result, we found that in bone marrow (BM) mesenchyme isolated from PIM1 knockout mice the number of SMA-positive cells was significantly reduced relatively to PIM1 wild type BM stroma (Fig. 6A–C). To further confirm that BHPrS1/PIM1-BPH1 cell mixtures are dependent on PIM1 activity for the expression of myofibroblastic phenotype, the pan-PIM1 inhibitor AZD1208 was applied to the co-cultures. The addition of the PIM1 inhibitor decreased the number of αSMA-positive fibroblasts (Fig. 5F and Fig. 7G and H) reduced both, the secreted COL1A1, COL4A, CAD11 levels and the expression of PDGFRβ and αSMA (Fig. 7E,F and H). These data suggest that the extent of the formation of myofibroblast phenotype during fibroblast-epithelial cell co-culturing was in part PIM1 dependent.
Figure 6. BM stroma lacking PIM1 expression exposes decreased number of myofibroblasts.
(A) Phase contrast microscopic images of bone marrow (BM) mesenchymal cells at passage 6 harvested from femurs of PIM1KO (upper panel) or PIM1 WT (lower panel) mice. The big, flat cells with myofibroblastic phenotype are indicated by arrows. (B) WB analysis of the lysates obtained from primary BM cells (passage2) harvested from PIM1 knockout mice or WT mice for indicated protein expression. (C) BM mesenchymal cells at passage 7 collected from mice with the indicated phenotypes were immunostained with CY3 conjugated anti αSMA antibodies to visualize αSMA expression. The number of αSMA positive cells in each cell population was estimated by counting of 200 cells from three independent fields.
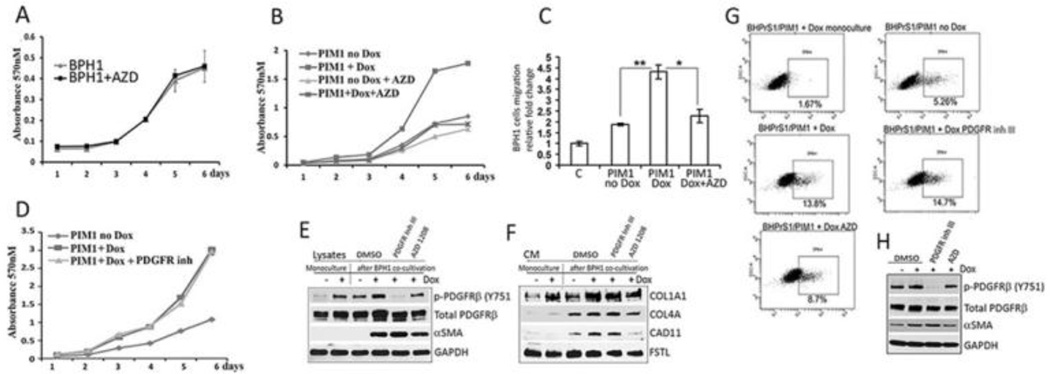
Figure 7. Co-cultures with prostate fibroblasts expressing PIM1 enhance the growth of BPH1 cells.
(A) The growth rate of BPH1 cells with and without the PIM1 inhibitor AZD1208 was measured by crystal violet staining as described in the supplementary 2.6. Materials and Methods. (B) BPH1/GFP epithelial cells were co-cultivated with the BHPrS1/PIM1 cells either with or without Dox (20ng/ml) and the PIM inhibitor AZD1208 using conditions described in the Fig. 5B as well as found in the 2.4 Materials and Methods. After cell separation, the GFP-positive BPH1 cell growth rate was measured using crystal violet staining at the indicated times. (C) BPH1/GFP epithelial cells were co-cultivated with the BHPrS1/PIM1 cells either with (Dox) or without PIM1 induction (no Dox) and with (AZD) or without PIM1 inhibition using conditions described in the Fig. 5B and Fig. 7B. At the end of the treatment the migration of epithelial cells were assayed using Boyden chambers. 5×104 GFP-positive epithelial BPH1 cells were seeded in a serum free media into upper chamber and the media containing 2% of serum was added into the lower chambers. Migration toward serum gradient was examined over 24 hours as described in supplementary 2.6 Materials and Methods. Figure legends indicate: Control (C) – BPH1/GFP cell migration without co-cultivation with BHPrS1; PIM1 no Dox – BPH1/GFP cells migration rate after co-cultivation with BHPrs1/PIM1 control cells without PIM1 induction; PIM1 Dox- BPH1/GFP cells migration rate after co-cultivation with BHPrS1 overexpressing PIM1 kinase, and PIM1 Dox + AZD – BPH1/GFP cells migration after co-cultivation with BHPrS1 cells over-expressing PIM1 kinase when PIM inhibitor 3µM AZD1208 was added into co-cultures. Values are average from the two independent experiments and the standard deviation from the mean is shown. . *, P< 0.05, **, P < 0.01; P values were calculated by t tests.
(D) The growth rate of BPH1/GFP cells co-cultivated with BHPrS1/PIM1 fibroblasts with or without PDGFR inhibitor III (1 µM, Santa Cruz) was determined as described in Fig. 7B and 2.4 Materials and Methods section. All experiments were done in triplicate and the average of these determinations +/− S.D is shown. Western blot analysis of the lysates (E) or CM (F) produced by BHPrS1/PIM1 fibroblasts after co-cultivation with BPH1 epithelial cells as described in B and D are shown for indicated proteins. (G) Flow cytometry analysis for αSMA expression in the BHPrS1/PIM1 fibroblasts after co-cultivation with BPH1/GFP epithelial cells using APC conjugated anti αSMA antibodies. The percentage of high αSMA positive cells is indicated. (H) Western blot analysis for indicated proteins in cell lysates obtained from the samples used in G.
3.6 The PIM1 protein kinase expressed in prostate fibroblasts can regulate the priming of BPH1 epithelial cell growth
To further examine the interaction between PIM1 expressing stromal fibroblasts and the BPH1 epithelial cells, BPH1 cells were re-isolated after the co-cultivation with BHPrS1 fibroblasts by flow sorting and then grown separately. Growth curves demonstrated that re-isolated BPH1 cells that had been co-cultivated with BHPrS1 fibroblasts over-expressing PIM1 kinase grew faster than control BPH1 cells or BPH1 cells co-cultivated with BHPrS1 cells expressing normal level of PIM1 (Fig. 5 B). To investigate whether the growth stimulation of BPH1 cells by PIM1 over-expressing fibroblasts was PIM1 dependent, pan-PIM inhibitor AZD1208 was added to the co-cultivated cells. Although this compound had no effect on BPH1 cell proliferation when cells were grown alone (Fig. 7A), the addition of AZD1208 to the cell co-culture abolished the ability of PIM1 over-expressed fibroblast cells to stimulate the growth of BPH1 cells (Fig. 7B). This result suggests that elevation of PIM1 kinase in the prostate fibroblasts caused changes in the epithelial cells that allowed them to grow at a faster rate (Fig. 5B and Fig. 7B). Additionally, we found that BPH1 cells after co-cultivation with BHPrS1/PIM1 fibroblasts demonstrated an increased migration rate, and this ability could be partially blocked by AZD1208 (Fig. 7C). In contrast to the PIM inhibitor, blocking the activity of the PDGFR with a small molecule inhibitor did not reduce BHPrS1/PIM1-induced stimulation of BPH1 epithelial cell growth (Fig. 7D). This data is in agreement with the observation that unlike PIM1 inhibition the blocking of PDGFRβ activity is not sufficient to reduce the expression of the myofibroblastic phenotype related markers αSMA, COL1A1 COL4A, and CAD11 (Fig. 7E–H), suggesting that formation of myofibroblasts in cell co-cultures can play a crucial role in priming of epithelial cell growth. Our results demonstrate that stromal fibroblasts when co-cultivated with epithelial cells undergo a transition to a myofibroblast/CAF-like phenotype. These differentiated cells are capable of priming epithelial cells for faster growth and migration. The fibroblast to myofibroblast transition and the priming activity of fibroblasts to stimulate prostate epithelial cells are both enhanced by increased PIM1 levels.
3.7. PIM1 stimulates increased translation of COL1A1 and PDGFRβ mRNAs
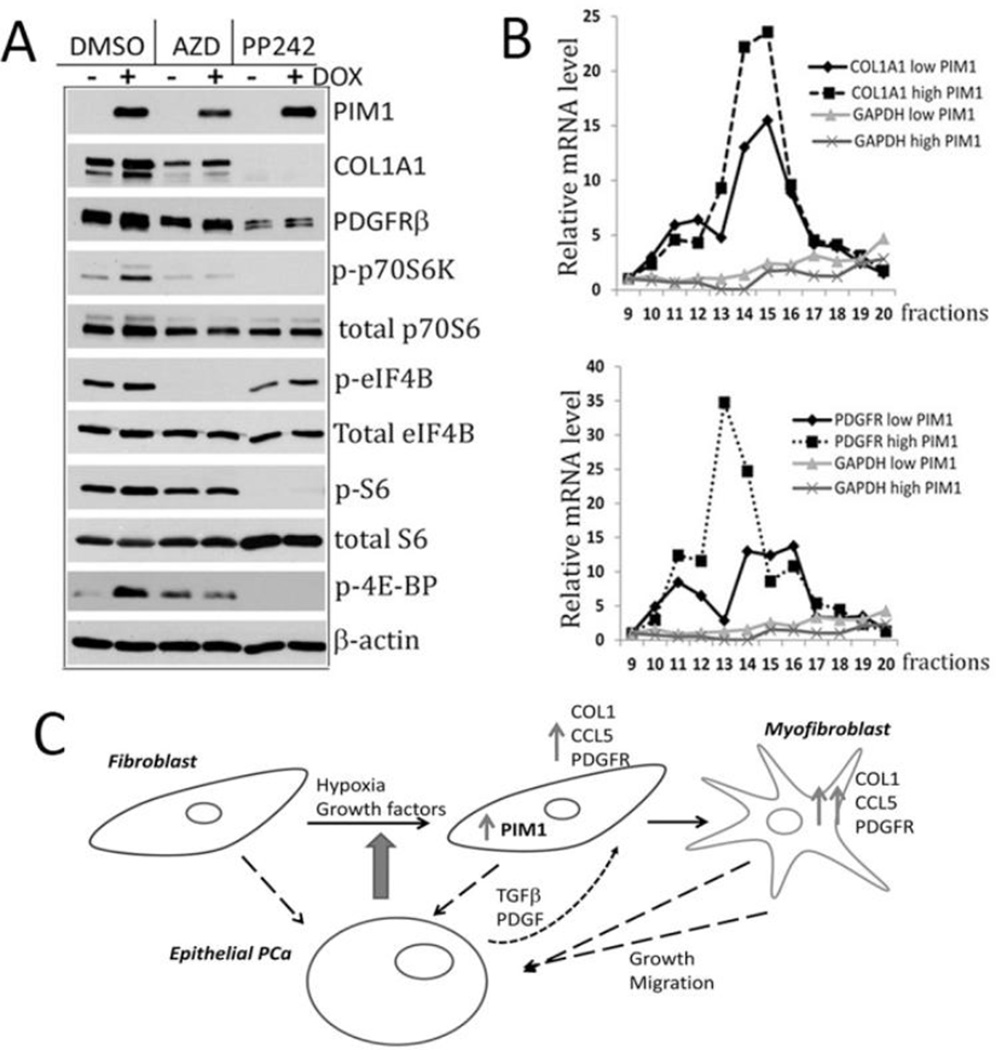
To investigate the biochemical mechanism by which PIM1 increases COL1A1 and PDGFRβ expression qRT-PCR analysis were carried out. Results demonstrated that neither the induction of PIM1 in stromal fibroblasts nor treatment with AZD1208 changed the level of the mRNAs encoding these proteins (Supplementary Fig. S3A and B). These results are in agreement with the observation that COL1A1 and PDGFRβ transcript levels were identical in a microarray analysis of TKO and WT MEFs (data not shown and Fig. 3G). To investigate whether PIM1 might be increasing these proteins by inhibiting their degradation, BHPrS1/PIM1 cells were treated with cycloheximide and the level of these proteins analyzed by Western blotting. These studies did not reveal any changes in the half-life of PDGFRβ or COL1A1 proteins when PIM1 expression was induced (Supplementary Fig. S3C). The PIM protein kinases have been shown to regulate translation through modulating the phosphorylation of PRAS40, TSC-2, 4E-BP1, and eIF4B (13, 27, 33, 34), leading to stimulation of mTOR activity and enhancing 5’Cap induced translation. Western blot analysis of extracts of PIM1 overexpressing BHPrS1 cells compared to control cells not only demonstrated an increase in COL1A1 and PDGFRβ protein levels, but also showed increased phosphorylation of the PIM1 target eIF4B (S406) (27), a protein that regulates the mRNA helicase eIF4A that is needed for 5’ Cap dependent translation (Fig. 8A). PIM1 over-expression in these cells caused marked increases in p70 S6 kinase and 4E-BP1 phosphorylation suggesting an activation of mTORC1 signaling. S6 protein is phosphorylated as a result of increased S6K activity (Fig. 8A). A similar observation of mTORC1 pathway activation was detected in PIM1 over-expressing WPMY1 fibroblasts (Supplementary Fig. S4). These increases in phosphorylation of proteins involved in the regulation of translation were blocked by the addition of PIM inhibitor AZD1208. Inhibition of mTOR activity by the TORC1/TORC2 inhibitor PP242 abolished PIM1 induced elevation of PDGFRβ and COL1A1 protein levels (Fig.8A). These data suggest that PIM1 kinase could regulate translation efficiency of PDGFRβ and COL1A1 transcripts through activation of mTOR pathway.
Figure 8. PIM1 controls the translation of PDGFRβ and COL1A1 in prostate stromal fibroblasts.
(A) BHPrS1/PIM1 cells with or without PIM1 induction by DOX were incubated with DMSO (vehicle control), PIM inhibitor AZD 1208 (3µM) or mTORC1, 2 inhibitor PP242 (2µM) for 16 hours. Cell lysates were probed by Western blotting for the indicated proteins. (B) BHPrS1/PIM1 fibroblasts were treated with or without Dox (20ng/ml) for 5 days to induce PIM1 expression. Total cell lysates were run on sucrose gradients to isolate ribosome fractions and the levels of COL1A1, PDGFRβ and GAPDH mRNA associated with each fraction was determined by qRT-PCR. (C) A model demonstrating the role of PIM1 in PCa-associated stromal fibroblasts.
Although prior studies had suggested a role for PIM1 in controlling translation, actual changes in the binding of specific mRNAs to the polysomes has not been evaluated. Because the overall translational efficiency of an individual mRNA is reflected by the amount of translated mRNA bound to light and heavy polysomes, we examined the distribution of COL1A1 and PDGFRβ mRNA using the cytosolic extracts of BHPrS1 with and without PIM1 induction. Cell extracts were subjected to sucrose density centrifugation, fractions collected and RNA prepared from each fraction (Supplementary Fig. S5). The amount of COL1A1, PDGFRβ transcripts was evaluated using GAPDH as a control, and mRNAs bound to polysomes in fractions 9 through 20 (mono and polyribosomes) were quantified by qRT-PCR. As shown in Fig. 8B, elevated PIM1 levels in prostate fibroblasts significantly increased binding of the both COL1A1 and PDGFRβ mRNA to polysomes, whereas accumulation of GAPDH mRNA did not change. Thus, the ability of PIM1 to elevate COLA1 and PDGFRβ protein levels appeared in part to be based on the ability of PIM1 to regulate the activity of the 5’Cap and enhance translation of these mRNAs. In summary, the PIM1 kinase through its ability to regulate the transcription of specific genes, i.e. CCL5, and the translation of specific proteins, i.e. PDGFRβ and COL1A1, primes prostate fibroblast cells to undergo a myofibroblast/CAF-like transition leading to stimulation of prostate epithelial cell growth and migration (Fig.8C).
4. Discussion
Although the PIM1 protein kinase has been implicated in the epithelial component of PCa progression, this is the first study highlighting the importance of this protein kinase in regulating the phenotype of prostate fibroblasts. Our finding that the PIM1 level is elevated in the fibroblasts of PCa patients suggested the potential importance of this kinase in regulating the function of prostate stromal fibroblasts. Transduction of the PIM1 cDNA into normal prostate fibroblasts either freshly isolated or immortalized induced increases in CCL5 secretion, intracellular and extracellular COL1A1, and activated PDGF receptors that are hall marks of the myofibroblast and CAF phenotype (2, 6, 31). As suggested by the low levels of αSMA, CAD11, and periostin, short-term expression of PIM1 kinase did not induce full differentiation to myofibroblasts. The fibroblasts with elevated PIM1 level demonstrated enlarged number of cells with a myofibroblast/CAFs-like phenotype when co-cultivated with epithelial cells, suggesting that PIM1 activity had a priming effect on the ability of these fibroblasts to differentiate into myofibroblasts in response to the stimuli produced by epithelial cells. This type of interaction between fibroblasts and epithelial cells, has been previously documented in fibroblast-keratinocytes or fibroblast-lung epithelial co-cultures (35, 36) where myofibroblast differentiation was induced. Also, a co-implantation assay demonstrated that human mammary fibroblasts progressively convert to myofibroblasts when fibroblast-epithelial cell mixtures were inoculated into animals (37). PDGF and TGFβ are thought to play an important role in stromal fibroblast differentiation (6, 31). However, PIM1 expression in prostate fibroblasts did not change the production of TGFβ ligands or TGF receptors (data not shown). Because PIM1 over-expressing fibroblasts produce elevated levels of COL1A1, it is possible that increases in ECM stiffness could play a role in enhancing the myofibroblast phenotypic conversion by improving the efficiency of latent TGFβ1 activation (8). PIM1 could sensitize stromal fibroblasts to the TGFβ and PDGF stimulation by modulating a combination of ECM stiffness and PDGFR levels.
It has been reported that the pro-inflammatory cytokine CCL5 induces both proliferation and migration of prostate epithelial cells (28). In vivo, CCL5 may favor tumor development in multiple ways, for example acting as a growth factor, stimulating angiogenesis, modulating ECM, inducing the recruitment of additional stromal and inflammatory cells, and taking part in immune evasion mechanisms, tumor motility, invasion and metastasis (29, 38). CCL5 is a target gene of NFκB activity (39). As shown in our previous study in PCa cells as well as experiments carried out by others, PIM1 kinase is able to activate NFκB through phosphorylation of RelA/p65 at serine 276, preventing the degradation of this protein and enhancing the activation of NFκB signaling (40, 41). Therefore, the increase in CCL5 transcription detected in the prostate fibroblasts could be explained by PIM1-mediated activation of NFκB.
Our data demonstrate that overexpression of PIM1 in prostate stromal fibroblasts does not increase the level of either PDGFRβ or COL1A1 mRNA or the half-life of these proteins (Supplementary Fig. S3). Thus, the molecular basis of PIM1-induced up-regulation of COL1A1 and PDGFRβ levels may be explained in part through the ability of regulation of cap-depended translation. The expression of type I collagen is predominantly regulated at the posttranscriptional level in part by a unique structural element in the 5’ UTR of the collagen mRNA via PI3K/AKT/p70S6K –mediated translational mechanism (42). We did not detect activation of AKT in PIM1 over-expressing fibroblasts (Supplementary Fig. S4). However, the ability of PIM1 kinase to regulate the 5’Cap binding by both modulating the phosphorylation of eIF4B (27) and control p70S6K/S6/4EBP1 phosphorylation through the regulation of TSC2 and PRAS40 (13, 33) pointed to PIM1 kinase as an alternative to AKT regulator of COL1A1 synthesis. Polysome profiling demonstrates for the first time that changes in PIM1 levels actually increase in the recruitment of COL1A1 and PDGFRβ mRNAs to the translational apparatus. It is possible that the PIM1-induced transcriptional up regulation of CCL5 could also play a role in stimulating the translation of COL1A1 and PDGFRβ proteins, as well. In T cells and MCF-7 breast cancer cells CCL5 chemokine stimulates mRNA translation through modulation of the mTOR/4E-BP axis leading to the increased translation of a subset of mRNAs (43, 44).
5. Conclusion
Our studies demonstrate that PIM1 kinase is over-expressed in myofibroblast/CAFs isolated from human prostate cancer tissues, and the kinase activity plays a role in the maintaining of myofibroblast population in the model cell cultures. Altogether these results suggest the potential utility of PIM-targeted inhibitors as unique agents that could block trans-differentiation of fibroblasts in PCa and reduce stroma-induced stimulation of tumor growth.
Supplementary Material
Highlight.
PIM1 is elevated in human prostate myofibroblast/CAF
PIM1 promotes growth of PCa
Acknowledgements
Financial support
DOD W81XWH-08-PCRP-IDA and R01 CA1732000 to A.S.K, an American Cancer Society Institutional Research Grant to M.Y.Z. awarded from the Hollings Cancer Center at the Medical University of South Carolina supported this work. This study was supported in part by NIH Grant 1K01DK085196 to B.C and the Cell Evaluation & Therapy Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313). We would like to thank Dr. Dennis Huszar and AstraZeneca for the gift of AZD1208, and Dr. Alan Ebens at Genentech for providing GNE-652. The gift of the BPH1 and BHPrS1 cells from Dr. Simon Hayward, Vanderbilt University, was critical to this work.
Abbreviations
- PIM1
Proviral Insertion site of the Moloney murine leukemia virus
- CAFs
cancer associated fibroblasts
- BM
bone marrow
- COL1A1
collagen type I alpha 1
- PDGFR
platelet-derived growth factor receptors
- CCL5
chemokine (C-C motif) ligand 5
- BPH
benign prostate hyperplasia
- PCa
prostate cancer
- CAD11
cadherin11
- COL4A
collagen type IV alpha
- αSMA
smooth muscle actin
- Dox
doxycycline
- CM
conditioning media
- qT-PCR
quantitative real-time polymerase chain reaction
- IHC
immunohistochemistry
- TMA
tissue microarray
- GFP
green fluorescein protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
No potential conflicts of interest were disclosed.
References
- 1.Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 2005;173:10–20. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 2.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 3.Josson S, Matsuoka Y, Chung LW, Zhau HE, Wang R. Tumor-stroma co-evolution in prostate cancer progression and metastasis. Semin Cell Dev Biol. 2010;21:26–32. doi: 10.1016/j.semcdb.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron DA, Rowley DR. The reactive stroma microenvironment and prostate cancer progression. Endocr Relat Cancer. 2012;19:R187–R204. doi: 10.1530/ERC-12-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 6.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 7.Tischler V, Fritzsche FR, Wild PJ, Stephan C, Seifert HH, Riener MO, Hermanns T, Mortezavi A, Gerhardt J, Schraml P, et al. Periostin is up-regulated in high grade and high stage prostate cancer. BMC Cancer. 2010;10:273. doi: 10.1186/1471-2407-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otranto M, Sarrazy V, Bonte F, Hinz B, Gabbiani G, Desmouliere A. The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr. 2012;6:203–219. doi: 10.4161/cam.20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen JD, Berns A. Complementation tagging of cooperating oncogenes in knockout mice. Semin Cancer Biol. 1996;7:299–306. doi: 10.1006/scbi.1996.0038. [DOI] [PubMed] [Google Scholar]
- 10.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Roh M, Abdulkadir SA. Pim1 promotes human prostate cancer cell tumorigenicity and c-MYC transcriptional activity. BMC Cancer. 2010;10:248. doi: 10.1186/1471-2407-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He HC, Be XC, Zheng ZW, Dai QS, Han ZD, Liang YX, Ye YK, Zeng GH, Zhu G, Zhong WD. Med. Oncol. 2009;26:303–308. doi: 10.1007/s12032-008-9120-9. [DOI] [PubMed] [Google Scholar]
- 13.Chen WW, Chan DC, Donald C, Lilly MB, Kraft AS. Pim family kinases enhance tumor growth of prostate cancer cells. Mol Cancer Res. 2005;3:443–451. doi: 10.1158/1541-7786.MCR-05-0007. [DOI] [PubMed] [Google Scholar]
- 14.Valdman A, Fang X, Pang ST, Ekman P, Egevad L. Pim-1 expression in prostatic intraepithelial neoplasia and human prostate cancer. Prostate. 2004;60:367–371. doi: 10.1002/pros.20064. [DOI] [PubMed] [Google Scholar]
- 15.Cibull T, Jones T, Li L, Eble J, Ann Baldridge L, Malott S, Luo Y, Cheng L. Overexpression of Pim-1 during progression of prostatic adenocarcinoma. Journal of Clinical Pathology. 2006;59:285–288. doi: 10.1136/jcp.2005.027672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Kobayashi M, Darmanin S, Qiao Y, Gully C, Zhao R, Yeung S, Lee M. Pim1 plays a pivotal role in hypoxia-induced chemoresistance. Oncogene. 2009;28:2581–2592. doi: 10.1038/onc.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Block KM, Hanke NT, Maine EA, Baker AF. IL-6 stimulates STAT3 and Pim-1 kinase in pancreatic cancer cell lines. Pancreas. 2012;41:773–781. doi: 10.1097/MPA.0b013e31823cdd10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lilly M, Le T, Holland P, Hendrickson SL. Sustained expression of the pim-1 kinase is specifically induced in myeloid cells by cytokines whose receptors are structurally related. Oncogene. 1992;7:727–732. [PubMed] [Google Scholar]
- 19.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 20.Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–8142. [PubMed] [Google Scholar]
- 21.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, 2nd, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peehl DM, Sellers RG. Induction of smooth muscle cell phenotype in cultured human prostatic stromal cells. Exp Cell Res. 1997;232:208–215. doi: 10.1006/excr.1997.3525. [DOI] [PubMed] [Google Scholar]
- 23.Mikkers H, Nawijn M, Allen J, Brouwers C, Verhoeven E, Jonkers J, Berns A. Mice deficient for all PIM kinases display reduced body size and impaired responses to hematopoietic growth factors. Mol Cell Biol. 2004;24:6104–6115. doi: 10.1128/MCB.24.13.6104-6115.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peltola K, Hollmen M, Maula SM, Rainio E, Ristamaki R, Luukkaa M, Sandholm J, Sundvall M, Elenius K, Koskinen PJ, et al. Pim-1 kinase expression predicts radiation response in squamocellular carcinoma of head and neck and is under the control of epidermal growth factor receptor. Neoplasia. 2009;11:629–636. doi: 10.1593/neo.81038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas R, True LD, Bassuk JA, Lange PH, Vessella RL. Differential expression of osteonectin/SPARC during human prostate cancer progression. Clin Cancer Res. 2000;6:1140–1149. [PubMed] [Google Scholar]
- 26.Keeton EK, McEachern K, Dillman KS, Palakurthi S, Cao Y, Grondine MR, Kaur S, Wang S, Chen Y, Wu A, et al. AZD1208, a potent and selective pan-Pim kinase inhibitor, demonstrates efficacy in preclinical models of acute myeloid leukemia. Blood. 2014;123:905–913. doi: 10.1182/blood-2013-04-495366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cen B, Xiong Y, Song JH, Mahajan S, DuPont R, McEachern K, DeAngelo DJ, Cortes JE, Minden MD, Ebens A, et al. The Pim-1 Protein Kinase Is an Important Regulator of MET Receptor Tyrosine Kinase Levels and Signaling. Mol Cell Biol. 2014;34:2517–2532. doi: 10.1128/MCB.00147-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66:124–134. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- 29.Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;2014:292376. doi: 10.1155/2014/292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cen B, Mahajan S, Wang W, Kraft AS. Elevation of receptor tyrosine kinases by small molecule AKT inhibitors in prostate cancer is mediated by Pim-1. Cancer Res. 2013;73:3402–3411. doi: 10.1158/0008-5472.CAN-12-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth--bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 33.Zhang F, Beharry ZM, Harris TE, Lilly MB, Smith CD, Mahajan S, Kraft AS. PIM1 protein kinase regulates PRAS40 phosphorylation and mTOR activity in FDCP1 cells. Cancer Biol Ther. 2009;8:846–853. doi: 10.4161/cbt.8.9.8210. [DOI] [PubMed] [Google Scholar]
- 34.Schatz JH, Oricchio E, Wolfe AL, Jiang M, Linkov I, Maragulia J, Shi W, Zhang Z, Rajasekhar VK, Pagano NC, et al. Targeting cap-dependent translation blocks converging survival signals by AKT and PIM kinases in lymphoma. J Exp Med. 2011;208:1799–1807. doi: 10.1084/jem.20110846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shephard P, Martin G, Smola-Hess S, Brunner G, Krieg T, Smola H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am J Pathol. 2004;164:2055–2066. doi: 10.1016/s0002-9440(10)63764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad S, Hogaboam CM, Jarai G. Deficient repair response of IPF fibroblasts in a co-culture model of epithelial injury and repair. Fibrogenesis Tissue Repair. 2014;7:7. doi: 10.1186/1755-1536-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 38.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 39.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Zemskova M, Sahakian E, Bashkirova S, Lilly M. The PIM1 kinase is a critical component of a survival pathway activated by docetaxel and promotes survival of docetaxel-treated prostate cancer cells. J Biol Chem. 2008;283:20635–20644. doi: 10.1074/jbc.M709479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nihira K, Ando Y, Yamaguchi T, Kagami Y, Miki Y, Yoshida K. Pim-1 controls NF-kappaB signalling by stabilizing RelA/p65. Cell Death Differ. 2010;17:689–698. doi: 10.1038/cdd.2009.174. [DOI] [PubMed] [Google Scholar]
- 42.Blackstock CD, Higashi Y, Sukhanov S, Shai SY, Stefanovic B, Tabony AM, Yoshida T, Delafontaine P. Insulin-like growth factor-1 increases synthesis of collagen type I via induction of the mRNA-binding protein LARP6 expression and binding to the 5' stem-loop of COL1a1 and COL1a2 mRNA. J Biol Chem. 2014;289:7264–7274. doi: 10.1074/jbc.M113.518951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murooka TT, Rahbar R, Platanias LC, Fish EN. CCL5-mediated T-cell chemotaxis involves the initiation of mRNA translation through mTOR/4E-BP1. Blood. 2008;111:4892–4901. doi: 10.1182/blood-2007-11-125039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murooka TT, Rahbar R, Fish EN. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem Biophys Res Commun. 2009;387:381–386. doi: 10.1016/j.bbrc.2009.07.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.