Abstract
While smokers are known to find smoking-related stimuli to be motivationally salient, the extent to which former smokers do so is largely unknown. In this study, we collected event-related potential (ERP) data from former and never smokers and compared them to a sample of current smokers interested in quitting who completed the same ERP paradigm prior to smoking cessation treatment. All participants (n = 180) attended one laboratory session where we recorded dense-array ERPs in response to cigarette-related, pleasant, unpleasant, and neutral pictures, and where we collected valence and arousal ratings of the pictures. We identified three spatial and temporal regions of interest, corresponding to the P1 (120-132 ms), early posterior negativity (EPN; 244-316 ms), and late positive potential (LPP; 384-800 ms) ERP components. We found that all participants produced larger P1 responses to cigarette-related pictures compared to the other picture categories. With the EPN component, we found that, similar to pleasant and unpleasant pictures, cigarette-related pictures attracted early attentional resources, regardless of smoking status. Both former and never smokers produced reduced LPP responses to cigarette-related and pleasant pictures compared to current smokers. Current smokers rated the cigarette-related pictures as being more pleasant and arousing than the former and never smokers. The LPP and picture rating results suggest that former smokers, like never smokers, do not find cigarette-related stimuli to be as motivationally salient as current smokers.
Keywords: smoking, ERP, former smokers, LPP, EPN
Smoking contributes to 30% of all cancer deaths in the United States, yet approximately 19% of the population still smokes (CDC, 2007). Each year 40% of smokers make a serious cessation attempt, but less than 6% of them are abstinent one year later (CDC, 1996). The difficulties that smokers face when trying to quit have been attributed in large measure to neuroadaptations that increase the salience of stimuli conditioned during drug use to the point that they influence attention, motivation, and ultimately behavior (Koob & Volkow, 2010; Robinson & Berridge, 1993; Volkow et al., 2010). However, the time course of the development of these neuroadaptations is unknown, as is the ability to recover from them once a person quits smoking.
The increased salience of drug cues is a key feature of many theories of drug dependence. In Robinson & Berridge’s (1993; 2000) incentive-sensitization model, the motivational system becomes sensitized by drug use, causing the user to assign excessive salience to drugs and their related cues. Volkow and colleagues (Koob & Volkow, 2010; Volkow et al., 2010) proposed that the development of addiction includes a key process whereby incentive salience increases to drug-related cues at the expense of the salience of non-drug-related intrinsically rewarding stimuli (e.g., food). Thus, drug-dependent individuals may be more likely to pay attention to and have their behavior motivated by conditioned drug-related cues than unconditioned naturally rewarding stimuli, making it difficult to abstain.
Evidence for an enhanced response to drug-related cues among the drug dependent compared to non-users is strong. Abstinent (Johnsen, Thayer, Laberg, & Asbjornsen, 1997) and nonabstinent (Munafò, Mogg, Roberts, Bradley, & Murphy, 2003) smokers produced slower reaction times to cigarette-related compared to neutral words in the modified Stroop task, suggesting an attentional bias towards smoking cues. A similar attentional bias to drug cues has been found for alcohol abusers (Jones & Bruce, 2006), heroin addicts (Franken, Kroon, Wiers, & Jansen, 2000; Lubman, Peters, Mogg, Bradley, & Deakin, 2000), and alcohol and marijuana users (Jones, Jones, Smith, & Copley, 2003), compared with respective drug nonusers. Event-Related Potential (ERP) studies have also found that substance users (Littel, Euser, Munafo, & Franken, 2012), including cigarette smokers (Littel & Franken, 2007), show greater responses to drug-related pictures than non-users on the late positive potential (LPP or P300/slow positive wave) component, an ERP component peaking approximately 400-700 ms after stimulus onset that is thought to index the motivational salience of a stimulus (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Keil et al., 2002; Schupp et al., 2000). Additionally, cocaine users (Dunning et al., 2011) and the nicotine dependent (Versace et al., 2011) have been shown to produce LPP responses to drug cues on par with intrinsically motivational pleasant and unpleasant stimuli. This evidence supports the notion that, among chronic drug users, drug-related cues are more salient relative to non-drug-related stimuli, particularly when compared to non-users.
The motivational relevance of drug cues among former users in unclear. On the one hand, evidence suggests that some former smokers experience occasional craving for months (Gritz, Carr, & Marcus, 1991) or even years (Hughes, 2010) after quitting, and have a 10% risk of relapse even after 30 years of abstinence (García-Rodríguez et al., 2013), suggesting that sensitivity to drug cues may persist among former smokers. However, the findings concerning the motivational relevance of drug cues among former smokers are mixed. In terms of cognitive measures of stimulus bias, some studies found significantly less attentional (Munafò et al., 2003) and approach bias (Wiers et al., 2013) to smoking cues for former compared with current smokers, but most found no difference (Ehrman et al., 2002; Munafò, Johnstone, & Mackintosh, 2005; Munafò & Johnstone, 2008; Nestor, McCabe, Jones, Clancy, & Garavan, 2011). With other drugs of abuse, one study revealed that former opiate users show less attentional bias to drug cues than current users (Constantinou et al., 2010). The one ERP study that investigated this question found that, similar to never smokers, former smokers produced significantly smaller LPP responses to cigarette pictures than current smokers, suggesting that former smokers found cigarette pictures to be less motivationally relevant than current smokers (Littel & Franken, 2007).
Given the lack of consensus concerning the extent to which former smokers find cigarette-related stimuli to be motivationally relevant, we examined ERP responses to passively viewed cigarette-related, intrinsically motivationally relevant (i.e., pleasant and unpleasant), and neutral pictures among former, never, and current smokers. We sought to extend the findings of Littel and Franken (2007) by using permutation analysis to identify likely temporal and spatial regions of interest (ROIs) where differences in the early processing of motivationally relevant pictures might be evident and potentially biologically important among the groups. We included intrinsically pleasant and unpleasant stimuli to provide comparisons to the cigarette-related stimuli that were more motivationally relevant than neutral stimuli. Additionally, we recruited our participants from the community, rather than from a university population, so that we could better generalize these findings to the larger population of current treatment-seeking and former smokers, who are typically older, less educated, and have a longer smoking history. Finally, we examined potential gender differences on ERP and self-reported affective responses to the pictures, given the equivocal findings about gender differences in cigarette cue reactivity (Field & Duka, 2004; Saladin et al., 2012).
Methods
Participants
All participants provided informed consent, and the current study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board (IRB).
Nonsmokers (Former and Never Smokers)
Former (n=60) and never (n=60) smokers were recruited using local (Houston metropolitan area) radio and newspaper advertisements requesting volunteers who were never or former smokers. They had to be aged 18-65 years, be fluent in English, have a working telephone, not have taken psychotropic, anticonvulsive, or narcotic medication in the past 30 days, have not used a nicotine product in the past year, not have used marijuana or other illicit drugs within the week preceding the screening, not have current visual or auditory problems that could interfere with the completion of study assessments, and have a baseline expired carbon monoxide (CO) less than 4 ppm. To be eligible for the never smokers group, participants must have smoked less than 100 cigarettes in their lifetime. To be eligible for the former smokers group, participants must have smoked 100 or more cigarettes in their lifetime and not be involved in any current smoking cessation activity. The “100 lifetime cigarettes” criterion was chosen because it is the oldest (Bondy, Victor, & Diemert, 2009) and most frequently used criterion for distinguishing never from ever smokers (e.g., CDC, 2011; Gilpin, Lee, & Pierce, 2004). Participants were paid $35 for completing the laboratory session and $5 for showing up on time.
Smokers
For the current smokers, a sample (n=60) that matched the nonsmokers on race, gender, and age was randomly selected using SAS PROC SURVEYSELECT (version 9.3; SAS Institute Inc., Cary, NC) from a larger sample (n=166) of treatment-seeking community volunteers who underwent an identical ERP picture-viewing paradigm prior to enrollment in a smoking cessation study. The eligibility criteria were identical to that used with nonsmokers, except that the current smokers had to report smoking at least 10 cigarettes per day (CPD) for the last 6 months and produce an expired CO of 10 or higher. Current smokers were reimbursed $60 for completing the laboratory session.
Procedures
The procedures were identical for the smokers and nonsmokers, except where indicated.
Telephone Screening
All eligibility requirements, except for the expired CO requirement, were assessed during a 15-min telephone pre-screen. All preliminarily eligible and interested participants were scheduled for an in-clinic laboratory session and requested to limit their intake of caffeinated beverages to no more than two cups for four hours prior to the session and to consume no alcohol for 12 hours prior to this session. Current smokers were instructed to smoke ad libitum prior to arriving for their laboratory session.
Laboratory Session
Following study orientation, interested participants provided informed consent. As a final check of eligibility, participants provided an expired CO sample (Bedfont EC50 Micro III Smokerlyzer; Bedfont Scientific, Medford, NJ). Eligible and interested participants next completed the study questionnaires, the passive picture viewing task, and the picture rating task. The entire laboratory session took approximately 3 hours to complete.
Questionnaires
Participants completed the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977) and the Fawcett-Clark Pleasure Scale (FCPS; Fawcett, Clark, Scheftner, & Gibbons, 1983), a measure of the capacity to enjoy daily activities, and the Environmental Tobacco Smoke Exposure Questionnaire (ETSEQ; Nondahl, Cruickshanks, & Schubert, 2005), a measure of exposure to second-hand smoke that we used as proxy for exposure to environmental smoking cues. Current smokers completed the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Former and never smokers also completed a population measure of depression vulnerability, the Depression Proneness Inventory (DPI; Strong, Brown, Kahler, Lloyd-Richardson, & Niaura, 2004), and former smokers also completed the retrospective Fagerström Test for Nicotine Dependence (FTND) to establish a level of dependence when they were last active smokers (Hudmon, Pomerleau, Brigham, Javitz, & Swan, 2005).
Passive Picture Viewing Task
During their laboratory session, participants viewed one of three randomly chosen sets of pictures presented with a PC computer using E-prime software (version 1.4; Psychology Software Tools, Inc., Pittsburgh, PA) on a 42-inch plasma screen placed approximately 1.5 m from the participant’s eyes, such that the images subtended at an approximately 24° horizontal viewing angle. These three equivalent sets consisted of pictures from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008), the International Smoking Image Series (ISIS; Gilbert & Rabinovich, 1999), the Normative Appetitive Picture System (NAPS; Stritzke, Breiner, Curtin, & Lang, 2004), and our lab (Carter et al., 2006), and are listed in a prior publication (Robinson et al., 2013). Each set included 4 picture categories, cigarette-related (CIG), neutral (NEU), pleasant (PLE), and unpleasant (UNP), with 24 slides each. The 96 slides per set were presented twice during each session, for a total of 192 pictures. During the slide presentation, images were presented in pseudo-random sequences with no more than two pictures of the same category presented consecutively. Each picture was presented for 4 seconds and was followed by a random inter-stimulus interval of 3-5 s. A quarter of the pictures co-occurred with a brief acoustic startle probe (2.5 or 3.5 s after picture onset) consisting of a 50-ms 100 dB(A) white noise with instantaneous rise time binaurally delivered through insert earphones. The entire picture presentation lasted approximately 30 min.
ERP Recording
During the passive slide viewing task, we recorded electroencephalogram (EEG) using a 129-channel Geodesic Sensor Net, amplified with an AC-coupled high input impedance (200 MΩ) amplifier (Geodesic EEG System 200; Electrical Geodesics Inc., Eugene, OR), and referenced to Cz. The sampling rate was 250 Hz, and data were filtered online by using 0.1 Hz high-pass and 100 Hz low-pass filters. Scalp impedances were kept below 70 KΩ, as suggested by the manufacturer.
Picture Ratings
At the end of the laboratory session, we obtained participants’ ratings of each picture using an instrument based on the self-assessment manikin (SAM; Lang, 1980) and administered using E-Prime software. We randomly presented each picture for 3 s, followed by graphic scales on which participants rated each picture on Valence (i.e., pleasantness) and Arousal (i.e., intensity) using a 9-point scale. The Valence scale showed a cartoon figure with expressions ranging from happy (9) to unhappy (1), and the Arousal scale showed a figure with expressions ranging from excited (9) to calm (1). Because of time constraints, each participant rated half of the 96 pictures that they viewed during the passive picture viewing task.
Data Analysis
ERP Scoring
After data collection, a 30-Hz low-pass filter was applied off-line. The data were visually inspected, and channels contaminated by artifacts for more than 50% of the recording were interpolated with the use of spherical splines. Eye blinks were then corrected by using a spatial filtering method as implemented in BESA (version 5.3; BESA GmbH, Gräfelfing, Germany). After eye blink correction, the EEG data were transformed to the average reference, and exported for segmentation and further artifact correction using Brain Vision Analyzer (version 2.0.4; Brain Products GmbH, Munich, Germany). The data were segmented into 900-ms segments starting 100 ms before onset of the picture, and baseline was defined as the 100-ms interval preceding the picture. Using the segmented data, artifacts affecting sensors within specific trials were identified, and a segment was excluded from the subsequent averages if more than 10% of the sensors were contaminated by artifacts. At the end of this process, the average ERPs were calculated at each scalp site for each category (i.e., CIG, NEU, PLE, and UNP). Histograms represent least-square means, and error bars represent SE.
Due to poor quality ERP data, primarily from excessive movement and eyeblink artifacts, 9 participants were excluded from further analysis, leaving 57 current, 58 former, and 56 never smokers who were included in the ERP analyses below.
Statistical analysis and permutation testing
To compare baseline participant characteristics by group, we analyzed continuous variables using one-way analysis of variance (ANOVA), with post hoc pairwise comparisons corrected using the Tukey method, and categorical variables using Fisher’s Exact Test. SAM Valence and Arousal picture ratings were separately analyzed using Group × Gender × Picture Type linear mixed models (SAS PROC MIXED version 9.3; SAS Institute Inc., Cary, NC), with subject as a random effect.
For the ERP data, we performed a permutation-based statistical testing to guide us in identifying time regions and channels (within time regions) to include in our main Group by Picture Type analyses. Permutation testing, also known as randomization testing, is a resampling method whereby 1) an empirical null distribution for hypothesis testing is derived by randomly assigning a large number of times the data matrix obtained for each participant within each experimental condition to different data vectors and 2) the p value of the statistic of interest is evaluated under the empirical null distribution. Permutation tests control the rate of Type I errors, can be used with any statistical test, do not require normality or independence among observations, and can provide exact probability statements because sampling without replacement is used to build the null distribution (Maris, 2004). Permutation tests can also be applied to multivariate data, and are increasingly recommended for evaluating ERP (Keil et al., 2014), which we have done in previous manuscripts (Versace et al., 2010; Versace et al., 2011).
To identify time regions where there were likely Group by Picture Type differences, we performed the following steps. First, we calculated mean global field power (GFP), the sum of the squared potential differences of all 129 channels (Lehmann & Skrandies, 1980), for each time point for each picture type for each participant. Second, we built a permutation distribution by randomly shuffling data labels (i.e., picture type) with data values (i.e., GFP voltage values) within each time point for each participant (Groppe, Urbach, & Kutas, 2011). We conducted a mixed Group (between-subjects) by Picture Type (within-subjects) ANOVA on each time point, storing the highest F value for each permutation of the data vector. This procedure was repeated 10,000 times. Third, the F values of the actual data were calculated at each time point for the Group by Picture Type ANOVA and compared to the 95th percentile of the stored F values generated from the permutation distribution. If the F value for any given time point of the actual data exceeded the 95th percentile of the permutation F’s, it was considered to be significant at the 0.05 level. Consecutive significant time points were considered belonging to the same time region of interest (ROI) and averaged.
Next, we built separate permutation distributions for each time ROI identified in the steps above to determine those channels where there were likely Group by Picture Type differences. First, we built a permutation distribution similar to above, but by randomly shuffling data labels (i.e., picture type) with data values (i.e., mean voltage values of the time ROIs) within each channel for each participant. Third, we identified channels showing significant differences across categories by comparing the actual channel F values to the permutation F.
Once we identified channels showing significant differences for each time ROI, we averaged these channels across the significant consecutive time points for each picture type for each participant. Because we were interested in whether groups showed ERP differences to smoking and affective pictures relative to NEU pictures, and not absolute ERP differences to group (i.e., “reactivity” scores), we standardized ERP responses, within subject. We standardized scores for each subject and each time ROI by subtracting the NEU mean from each motivationally relevant picture mean (i.e., CIG, PLE, and UNP) and by dividing by the standard deviation of the NEU pictures. We entered these standardized scores into separate 3 (Group) × 2 (Gender) × 3 (Picture Type) mixed models analyses, with subject modeled as a random effect, for each time ROI.
For all significant linear mixed models, we examined post hoc differences between least-square means (LSM) using tests of simple effects. To correct for the effects of multiple comparisons on type I error rate, the family-wise α levels (p < .05) of post hoc contrasts were adjusted using the Holm-Bonferroni correction (Seaman, Levin, & Serlin, 1991). To determine whether each picture type differed from NEU, we compared the NEU-standardized LSM of the picture types to zero (i.e., the NEU mean) for each component.
Results
Baseline Participant Characteristics
The participants in this study were primarily African-American men who were 46 years old and unemployed, on average (see Table 1). Current smokers produced significantly higher scores on the FTND than former smokers did on the Retrospective FTND, F(1,114)=84.04, p<.0001. Former smokers reported more lifetime cigarettes smoked than never smokers, F(1,118)=44.50, p<.0001, and less years since last cigarette, F(1,90)=30.28, p<.0001 (never smokers who reported not smoking any cigarettes were excluded from this analysis). There was a significant main effect of group of FCPS, F(1,173)=4.96, p<.01, with Tukey post hoc analysis (p<.05) indicating that never smokers produced a higher FCPS score (i.e., more hedonic capacity) than current smokers. None of the other comparisons differed significantly.
Table 1.
Baseline Demographic and Smoking Characteristics.
| Variable | Never Smokers (n=60) |
Former Smokers (n=60) |
Current Smokers (n=60) |
|---|---|---|---|
| Race/Ethnicity | % (N) | % (N) | % (N) |
| African-American, Non-Hispanic | 58.3% (35) | 51.7% (31) | 55.0% (33) |
| White, Non-Hispanic | 21.7% (13) | 33.3% (20) | 31.7% (19) |
| Other | 20.0% (12) | 15.0% (9) | 13.3% (8) |
| Gender | |||
| Female | 46.7% (28) | 36.7% (22) | 41.7% (25) |
| Unemployed | 61.7% (37) | 51.7% (31) | 53.3% (32) |
| High Environmental Tobacco Smoke Exposure (ETSEQ) |
23.2% (13) | 34.6% (18) | N/A |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 46.09 (11.2) | 46.63 (11.3) | 45.35 (10.9) |
| Nicotine Dependence (FTND) a | N/A | 3.04 (2.3) | 6.29 (1.6)** |
| FTND Cigarettes Smoked per Day a | N/A | 15.10 (12.0) | 18.25 (7.2) |
| Lifetime cigarettes | 7.30 (18.0) | 73232.43 (85029.0) | N/A** |
| Years since last cigarette | 25.16 (14.7) | 9.85 (11.5) | N/A** |
| Depressive Symptoms (CES-D) | 7.57 (7.5) | 11.19 (10.3) | 8.89 (7.9) |
| Depression Proneness (DPI) | 2.38 (1.0) | 2.46 (1.1) | N/A |
| Pleasure Responsiveness (FCPS) | 4.06 (0.4) | 3.87 (0.5) | 3.82 (0.5) b* |
Note. All frequencies are calculated within group (column).
Measured using the Retrospective FTND for former smokers, and the FTND for current smokers.
A Tukey post hoc analysis indicated a significant difference between never and current smokers, p<.05. ANOVA significance
p<.01
p<.0001
ERP Results
Permutation Tests
Time ROIs
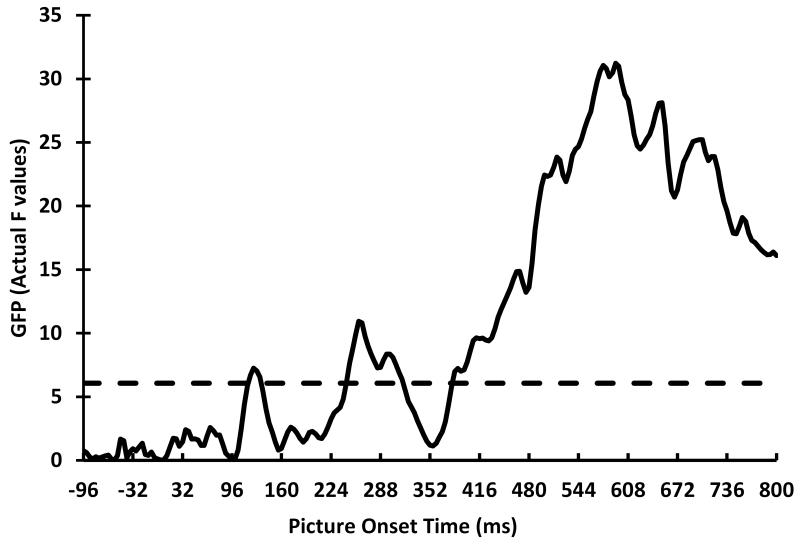
The permutation test of individual time point GFP values for the Group × Picture Type interaction yielded a permutation F of 6.07. Graphed against the actual Group × Picture Type F values, by each time point, the permutation test indicated that there were three significant time ROIs, 120-132 ms, 244-316 ms, and 384-800 ms (see Figure 1), which we refer to as the P1, early posterior negativity (EPN), and late positive potential (LPP) components, respectively. We calculated mean voltages for each channel for each component for inclusion in the permutation test of channel ROIs.
Figure 1.
Actual GFP F values for the Group by Picture Type interaction for each time point. The solid line shows the actual F value at each time point. The dash line indicates the permutation F value.
The P1, EPN, and LPP components are well established in the visual processing literature and have been found to be sensitive to the motivational relevance of visual stimuli (see Olofsson, Nordin, Sequeira, & Polich, 2008, for review). The P1 is a component that has been found to reflect early (90-160 ms) sensory processing with the visual cortex (Di Russo, Martinez, & Hillyard, 2003). The EPN, a measure of selective attention, has been found to negatively peak between 200-350 ms at temporal and occipital sites when viewing visual stimuli and is thought to reflect the early discrimination of motivationally relevant stimuli (Schupp, Junghofer, Weike, & Hamm, 2003; Schupp et al., 2007). The LPP, which overlaps with the P300 reported in non-affective studies, is a component that typically peaks between 350-750 ms over central and parietal sites and is thought to reflect selective processing of motivationally relevant stimuli (Cuthbert et al., 2000; Schupp et al., 2000).
Channel ROIs
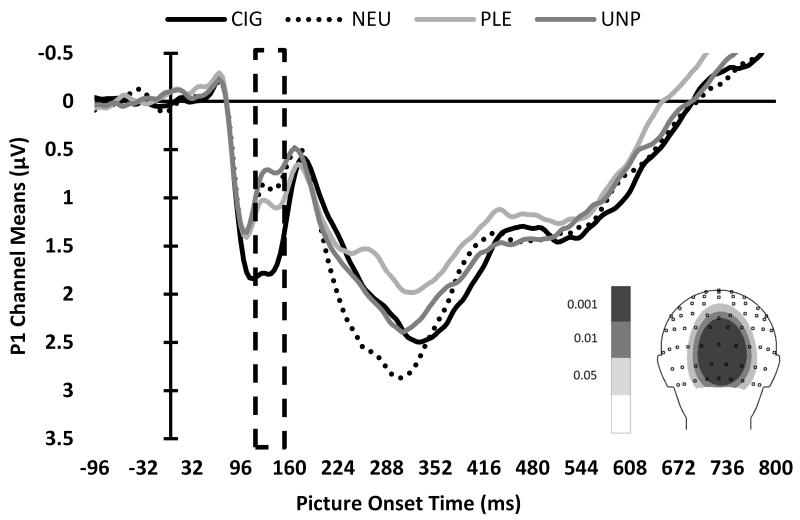
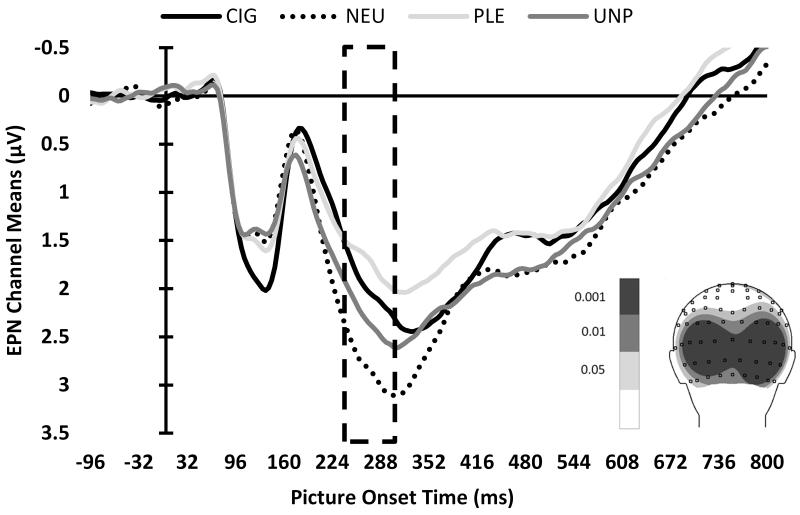
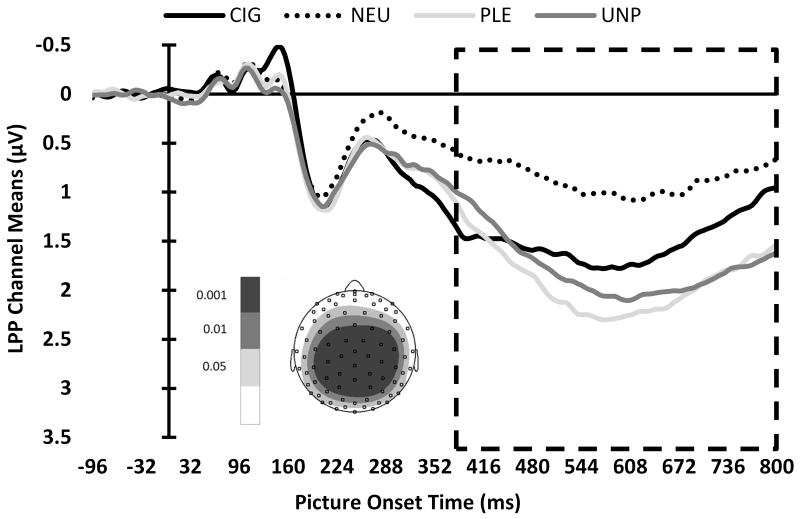
The permutation test of individual channel voltage values for the Group by Picture Type interaction, separately for the mean P1, EPN, and LPP components, yielded permutation Fs of 6.90, 6.48, and 6.46, respectively. We graphed the topographic distributions of the F values to visualize which channels showed significant differences (i.e., channel ROIs) for each component (see Figure 2, insets). Means of channel ROIs were calculated for each component. To further restrict the channels selected for inclusion in these channel ROI means, we only included channels exceeding a more restrictive permutation F threshold, corresponding to the 99.9th percentile.
Figure 2.
ERP waveforms of mean channel ROIs, by picture type, for the (A) P1, (B) EPN, and (C) LPP components. The boxes indicate the time ROIs for each component. Inset: Topographic distributions of the F values to visualize which channels showed significant differences for each component. Channels that exceeded the 99.9th percentile permutation F threshold, indicated by the darkest shading, were included in subsequent analyses.
Group by Picture Type Analyses
We graphed the ERP waveforms of the mean channel ROIs, by picture type, for the P1, EPN, and LPP components (see Figure 2). After standardizing ERP responses relative to NEU pictures (see Statistical Analysis and Permutation Testing, above), we analyzed the standardized scores using a 3 (Group) × 2 (Gender) × 3 (Picture Type) linear mixed model separately for each ERP component.
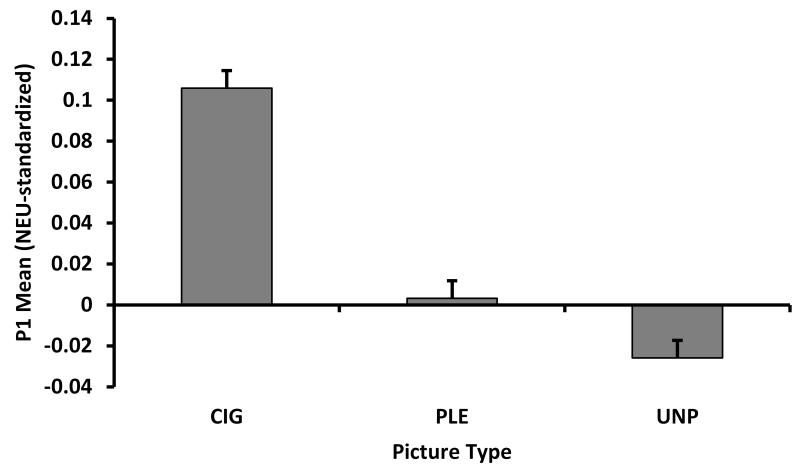
P1 component (120-132 ms)
For the P1 component, we found a significant main effect of picture type, F(2,340)=426.20, p<.0001 (see Figure 3A). Pairwise comparisons indicated that CIG evoked greater standardized scores than PLE, t(340)=21.65, p<.0001, and UNP, t(340)=27.79, p<.0001, and that PLE was greater than UNP, t(340)=6.15, p<.0001. There was no significant group main effect or Group × Picture Type interaction for the P1. When we compared the NEU-standardized LSM of the picture types to zero (i.e., the mean of NEU), CIG t(340)=12.34, p<.0001, and UNP, t(340)=−3.02, p=.0027, significantly differed from zero, indicating that the P1 of CIG and UNP differed from NEU. There were no interactions or main effects of gender on the P1 component.
Figure 3.
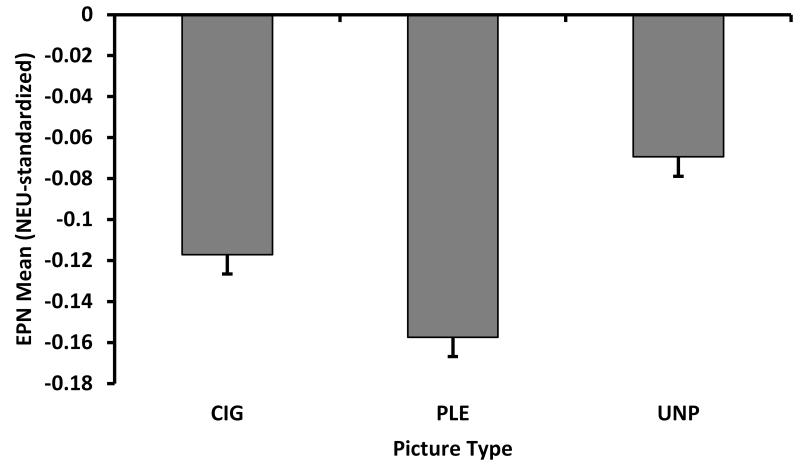
Analysis of the mean standardized scores, by ERP component: (A) The significant picture type main effect for the P1 component (120-132 ms); (B) The significant picture type main effect for the EPN (244-316 ms); and (C) The significant Group × Picture Type interaction for the LPP components (384-800 ms). CIG=cigarette; PLE=pleasant; UNP=unpleasant picture type.
EPN component (244-316 ms)
We found a significant picture type main effect for the EPN component, F(2,340)=265.69, p<.0001 (see Figure 3B). Pairwise comparisons indicated that PLE evoked greater negative standardized scores than CIG, t(340)=−10.53, p<.0001, and UNP, t(340)=−23.02, p<.0001, and CIG evoked greater negative standardized scores than UNP, t(340)=-12.39, p<.0001. There was no significant group main effect or Group × Picture Type interaction for the EPN. When we compared the NEU-standardized LSM of the picture types to zero, all picture types differed from zero (p’s<.0001), indicating that the EPN of CIG, PLE, and UNP differed from NEU. There were no interactions or main effects of gender on the EPN component.
LPP component (384-800 ms)
We found a significant Group × Picture Type interaction for the LPP component, F(4,336)=7.72, p<.0001 (see Figure 3C). Pairwise comparisons, within picture type, indicated that CIG evoked larger standardized scores among current smokers than former, t(336)=2.94, p=.0035, and never smokers, t(336)=3.11, p=.002. Likewise, PLE evoked larger standardized scores among current smokers than former, t(336)=2.39, p=.0176, and never smokers, t(336)=2.60, p=.0096. The UNP pairwise comparisons, within picture type, were not significant after applying the Holm-Bonferroni correction. When we compared the NEU-standardized LSM of the picture types to zero, all picture types differed from zero (p’s<.0001), indicating that the LPP of CIG, PLE, and UNP differed from NEU. There were no interactions or main effects of gender on the LPP component.
Potential covariates of ERP response
To determine whether any of the baseline smoking, affective, or demographic variables functioned as a covariate of ERP response, we reran the above Group × Picture Type Analyses, by ERP component, and included all of the potential moderators detailed in Table 1 as covariates in the models. Because not all measures were administered to every group, we analyzed measures common between groups using separate analyses. In the models comparing all 3 groups for each ERP component, we included age, race/ethnicity, employment status (yes/no), depressive symptoms (CES-D), and Pleasure Responsiveness (FCPS) as covariates. In the models comparing Never vs. Former smokers, we included environmental tobacco smoke exposure (ETSEQ; high vs. low), lifetime cigarettes smoked, years since last cigarette, and depression proneness (DPI). In the models comparing Former vs. Never smokers, we included nicotine dependence (retrospective FTND and FTND, respectively) and cigarettes smoked per day (from the FTND). We found no main effects for any of the baseline measure on any of the three ERP components, indicating that none of the baseline measures in Table 1 covaried with ERP response for any of the 3 time ROIs. Likewise, the presence of the covariates did not alter the significant main effect of picture type main effect for the P1 or EPN components, or the significant Group × Picture Type interaction for the LPP component.
SAM Ratings
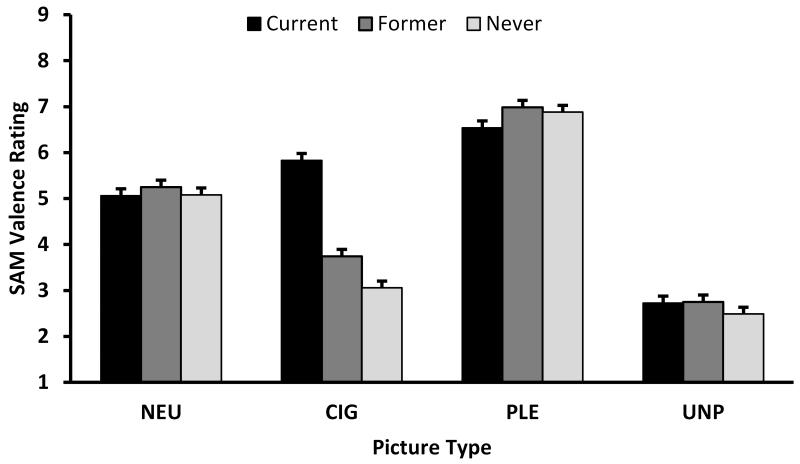
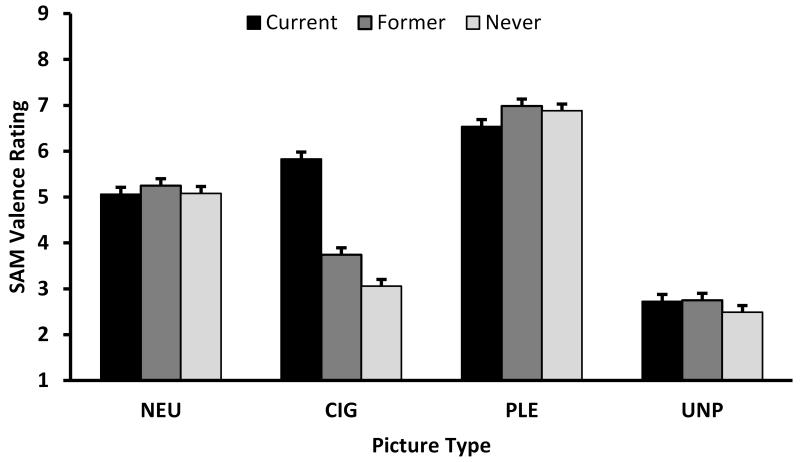
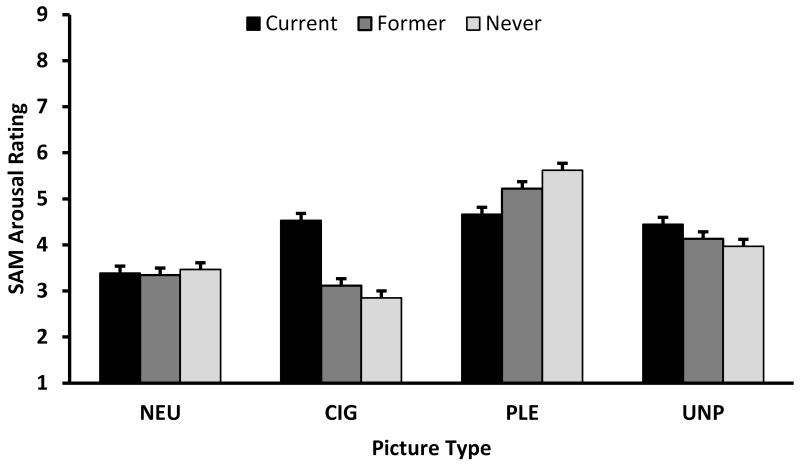
There were significant Group × Picture Type interactions for the SAM Valence, F(6,700)=25.56, p<.0001, and Arousal ratings, F(6,696)=5.89, p<.0001 (see Figure 4). Pairwise comparisons indicated that never smokers rated the CIG pictures as significantly less pleasant compared to former, t(700)=3.22, p=.0013, and current, t(700)=13.06, p<.0001, smokers, and former smokers rated the CIG pictures as significantly less pleasant than current smokers, t(700)=9.80, p<.0001. There were no main effects or interactions involving gender. In terms of Arousal ratings, current smokers rated CIG pictures as being more arousing than former (t[696]=4.22, p<.0001) or never (t[700]=5.01, p<.0001) smokers, who did not differ on their ratings. Additionally, current smokers rated PLE pictures as less arousing than never smokers (t[696]=2.85, p=.0045). We found a significant Gender × Picture Type interaction, F(3,698)=5.03, p=.0019, with pairwise comparisons indicating that women (LSM=4.55, SE=0.21) rated UNP pictures as more arousing than men (LSM=3.92, SE=0.18), t(698)=2.25, p=.0245, and that men (LSM=5.48, SE=0.18) rated PLE pictures as more arousing than women (LSM=4.73, SE=0.21), t(698)=2.66, p=.0081.
Figure 4.
Interaction of Group by Picture Type on (A) Valence and (B) Arousal SAM Ratings. CIG=cigarette; NEU=neutral; PLE=pleasant; UNP=unpleasant picture type. A score of 9 represents happy and excited for the valence and arousal scales, respectively. Histograms represent least-square means, and error bars represent SE.
Discussion
Our LPP findings suggest that former smokers, like never smokers, found cigarette-related and intrinsically pleasant stimuli to be less motivationally salient than current smokers. The EPN results suggest that cigarette-related stimuli, much like intrinsically pleasant and unpleasant stimuli, attract early attentional resources, regardless of a person’s smoking status. The P1 findings suggest that images of cigarettes possess perceptual features that facilitate early sensory processing, again regardless of a person’s smoking status. Together, these results extend the findings of Littel and Franken (2007) by demonstrating that these differences between former and current smokers are generalizable to appetitive stimuli (i.e., CIG and PLE pictures), are specific to later ERP components thought to reflect selective processing of motivationally relevant stimuli (i.e., the LPP), and are found among an older, presumably more diverse and dependent group of smokers seeking treatment and former smokers. The LPP findings have important theoretical implications, as they suggest that the increased motivational salience of drug cues that develops through repetitive drug use and that helps maintain drug dependence weakens and eventually “normalizes” among former users.
Replicating previous work that compared cigarette smokers (Littel & Franken, 2007; Minnix et al., 2013; Warren & McDonough, 1999), alcoholics (Namkoong, Lee, Lee, Lee, & An, 2004), cocaine users (van de Laar, Licht, Franken, & Hendriks, 2004), and heroin users (Franken, Stam, Hendriks, & van den Brink, 2003; Lubman, Allen, Peters, & Deakin, 2008; Lubman et al., 2009) with non-users, we found that current smokers produced enhanced LPP ERP responses to passively viewed drug-related (i.e., CIG) pictures compared to non-users. More importantly to the aims of this study, and consistent with a previous study (Littel & Franken, 2007), we found that former smokers produced LPP responses to CIG pictures comparable to never smokers, which suggests that both former and never smokers found cigarette pictures to be less motivationally salient than current smokers.
The results for the EPN component suggest that CIG pictures attracted early attentional resources among both non-smokers (i.e., former and never smokers) and current smokers compared to NEU pictures. This is consistent with the only other study to examine the impact of drug-related stimuli on this component, which found no difference in EPN to cocaine-related pictures among cocaine current, abstinent, and non-users (Dunning et al., 2011). Given that most previous studies have found that intrinsically motivationally relevant visual stimuli produce larger EPNs than neutral stimuli (Flaisch, Stockburger, & Schupp, 2008; Foti, Hajcak, & Dien, 2009; Schupp et al., 2003; Schupp, Junghofer, Weike, & Hamm, 2004; Versace et al., 2011), our EPN results suggest that cigarette-related pictures attract early attentional resources much like intrinsically motivationally relevant pictures.
Our main effect for picture type for the P1 component suggests cigarette-related picture facilitate early sensory processing, regardless of smoking status. These findings are consistent with a previous study in which we found significant P1 enhancement to CIG pictures compared to pleasant and unpleasant pictures in current smokers (Versace et al., 2011). In that study, we concluded that the enhanced P1 to CIG pictures was due smokers’ attentional bias toward the one unique perceptual features common to all pictures in that category, a cigarette. Unlike later ERP components, the P1 reflects feature-based selective modulation of neural activity through the visual cortex during feed-forward processing (Zhang & Luck, 2009). Our current findings suggest that this early attentional bias to depictions of cigarettes occurs regardless of smoking history and is likely due to the distinct perceptual features of cigarettes.
The SAM valence and arousal ratings to CIG pictures were consistent with our LPP findings. Current smokers rated CIG pictures as more pleasant and arousing than former and never smokers, consistent with previous studies that compared ratings to drug-specific pictures between smokers and nonsmokers (Engelmann, Gewirtz, & Cuthbert, 2011; Littel & Franken, 2011) and between the opiate dependent individuals and controls (Lubman et al., 2009). Unlike Lubman and colleagues (2009), our SAM arousal ratings do not suggest that current users found the drug-related pictures to be more arousing than the PLE pictures. However, the current smokers in our study did rate the PLE pictures as less arousing than the never smokers.
In contrast to our SAM arousal ratings, none of our ERP findings suggest that the intrinsically rewarding stimuli (i.e., PLE) were less salient among current compared to non-smokers. Our LPP PLE results are consistent with our previous study that found that current smokers produced larger LPP to pleasant pictures than never smokers (Minnix et al., 2013), but are inconsistent with other studies that found no LPP differences when comparing smokers to non-smokers (Dunning et al., 2011; Littel & Franken, 2011). Additionally, our LPP findings are inconsistent with studies of other drugs of abuse, using ERP (Lubman et al., 2008; Lubman et al., 2009), fMRI (Asensio et al., 2010; Garavan et al., 2000), and PET (Volkow et al., 1997; Volkow et al., 2008) methodologies, which found that chronic drug users attributed less motivational salience to pleasant cues compared to non-users. One explanation for these inconsistent findings could be that there are individual differences in hedonic capacity, the ability to extract enjoyment from natural reinforcers, that were unaccounted for among the studies. Smokers with greater trait anhedonia reported diminished positive affect and a greater urge to smoke to enhance pleasure (Cook, Spring, McChargue, & Hedeker, 2004; Leventhal, Waters, Kahler, Ray, & Sussman, 2009), consistent with nicotine’s ability to increase the incentive value of stimuli accompanying nicotine delivery (Caggiula et al., 2009). Additionally, we have found that smokers with reduced LPP to pleasant stimuli (i.e., lower hedonic capacity) were more vulnerable to relapse (Versace et al., 2012). Thus, future studies might better address whether intrinsically rewarding stimuli are less salient among current compared to non-smokers by taking into account this individual difference in hedonic capacity.
Our results, across all three of our ERP components, suggest that cigarette-related pictures attract greater attentional resources than neutral pictures, regardless of participant smoking status. While it might seem surprising that this difference exists among non-smokers, our findings are consistent with ERP (Bloom, Potts, Evans, & Drobes, 2013; McDonough & Warren, 2001), fMRI (Vollstädt-Klein et al., 2011), and reaction time studies (Oliver & Drobes, 2012) that found attentional bias toward CIG compared to neutral images among non-smokers. One reason for this universal bias could be that the cigarette cues were less complex visual stimuli than the neutral stimuli (i.e., all of cigarette pictures contained a cigarette, while the neutral pictures were of varying content), but other work suggests that LPPs to picture stimuli are sensitive to motivational relevance but not stimulus complexity (Bradley, Hamby, Löw, & Lang, 2007; Franken, Van Strien, Bocanegra, & Huijding, 2011). Another reason could be that cigarette stimuli were primed because of experimental demand effects (i.e., participants were all recruited into a study advertised as being smoking-related). Finally, the cigarette pictures might have been motivationally relevant to never and former smokers because they were unpleasant for those groups, a hypothesis supported by our SAM valence rating results.
An important question raised by our LPP findings is at what point do former smokers cease responding to CIG pictures like current smokers? Unfortunately, we did not select the former smokers with regards to previous smoking duration, beyond one year, meaning that we were unable to identify the time course of reduced responding to cigarette-related cues among former smokers. Identifying this time course would necessitate using a longitudinal or cohort design to systematically sample former smokers in the months and years following quitting. One cohort study found that prolonged former smokers (mean years since last cigarette = 18) showed no attentional bias on a visual dot-probe task to CIG compared to NEU pictures, but that intermediate (7 yrs since last cigarette) and recent former smokers (1.2 yrs since last cigarette) did (Peuker & Bizarro, 2014). Future studies should investigate whether this transition is abrupt or gradual, is linear or nonlinear, or varies by moderating factors.
One such moderating factor might be level of nicotine dependence. However, one shortcoming with our study is that the sample of former smokers may not have been as nicotine dependent when they were actively smoking as the current smokers were at the time of the study, based on the finding that the former smokers’ Retrospective FTND scores were significantly lower than the current smokers’ FTND scores. This imbalance likely came about because while we excluded intermittent and light smokers from our current smokers group due to our CPD and CO requirements, we did not identify and exclude them from our former smokers group. It is not clear how the former smokers would have responded to the cigarette-related pictures had they been as nicotine dependent when smoking as the current smokers were. Future studies should take care in matching former and current smokers on important characteristics such as nicotine dependence.
We found no evidence that gender moderated the effect of smoking status on ERP response to smoking-related (or intrinsically affective) cues. This is perhaps unsurprising given the equivocal findings regarding gender differences and smoking cue reactivity. While some studies have found greater smoking cue reactivity among women than men (Field & Duka, 2004; Knott et al., 2009; Waters et al., 2004), others have found no gender differences (Carter & Tiffany, 2001; Robinson et al., 2007; Saladin et al., 2012). In terms of ERP responses to smoking-related cues, we are unaware of any study in this area that reported examining gender differences, so it is unknown whether our lack of gender findings are typical. Our findings that women rated UNP pictures as more arousing but PLE pictures as less arousing than men is consistent with other self-report findings about these affective picture types (e.g., Bradley, Codispoti, Sabatinelli, & Lang, 2001).
We did not evaluate an acutely nicotine-deprived (i.e., overnight) group of current smokers, given that all of the current smokers were instructed to smoke ad libitum before the lab session. Evidence suggests that the administration of nicotine, even among never and former smokers, improves several aspects of attentional and cognitive performance, primarily speed (Heishman, Kleykamp, & Singleton, 2010). However, a meta-analysis of LPP response to drug-related compared to neutral stimuli among the drug dependent found no effects of drug administration or deprivation (Littel et al., 2012). Thus, it is unlikely that the degree of acute drug satiation influenced the pattern of ERP response in our study. Additionally, we did not include non-treatment-seeking current smokers in this study. The treatment-seeking smokers we included in our current smokers group may differ demographically from smokers not interested in quitting or who do not seek out assistance with quitting (Shiffman, Brockwell, Pillitteri, & Gitchell, 2008), which could result in differential responses to smoking and affective cues.
Besides smoking status, there are individual differences that may influence response to drug-related stimuli. Sensitivity to smoking cues has been found to vary by genotype, including the serotonin transporter SLC6A4 among smokers (Munafò et al., 2005), the dopamine D4 receptor variable number of tandem repeats among former smokers (Munafò & Johnstone, 2008), and the CHRNA3 rs578776 among current smokers (Robinson et al., 2013). Additionally, we have found that individual differences in response to CIG relative to PLE stimuli predict smoking cessation outcome using ERP (Versace et al., 2012) and fMRI (Versace et al., 2014) measures.
In summary, we found that both former and never smokers produced reduced LPP responses and SAM valence and arousal ratings to cigarette-related pictures compared to current smokers. With the EPN component, we found that, similar to pleasant and unpleasant pictures, cigarette-related pictures attracted early attentional resources among both non-smokers (i.e., former and never smokers) and current smokers. All participants, irrespective of smoking status, produced larger P1 responses to cigarette-related pictures compared to the other picture categories. The LPP and SAM results, which reflect selective processing and semantic evaluation, respectively, suggest that former smokers, like never smokers, do not find cigarette-related stimuli to be as motivationally salient as current smokers. The EPN results suggest that cigarette-related pictures attract attentional resources much like intrinsically motivationally relevant pictures. The P1 findings suggest that depictions of cigarettes facilitate sensory processing for all people, probably for their perceptual characteristics. Future studies should investigate the process by which cigarette-related stimuli lose motivational significance for former smokers by using longitudinal or cohort designs.
Acknowledgements
This project was supported by a grant from The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment and by NIH grants R01DA032581 and UO1DA020830.
The authors wish to thank Kimberly Claiborne, Janeene Frerking, Danika Dirba, Lauren Baker, Christine Jeria, Kevin Winslow, and Jennifer Ferguson for their assistance in data collection.
Footnotes
Disclosures
All authors contributed in a significant way to the manuscript and have read and approved the final manuscript.
All of the authors declare that they have no conflicts of interest.
Reference List
- Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, et al. Altered neural response of the appetitive emotional system in cocaine addiction: An fMRI study. Addiction Biology. 2010;15:504–516. doi: 10.1111/j.1369-1600.2010.00230.x. doi: 10.1111/j.1369-1600.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- Bloom EL, Potts GF, Evans DE, Drobes DJ. Cue reactivity in smokers: An event-related potential study. International Journal of Psychophysiology. 2013;90:258–264. doi: 10.1016/j.ijpsycho.2013.08.005. doi: 10.1016/j.ijpsycho.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy SJ, Victor JC, Diemert LM. Origin and use of the 100 cigarette criterion in tobacco surveys. Tobacco Control. 2009;18:317–323. doi: 10.1136/tc.2008.027276. doi: 10.1136/tc.2008.027276. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–319. doi: 10.1037/1528-3542.1.3.276. [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Löw A, Lang PJ. Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology. 2007;44:364–373. doi: 10.1111/j.1469-8986.2007.00520.x. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: A dual-reinforcement model. In: Bevins RA, Caggiula AR, editors. The motivational impact of nicotine and its role in tobacco use, Nebraska symposium on motivation. Vol. 55. Springer-Verlag; New York: 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Day SX, Tsan JY, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine & Tobacco Research. 2006;8:361–369. doi: 10.1080/14622200600670215. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. doi: 10.1037/1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- CDC Cigarette smoking among adults - United States, 1994. MMWR: Morbidity and Mortality Weekly Report. 1996;45:588–590. [PubMed] [Google Scholar]
- CDC Cigarette smoking among adults, United States, 2006. MMWR: Morbidity and Mortality Weekly Report. 2007;56/44:1157–1161. [PubMed] [Google Scholar]
- CDC Vital Signs: Current Cigarette Smoking Among Adults Aged >18 Years -- United States, 2005-2010. MMWR: Morbidity and Mortality Weekly Report. 2011;60:1207–1212. [PubMed] [Google Scholar]
- Constantinou N, Morgan CJ, Battistella S, O’Ryan D, Davis P, Curran HV. Attentional bias, inhibitory control and acute stress in current and former opiate addicts. Drug and Alcohol Dependence. 2010;109:220–225. doi: 10.1016/j.drugalcdep.2010.01.012. doi: 10.1016/j.drugalcdep.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine & Tobacco Research. 2004;6:39–47. doi: 10.1080/14622200310001656849. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. doi: 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cerebral Cortex. 2003;13:486–499. doi: 10.1093/cercor/13.5.486. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, Alia-Klein N, Woicik PA, et al. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users - an ERP study. European Journal of Neuroscience. 2011;33:1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O’Brien CP. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug and Alcohol Dependence. 2002;67:185–191. doi: 10.1016/s0376-8716(02)00065-0. doi: 10.1016/S0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Gewirtz JC, Cuthbert BN. Emotional reactivity to emotional and smoking cues during smoking abstinence: Potentiated startle and P300 suppression. Psychophysiology. 2011;48:1656–1668. doi: 10.1111/j.1469-8986.2011.01235.x. doi: 10.1111/j.1469-8986.2011.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients. Archives of General Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T. Cue reactivity in smokers: The effects of perceived cigarette availability and gender. Pharmacology, Biochemistry & Behavior. 2004;78:647–652. doi: 10.1016/j.pbb.2004.03.026. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Flaisch T, Stockburger J, Schupp HT. Affective prime and target picture processing: an ERP analysis of early and late interference effects. Brain Topogr. 2008;20:183–191. doi: 10.1007/s10548-008-0045-6. doi: 10.1007/s10548-008-0045-6. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Franken IH, Stam CJ, Hendriks VM, van den Brink W. Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology. 2003;170:205–212. doi: 10.1007/s00213-003-1542-7. doi: 10.1007/s00213-003-1542-7. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Kroon LY, Wiers RW, Jansen A. Selective cognitive processing of drug cues in heroin dependence. Journal of Psychopharmacology. 2000;14:395–400. doi: 10.1177/026988110001400408. doi: 10.1177/026988110001400408. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Van Strien JW, Bocanegra BR, Huijding J. The P3 event-related potential as an index of motivational relevance. Journal of Psychophysiology. 2011;25:32–39. doi: 10.1027/0269-8803/a000030. [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- García-Rodríguez O, Secades-Villa R, Flórez-Salamanca L, Okuda M, Liu SM, Blanco C. Probability and predictors of relapse to smoking: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug and Alcohol Dependence. 2013;132:479–485. doi: 10.1016/j.drugalcdep.2013.03.008. doi: 10.1016/j.drugalcdep.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. The international smoking image series (with neutral counterparts), v. 1.2. Department of Psychology, Southern Illinois University; Carbondale, IL: 1999. [Google Scholar]
- Gilpin EA, Lee L, Pierce JP. Changes in population attitudes about where smoking should not be allowed: California versus the rest of the USA. Tobacco Control. 2004;13:38–44. doi: 10.1136/tc.2003.004739. doi: 10.1136/tc.2003.004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz ER, Carr CR, Marcus AC. The tobacco withdrawal syndrome in unaided quitters. British Journal of Addiction. 1991;86:57–69. doi: 10.1111/j.1360-0443.1991.tb02629.x. doi: 10.1111/j.1360-0443.1991.tb02629.x. [DOI] [PubMed] [Google Scholar]
- Groppe DM, Urbach TP, Kutas M. Mass univariate analysis of event-related brain potentials/fields I: A critical tutorial review. Psychophysiology. 2011;48:1711–1725. doi: 10.1111/j.1469-8986.2011.01273.x. doi: 10.1111/j.1469-8986.2011.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon KS, Pomerleau CS, Brigham J, Javitz H, Swan GE. Validity of retrospective assessments of nicotine dependence: a preliminary report. Addictive Behaviors. 2005;30:613–617. doi: 10.1016/j.addbeh.2004.08.006. doi: 10.1016/j.addbeh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Craving among long-abstinent smokers: an Internet survey. Nicotine & Tobacco Research. 2010;12:459–462. doi: 10.1093/ntr/ntq009. doi: 10.1093/ntr/ntq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen BH, Thayer JF, Laberg JC, Asbjornsen AE. Attentional bias in active smokers, abstinent smokers, and nonsmokers. Addictive Behaviors. 1997;22:813–817. doi: 10.1016/s0306-4603(97)00010-5. doi: 10.1016/S0306-4603(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Jones BT, Bruce G. Methods, measures and findings of attentional bias in substance use, abuse and dependence. In: Wiers RW, Stacy AW, editors. Sage; Thousand Oaks, CA: 2006. pp. 309–338. [Google Scholar]
- Jones BT, Jones BC, Smith H, Copley N. A flicker paradigm for inducing change blindness reveals alcohol and cannabis information processing biases in social users. Addiction. 2003;98:235–244. doi: 10.1046/j.1360-0443.2003.00270.x. doi: 10.1046/j.1360-0443.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. doi: 10.1111/1469-8986.3950641. [DOI] [PubMed] [Google Scholar]
- Keil A, Debener S, Gratton G, Junghofer M, kappenman ES, Luck SJ, et al. Committee report: Publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology. 2014;51:1–21. doi: 10.1111/psyp.12147. doi: 10.1111/psyp.12147. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Naccache L, Cyr E, Fisher DJ, McIntosh JF, Millar AM, Villeneuve CM. Craving-induced EEG reactivity in smokers: effects of mood induction, nicotine dependence and gender. Neuropsychobiology. 2009;58:187–199. doi: 10.1159/000201716. doi: 10.1159/000201716. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: Computer applications. In: Sidowski JB, Johnson JH, Williams TA, editors. Technology in mental health care delivery systems. Ablex; Norwood, NJ: 1980. pp. 119–137. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida; Gainesville, FL: 2008. [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalography and Clinical Neurophysiology. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine & Tobacco Research. 2009;11:1047–1054. doi: 10.1093/ntr/ntp098. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littel M, Euser AS, Munafo MR, Franken IH. Electrophysiological indices of biased cognitive processing of substance-related cues: A meta-analysis. Neuroscience and biobehavioral reviews. 2012;36:1803–1816. doi: 10.1016/j.neubiorev.2012.05.001. doi: 10.1016/j.neubiorev.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Littel M, Franken IH. The effects of prolonged abstinence on the processing of smoking cues: An ERP study among smokers, ex-smokers and never-smokers. Journal of Psychopharmacology. 2007;21:873–882. doi: 10.1177/0269881107078494. doi: 10.1177/0269881107078494. [DOI] [PubMed] [Google Scholar]
- Littel M, Franken IH. Implicit and explicit selective attention to smoking cues in smokers indexed by brain potentials. Journal of Psychopharmacology. 2011;25:503–513. doi: 10.1177/0269881110379284. doi: 10.1177/0269881110379284. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. Journal of Psychophysiology. 2008;22:836–842. doi: 10.1177/0269881107083846. doi: 10.1177/0269881107083846. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Peters LA, Mogg K, Bradley BP, Deakin JF. Attentional bias for drug cues in opiate dependence. Psychological Medicine. 2000;30:169–175. doi: 10.1017/s0033291799001269. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: Associations with later heroin use. Archives of General Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Maris E. Randomization tests for ERP topographies and whole spatiotemporal data matrices. Psychophysiology. 2004;41:142–151. doi: 10.1111/j.1469-8986.2003.00139.x. doi: 10.1111/j.1469-8986.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- McDonough BE, Warren CA. Effects of 12-h tobacco deprivation on event-related potentials elicited by visual smoking cues. Psychopharmacology. 2001;154:282–291. doi: 10.1007/s002130000647. doi: 10.1007/s002130000647. [DOI] [PubMed] [Google Scholar]
- Minnix JA, Versace F, Robinson JD, Lam CY, Engelmann JM, Cui Y, et al. The late positive potential (LPP) in response to varying types of emotional and cigarette stimuli in smokers: A content comparison. International Journal of Psychophysiology. 2013;89:18–25. doi: 10.1016/j.ijpsycho.2013.04.019. doi: SO10167-8760(13)00121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò M, Mogg K, Roberts S, Bradley BP, Murphy M. Selective processing of smoking-related cues in current smokers, ex-smokers and never-smokers on the modified Stroop task. Journal of Psychopharmacology. 2003;17:310–316. doi: 10.1177/02698811030173013. doi: 10.1177/02698811030173013. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Johnstone EC. Smoking status moderates the association of the dopamine D4 receptor (DRD4) gene VNTR polymorphism with selective processing of smoking related cues. Addiction Biology. 2008;13:435–439. doi: 10.1111/j.1369-1600.2008.00098.x. doi: 10.1111/j.1369-1600.2008.00098.x. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Johnstone EC, Mackintosh B. Association of serotonin transporter genotype with selective processing of smoking-related stimuli in current smokers and ex-smokers. Nicotine & Tobacco Research. 2005;7:773–778. doi: 10.1080/14622200500259861. doi: 10.1080/14622200500259861. [DOI] [PubMed] [Google Scholar]
- Namkoong K, Lee E, Lee CH, Lee BO, An SK. Increased P3 amplitudes induced by alcohol-related pictures in patients with alcohol dependence. Alcoholism, Clinical and Experimental Research. 2004;28:1317–1323. doi: 10.1097/01.alc.0000139828.78099.69. doi: 10.1097/01.ALC.0000139828.78099.69. [DOI] [PubMed] [Google Scholar]
- Nestor L, McCabe E, Jones J, Clancy L, Garavan H. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage. 2011;56:2258–2275. doi: 10.1016/j.neuroimage.2011.03.054. doi: 10.1016/j.neuroimage.2011.03.054. [DOI] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Schubert CR. A questionnaire for assessing environmental tobacco smoke exposure. Environmental research. 2005;97:76–82. doi: 10.1016/j.envres.2004.02.005. doi: 10.1016/j.envres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Oliver JA, Drobes DJ. Visual search and attentional bias for smoking cues: The role of familiarity. Experimental and Clinical Psychopharmacology. 2012;20:489–496. doi: 10.1037/a0029519. doi: 10.1037/a0029519. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuker AC, Bizarro L. Attentional avoidance of smoking cues in former smokers. Journal of Substance Abuse Treatment. 2014;46:183–188. doi: 10.1016/j.jsat.2013.08.014. doi: 10.1016/j.jsat.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [Google Scholar]
- Robinson JD, Cinciripini PM, Tiffany ST, Carter BL, Lam CY, Wetter DW. Gender differences in affective response to acute nicotine administration and deprivation. Addictive Behaviors. 2007;32:543–561. doi: 10.1016/j.addbeh.2006.05.021. doi: 10.1016/j.addbeh.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JD, Versace F, Lam CY, Minnix JA, Engelmann JM, Cui Y, et al. The CHRNA3 rs578776 variant is associated with an intrinsic reward sensitivity deficit in smokers. Frontiers in Psychiatry. 2013;4:1–11. doi: 10.3389/fpsyt.2013.00114. doi: 10.3389/fpsyt.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction. 2000;95(Suppl. 2):91–117. doi: 10.1080/09652140050111681. doi: 10.1046/j.1360-0443.95.8s2.19.x. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. American Journal on Addictions. 2012;21:210–220. doi: 10.1111/j.1521-0391.2012.00232.x. doi: 10.1111/j.1521-0391.2012.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. doi: 10.1111/1469-8986.3720257. [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psychological Science. 2003;14:7–13. doi: 10.1111/1467-9280.01411. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghofer M, Weike AI, Hamm AO. Selective visual attention to emotion. Journal of Neuroscience. 2007;27:1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MA, Levin JR, Serlin RC. New developments in pairwise multiple comparisons: Some powerful and practicable procedures. Psychological Bulletin. 1991;110:577–586. doi: 10.1037/0033-2909.110.3.577. [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Individual differences in adoption of treatment for smoking cessation: Demographic and smoking history characteristics. Drug and Alcohol Dependence. 2008;93:121–131. doi: 10.1016/j.drugalcdep.2007.09.005. doi: 10.1016/j.drugalcdep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Stritzke WG, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: Advances in reliability, specificity, and validity. Psychology of Addictive Behaviors. 2004;18:148–159. doi: 10.1037/0893-164X.18.2.148. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Strong DR, Brown RA, Kahler CW, Lloyd-Richardson EE, Niaura R. Depression proneness in treatment-seeking smokers: A taxometric analysis. Personality and Individual Differences. 2004;36:1155–1170. doi: 10.1016/S0191-8869(03)00207-1. [Google Scholar]
- van de Laar MC, Licht R, Franken IH, Hendriks VM. Event-related potentials indicate motivational relevance of cocaine cues in abstinent cocaine addicts. Psychopharmacology. 2004;177:121–129. doi: 10.1007/s00213-004-1928-1. doi: 10.1007/s00213-004-1928-1. [DOI] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Robinson JD, Jackson EF, Green CE, Lam CY, et al. Pre-quit fMRI responses to pleasant and cigarette cues predict cessation outcome. Nicotine & Tobacco Research. 2014;16:697–708. doi: 10.1093/ntr/ntt214. doi: 10.1111/j.1460-9568.2011.07915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Lam CY, Engelmann JM, Robinson JD, Minnix JA, Brown VL, Cinciripini PM. Beyond cue reactivity: Blunted brain responses to pleasant stimuli predict long term smoking abstinence. Addiction Biology. 2012;17:991–1000. doi: 10.1111/j.1369-1600.2011.00372.x. doi: 10.1111/j.1369-1600.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Minnix JA, Robinson JD, Lam CY, Brown VL, Cinciripini PM. Brain reactivity to emotional, neutral and cigarette-related stimuli in smokers. Addiction Biology. 2011;16:296–307. doi: 10.1111/j.1369-1600.2010.00273.x. doi: 10.1111/j.1369-1600.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Robinson JD, Lam CY, Minnix JA, Brown VL, Carter BL, et al. Cigarette cues capture smokers’ attention: Evidence from event-related potentials. Psychophysiology. 2010;47:435–441. doi: 10.1111/j.1469-8986.2009.00946.x. doi: 10.1111/j.1469-8986.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: Decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Kobiella A, Buhler M, Graf C, Fehr C, Mann K, Smolka MN. Severity of dependence modulates smokers’ neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addiction Biology. 2011;16:166–175. doi: 10.1111/j.1369-1600.2010.00207.x. doi: 10.1111/j.1369-1600.2010.00207.x. [DOI] [PubMed] [Google Scholar]
- Warren CA, McDonough BE. Event-related brain potentials as indicators of smoking cue-reactivity. Clinical Neurophysiology. 1999;110:1570–1584. doi: 10.1016/s1388-2457(99)00089-9. doi: 10.1016/S1388-2457(99)00089-9. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Kuhn S, Javadi AH, Korucuoglu O, Wiers RW, Walter H, et al. Automatic approach bias towards smoking cues is present in smokers but not in ex-smokers. Psychopharmacology. 2013;229:187–197. doi: 10.1007/s00213-013-3098-5. doi: 10.1007/s00213-013-3098-5. [DOI] [PubMed] [Google Scholar]
- Zhang W, Luck SJ. Feature-based attention modulates feedforward visual processing. Nature Neuroscience. 2009;12:24–25. doi: 10.1038/nn.2223. doi: 10.1038/nn.2223. [DOI] [PubMed] [Google Scholar]