Abstract
Herein, we report the synthesis of DNA-functionalized infinite coordination polymer (ICP) nanoparticles as biocompatible gene regulation agents. ICP nanoparticles were synthesized from ferric nitrate and a ditopic 3-hydroxy-4-pyridinone (HOPO) ligand bearing a pendant azide. Addition of FeIII to a solution of the ligand produced nanoparticles, which were colloidally unstable in the presence of salts. Conjugation of DNA to the FeIII-HOPO ICP particles, via copper-free click chemistry, afforded colloidally stable nucleic acid nanoconstructs. The DNA-ICP particles, when cross-linked through sequence-specific hybridization, exhibit narrow, highly cooperative melting transitions consistent with dense DNA surface loading. The ability of the DNA-ICP particles to enter cells and alter protein expression was also evaluated. Our results indicate these novel particles carry nucleic acids into mammalian cells without the need for transfection agents and are capable of efficient gene knockdown.
Keywords: antisense, knockdown, nanoparticles, iron(III), coordination polymer
Spherical nucleic acids (SNAs) have emerged as an interesting new class of materials that have shown promise in programmable materials synthesis,[1] bio-detection,[2] and intracellular gene regulation.[3] Such structures are often comprised of a nanoparticle core functionalized with a dense layer of oligonucleotides, although hollow, core-free versions have been developed.[4] The earliest example of SNAs involved gold nanoparticles modified with a dense layer of alkylthiol-functionalized DNA,[5] but iron oxide,[6] silver,[7] semiconductor quantum dot,[8] and organic cores have been explored as well.[9] Notably, the chemical and biological properties of SNAs are markedly different from their linear counterparts. SNAs exhibit cooperative binding and sharp thermal denaturation profiles, enter cells without the need for cationic transfection agents, and have the ability to bind to receptors in a polyvalent fashion.[10] Consequently, they are powerful new entities for manipulating cellular processes through gene regulation,[11] drug delivery,[12] and immunomodulatory pathways.[13] The active uptake of SNAs occurs via caveolin-mediated endocytosis, triggered by their binding to class A scavenger receptors (SR-As).[14] Although SNAs made from gold have shown commercial promise as medical diagnostic and research tools and have shown no acute toxicity in vivo,[15] there are concerns about the potential long term toxicity of gold nanoparticles and their metabolic fate.[16] Consequently, new forms of SNAs with cores made of biocompatible materials are highly sought after. Herein, we report a strategy that employs the use of infinite coordination polymer (ICP) nanoparticles made from ferric ions and a rigid ditopic chelating ligand to synthesize novel SNA nanoparticle conjugates. These DNA-ICPs are designed from chemical building blocks approved by the FDA for other pharmaceutical uses, exhibit cooperative binding, and can readily cross mammalian cell membranes and inhibit protein expression in a targeted fashion.
ICP nanoparticles consist of amorphous networks of organic ligands bridged by metal nodes.[17] They are promising materials for SNA construction as the ligand/metal combination that defines the ICP structure can be rationally designed to optimize the toxicological and pharmacokinetic profiles of the DNA-ICP conjugate. One major limitation of many ICPs designed for medicinal applications is their instability in aqueous buffers. Some researchers have circumvented this limitation by encapsulating the particle core in silica[18] or a shell of lipids.[19] In contrast, we have sought to design ICP particles that could be synthesized, purified, and stored indefinitely under aqueous conditions and without specialized equipment or reagents. Furthermore, the use of relatively nontoxic metal ions is a crucial requirement for biological applications. These goals were accomplished by synthesizing ICP nanoparticles from strongly chelating 3-hydroxy-4-pyridinone (3,4-HOPO) ligands in combination with FeIII, the most abundant transition metal in the body. The coordination chemistry and pharmacology of the 3,4-HOPOs have been systematically investigated,[20] and the 1,2-dimethyl derivative (deferiprone) is FDA-approved for the treatment of iron overload in humans.[21] Furthermore, the Fe(HOPO)3 complex is known to dissociate below physiological pH.[22] This provides a potential release mechanism for delivering DNA into the cytosol following cell entry, a novel property not typically associated with SNAs prepared to date.
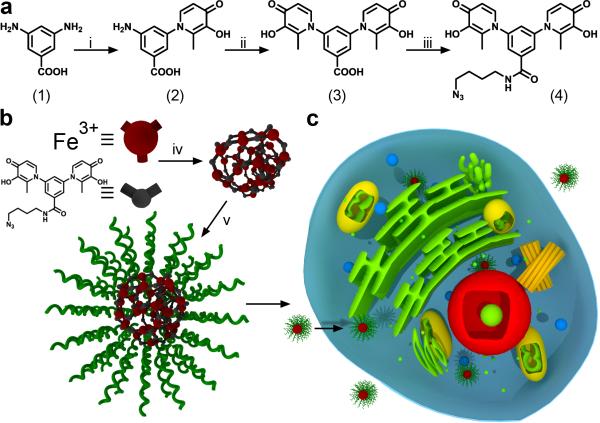
It is known that ditopic HOPO and catechol ligands, being isoelectronic, can form insoluble coordination polymers with oxophilic metal cations such as FeIII, CrIII, GaIII and others, however, such polymers are poorly understood and have not been well-studied in the literature.[23] These ligands have mainly been studied for metal sequestration as opposed to materials synthesis. Therefore, we saw an opportunity to construct a novel nanoparticle scaffold for modification with DNA. Specifically, we synthesized a new ditopic ligand DABA-bis-HP-N3 (4), which deliberately employs the inexpensive building blocks maltol and 3,5-diaminobenzoic acid (DABA, 1) (Scheme 1a). Two sequential acid-catalyzed condensations of maltol with DABA (1 to 2; 2 to 3) followed by HATU-mediated amidation of the carboxylic acid afforded the azide-bearing ditopic ligand 4. Importantly, the carboxylic acid in 3 may be amidated with a wide variety of amine building blocks, affording ICP particles with tailorable post-synthetic chemistry dictated by the pendant functional groups.
Scheme 1.
Synthesis and assembly of ICP particles and their cellular uptake. a) Synthetic scheme for bis-3,4-HOPO azide (4). b) Assembly of ICP particles from Fe(NO3)3 and compound 4, followed by conjugation with DNA via a Cu free ‘Click’ reaction. c) Scheme depicting the cellular uptake of ICP-DNA conjugates. Reaction conditions: i) maltol, n-propanol, reflux, 16 h. ii) maltol, ethoxyethanol, 64 h, reflux. iii) 4-azido-butan-1-amine, HATU, diisopropylethylamine, DMSO, RT, 4 h. iv) Fe(NO3)3·9H2O, NaOH (aq.), RT, 10 min. v) Dibenzocyclooctyne-DNA, 0.5M NaCl, RT, 16 h
To synthesize ICP nanoparticles from ligand 4, we prepared a dilute NaOH solution of ligand 4 (1.07 mM ligand, 1877 μL) and injected a solution of ferric nitrate (10.8 mM, 123 μL) into it (Scheme 1b). Particle formation occurs instantaneously and the color of the solution turns from clear to red due to the ligand-metal charge transfer band (LMCT) of the tris-HOPO-FeIII complex (λmax ≈ 460 nm).[24] The resulting ICP-N3 nanoparticles were colloidally unstable in the presence of low concentrations of salts (NaCl, Tris·HCl), leading to gradual precipitation of a red, insoluble material. The crude ICP-N3 particles were purified by centrifugal filtration (100 kDa molecular weight cut-off) and re-suspended in H2O. The particles were retained on the filter, as they were too large to pass through. Minimal loss of material through the filter indicated a colloidal dispersion of high molecular weight species was obtained. In deionized H2O, the as-synthesized particles were stable, with a mean hydrodynamic diameter of 10-20 nm, determined by dynamic light scattering (DLS) (Figure 1). TEM and AFM imaging revealed aggregates of small nanoparticles, with some degree of fusion occurring upon drying (see Supporting Information Figure S4). Furthermore, the composition of the ICP-N3 particles was probed spectroscopically. Aliquots containing a fixed concentration of DABA-bis-HP-N3 ligand in H2O were prepared and treated with increasing amounts of iron ranging from 0 to 1.1 equivalents. The absorbance at 460 nm increased until 0.66 equivalents of FeIII were added, consistent with a metal-ligand stoichiometry of Fe2L3 (see Supporting Information Figure S1).
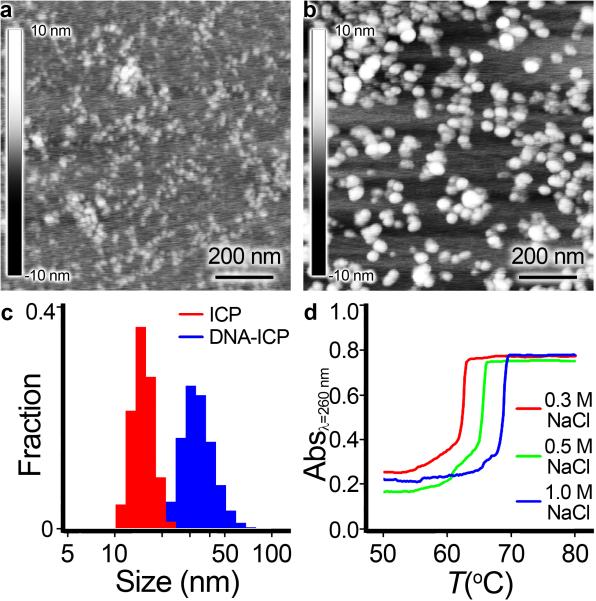
Figure 1.
Characterization of DNA-ICP particles. AFM image of (a) Bare ICP particles drop-cast and dried on mica. b) DNA-functionalized ICP particles drop-cast and dried on mica. c) DLS histograms comparing size distributions of bare and DNA-functionalized ICPs. d) Cooperative melting of ICP-DNA aggregates.
For conjugation to bare ICP-N3 particles, all oligonucleotides were made on an automated DNA synthesizer, purified by reverse-phase HPLC, and characterized by MALDI-ToF. Dibenzocyclooctyne (DBCO) phosphoramidites are commercially available and easily incorporated onto the 5’ termini of the oligonucleotides. DNA strands modified with a Cyanine 5 (Cy5) dye were used for intracellular imaging studies. DNA strands modified with a 5’ alkylthiol were used to construct AuNP-SNAs for comparison with DNA-ICP particles (Table S1). DBCO-bearing oligonucleotides were conjugated to ICP-N3 particles by simply mixing the two reactants in aqueous NaCl (0.5M) followed by repeated filtration to remove unreacted DNA. The resulting DNAICP particles were suspended in Tris·HCl buffer (100 mM, pH 8.0) and remained colloidally stable when stored at 5°C or when heated up to 80 °C over the course of a melting analysis.
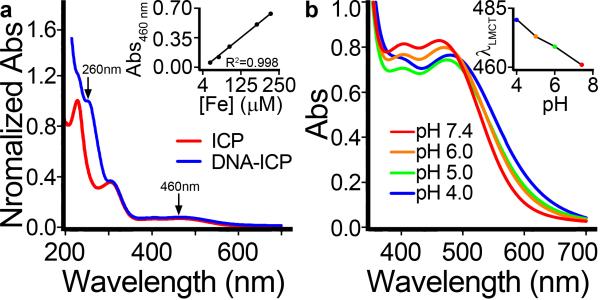
In addition to increasing colloidal stability, the conjugation of DNA to the surface of ICP-N3 particles resulted in changes to particle size, surface charge, and morphology (Figure 1). DLS and zeta potential measurements showed a consistent increase in hydrodynamic diameter and surface charge, respectively. Particles were imaged by AFM to visualize changes in size and morphology. UV-Vis spectroscopy was used to calculate the relative contribution of DNA to the absorbance at 260 nm, and hence the DNA concentration was determined. Inductively-coupled plasma mass spectrometry (ICP-MS) was used to calculate directly the extinction coefficient ε460 of the ICP particles (Figure 2a). Lastly, incubation of DNA-ICP particles in aqueous buffers ranging from physiological pH (7.4) to low lysosomal pH (4.0) showed a clear red-shift in the LMCT λmax, indicating partial dissociation of the tris-coordinated FeIII nodes comprising the particle (Figure 2b). Other supramolecular systems based on HOPOs and catechols exhibit similar pH dependence.[25]
Figure 2.
UV-Vis analysis of DNA-ICP particles. a) Comparison of bare ICP particles with DNA-ICP particles showing the DNA absorbance at 260 nm. Inset: determination of LMCT ε460. b) pH dependence of LMCT absorbance. The red-shift of λmax with decreasing pH is indicative of complex dissociation (see inset).
In order to probe the surface density of oligonucleotides on the DNA-ICP particles, thermal denaturation experiments were carried out wherein ICPs with complementary sequences (A-ICP and B-ICP) were mixed, allowed to hybridize, and then heated above the melting transition of the duplex. The free double-stranded DNA duplex possesses a 17 base-pair overlap with Tm = 54.0°C in 0.3M NaCl. In contrast, the same complementary strands form duplexes with a Tm = 66.9°C when conjugated to ICP-N3 particles, an increase of nearly 13°C. The melting transition of the DNA-ICP particle aggregates is extremely narrow, an indication of cooperativity; the full width at half-maximum of the melting curve is typically <2°C, compared to 10-20°C for free double-stranded DNA (Figure 1d). A-ICP particles alone exhibited no aggregation or melting under the experimental conditions, nor did A-ICP particles mixed with non-complementary particles (NonTarget-ICP). We also studied the interaction of DNA-ICP particles with conventional AuNP-SNAs (A-AuNP) that were prepared and purified according to established protocols.[26] Similar aggregation and meltingbehavior was observed between A-AuNP and B-ICP particles mixed in a 1:1 ratio (see Supporting Information Figure S3). Overall, these studies suggest high DNA surface loading on the ICP-N3 particles.[6a]
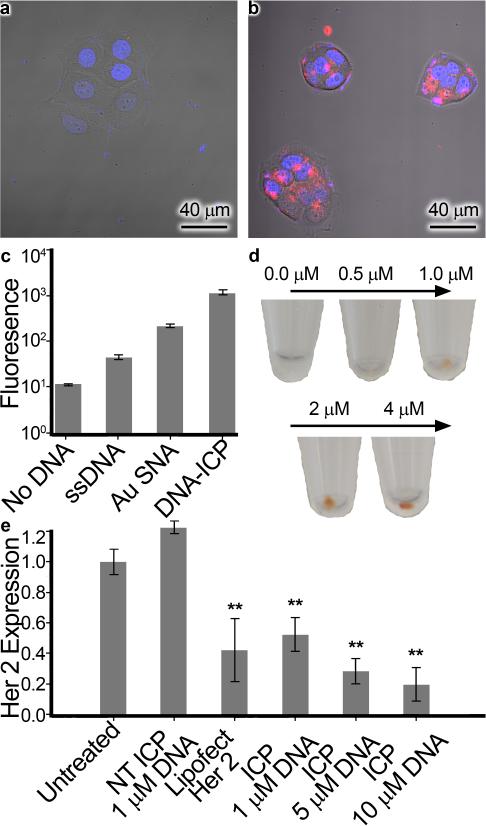
Due to the high apparent oligonucleotide density on the DNAICP surface, we hypothesized that they would function as efficient gene delivery agents, much like their gold predecessors.[3] To test this assumption, ICP-N3 particles were functionalized with the poly(CCT) oligonucleotide Cy5-DBCO bearing an internal fluorophore-label to afford Cy5-ICP particles. Likewise, gold nanoparticles (15 nm) were functionalized with the analogous Cy5-SH oligonucleotide to afford Cy5-AuNP particles having a loading of approximately 113 strands/AuNP, as determined by fluorescence (see Supporting Information, page S6). To test our hypothesis, uptake was examined in HeLa cervical cancer cells (Figure 3). The DNA-ICP particles were found to cross cell membranes more efficiently than the free DNA strands, and exhibited comparable uptake to AuNP-SNA nanoparticles[27] bearing the same sequence.
Figure 3.
Cellular uptake and gene knockdown. Confocal microscopy image of HeLa cells treated with (a) Cy5-ssDNA and (b) DNA-ICP particles (100 nM DNA in each case). Hoechst stain denotes the nucleus in blue while the Cy5 dye attached to the DNA is red. c) Fluorescence intensity of Cy5 dye quantified by flow cytometry. d) Naked-eye visualization of DNA-ICPs taken up in pelleted SKOV-3 ovarian cancer cells e) Expression of HER2 protein in SKOV-3 cells treated with non-targeting DNA-ICPs, HER2 targeting ssDNA + Lipofectamine (25 nM DNA basis), and HER2 targeting DNA-ICPS. Starred bars (**) indicate knockdown was significant (p<0.05) as determined by unpaired student's T test.
These results suggest that DNA-ICP nanoparticles have the potential to transport large amounts of DNA to the cytosol. A dose-dependent increase in iron concentration was found after incubation of DNAICPs in SKOV-3 ovarian cancer and MCF-7 breast cancer cells for 24 hours. The color of the iron complex could be seen by the naked eye in pelleted cells treated with DNA-ICPs. Confocal microscopy experiments with SKOV-3 and C166 cells confirmed that DNAICPs enter such cell lines, demonstrating that these structures exhibit comparable uptake characteristics to AuNP-SNAs (See Supporting Information Page S12).
Having demonstrated the ability of DNA-ICP conjugates to enter cells in a manner analogous to AuNP-SNAs, we probed their ability to alter protein expression by targeting a known cancer-related mRNA transcript. SKOV-3 ovarian cancer cells were chosen as they over-express human epithelial growth factor receptor 2 (HER2), which is involved in signal transduction pathways leading to malignant cell growth and differentiation.[28] We performed a series of gene knockdown experiments utilizing anti-HER2 DNA-ICPS. SKOV-3 cells were incubated with different concentrations of antisense DNA-ICPS (HER2-ICP) or non-targeting DNA-ICPS (NonTarget-ICP), with free anti-HER2 DNA complexed with Lipofectamine® (Life Technologies) as a positive control. After 3 days, cells were harvested and HER2 expression was determined by Western blot analysis (Figure 3e). Treatment with anti-HER2 DNAICPs reduced HER2 expression by up to 81%, in a dose dependent fashion. This is comparable to results achieved with commercial transfection kits, and with no change in HER2 expression observed with non-targeting DNA-ICPs. Lastly, no toxic effects or cell death resulted from treatment with DNA-ICPs, as predicted by MTT assays (see Supporting Information, page S10).
In conclusion, we have reported a facile method to synthesize biocompatible, DNA-decorated infinite coordination polymer nanoparticles that are capable of cell entry and gene regulation without transfection agents. Iron(III)-based ICP nanoparticles, synthesized in water, can be conjugated directly to oligonucleotides and carry them across cell membranes. Furthermore, the core is comprised of benign building blocks that are not expected to pose significant health hazards. This work represents a major step towards the construction of clinically viable gene regulation constructs for in vivo applications in the treatment of cancer and other genetic diseases.
Supplementary Material
Acknowledgments
This project is sponsored by the following awards: Defense Advanced Research Projects Agency under grant number HR0011-13-2-0018, Center for Cancer Nanotechnology Excellence initiative of the National Institutes of Health U54 CA151880, and the U.S. Army under contract/grant number W911NF-11-1-0229. T.J.M. acknowledges an IBNAM- Baxter Early Career Development Award in Bioengineering and support from Northwestern University's International Institute for Nanotechnology. W. E. B. acknowledges funding provided by a Patrick G. and Shirley W. Ryan Fellowship. P. S. R. acknowledges support from the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1324585. S. P. N. acknowledges DoD, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a. J.L.R. acknowledges a Postdoctoral Fellowship from the PhRMA foundation. A.W.S. acknowledges CCNE initiative of supplemental NCI/NIH Award 3U54CA151880. The content of the information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
Contributor Information
Colin M. Calabrese, Department of Chemistry Northwestern University, Evanston, IL (USA)
Timothy J. Merkel, International Institute for Nanotechnology Northwestern University, Evanston, IL (USA)
William E. Briley, Interdepartmental Biological Sciences Northwestern University, Evanston, IL (USA)
Pratik S. Randeria, Department of Biomedical Engineering Northwestern University, Evanston, IL (USA)
Suguna P. Narayan, Department of Biomedical Engineering Northwestern University, Evanston, IL (USA)
Jessica L. Rouge, International Institute for Nanotechnology Northwestern University, Evanston, IL (USA)
David A. Walker, International Institute for Nanotechnology Northwestern University, Evanston, IL (USA) Department of Chemical and Biological Engineering Northwestern University, Evanston, IL (USA).
Alexander W. Scott, Department of Biomedical Engineering Northwestern University, Evanston, IL (USA)
Chad A. Mirkin, Department of Chemistry Northwestern University, Evanston, IL (USA); International Institute for Nanotechnology Northwestern University, Evanston, IL (USA); Department of Chemical and Biological Engineering Northwestern University, Evanston, IL (USA).
References
- 1.a Macfarlane RJ, Lee B, Jones MR, Harris N, Schatz GC, Mirkin CA. Science. 2011;334:204–208. doi: 10.1126/science.1210493. [DOI] [PubMed] [Google Scholar]; b Nykypanchuk D, Maye MM, van der Lelie D, Gang O. Nature. 2008;451:549–552. doi: 10.1038/nature06560. [DOI] [PubMed] [Google Scholar]
- 2.a Zheng D, Seferos DS, Giljohann DA, Patel PC, Mirkin CA. Nano Lett. 2009;9:3258–3261. doi: 10.1021/nl901517b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Seferos DS, Giljohann DA, Hill HD, Prigodich AE, Mirkin CA. J. Am. Chem. Soc. 2007;129:5477. doi: 10.1021/ja0776529. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Prigodich AE, Randeria PS, Briley WE, Kim NJ, Daniel WL, Giljohann DA, Mirkin CA. Anal. Chem. 2012;84:2062–2066. doi: 10.1021/ac202648w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]; b Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. J. Am. Chem. Soc. 2009;131:2072. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a Cutler JI, Zhang K, Zheng D, Auyeung E, Prigodich AE, Mirkin CA. J. Am. Chem. Soc. 2011;133:9254–9257. doi: 10.1021/ja203375n. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Young KL, Scott AW, Hao L, Mirkin SE, Liu G, Mirkin CA. Nano Lett. 2012;12:3867–3871. doi: 10.1021/nl3020846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 6.a Cutler JI, Zheng D, Xu X, Giljohann DA, Mirkin CA. Nano Lett. 2010;10:1477–1480. doi: 10.1021/nl100477m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wagner K, Kautz A, Roder M, Schwalbe M, Pachmann K, Clement JH, Schnabelrauch M. Appl. Organomet. Chem. 2004;18:514–519. [Google Scholar]
- 7.a Thompson DG, Enright A, Faulds K, Smith WE, Graham D. Anal. Chem. 2008;80:2805–2810. doi: 10.1021/ac702403w. [DOI] [PubMed] [Google Scholar]; b Dougan JA, Karlsson C, Smith WE, Graham D. Nucleic Acids Res. 2007;35:3668–3675. doi: 10.1093/nar/gkm237. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lee JS, Lytton-Jean AK, Hurst SJ, Mirkin CA. Nano Lett. 2007;7:2112–2115. doi: 10.1021/nl071108g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a Li Y, Duan X, Jing L, Yang C, Qiao R, Gao M. Biomaterials. 2011;32:1923–1931. doi: 10.1016/j.biomaterials.2010.11.024. [DOI] [PubMed] [Google Scholar]; b Sun DZ, Gang O. Langmuir. 2013;29:7038–7046. doi: 10.1021/la4000186. [DOI] [PubMed] [Google Scholar]
- 9.a Rush AM, Thompson MP, Tatro ET, Gianneschi NC. ACS Nano. 2013;7:1379–1387. doi: 10.1021/nn305030g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chien MP, Thompson MP, Gianneschi NC. Chem. Commun. (Cambridge, U. K.) 2011;47:167–169. doi: 10.1039/c0cc02291h. [DOI] [PubMed] [Google Scholar]; c Li Z, Zhang Y, Fullhart P, Mirkin CA. Nano Lett. 2004;4:1055–1058. [Google Scholar]
- 10.Cutler JI, Auyeung E, Mirkin CA. J. Am. Chem. Soc. 2012;134:1376–1391. doi: 10.1021/ja209351u. [DOI] [PubMed] [Google Scholar]
- 11.a Lytton-Jean AK, Langer R, Anderson DG. Small. 2011;7:1932–1937. doi: 10.1002/smll.201100761. [DOI] [PubMed] [Google Scholar]; b Rush AM, Nelles DA, Blum AP, Barnhill SA, Tatro ET, Yeo GW, Gianneschi NC. J. Am. Chem. Soc. 2014;136:7615–7618. doi: 10.1021/ja503598z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a Dhar S, Daniel WL, Giljohann DA, Mirkin CA, Lippard SJ. J. Am. Chem. Soc. 2009;131:14652. doi: 10.1021/ja9071282. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang X-Q, Xu X, Lam R, Giljohann D, Ho D, Mirkin CA. ACS Nano. 2011;5:6962–6970. doi: 10.1021/nn201446c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei M, Chen N, Li J, Yin M, Liang L, He Y, Song H, Fan C, Huang Q. Angew Chem Int Ed Engl. 2012;51:1202–1206. doi: 10.1002/anie.201105187. [DOI] [PubMed] [Google Scholar]
- 14.a Patel PC, Giljohann DA, Daniel WL, Zheng D, Prigodich AE, Mirkin CA. Bioconjugate Chem. 2010;21:2250–2256. doi: 10.1021/bc1002423. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Choi CHJ, Hao L, Narayan SP, Auyeung E, Mirkin CA. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7625–7630. doi: 10.1073/pnas.1305804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, Scott AW, Rotz MW, Meade TJ, Giljohann DA, Mirkin CA, Stegh AH. Science Translational Medicine. 2013;5 doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alkilany AM, Murphy CJ. Journal of Nanoparticle Research. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a Spokoyny AM, Kim D, Sumrein A, Mirkin CA. Chem. Soc. Rev. 2009;38:1218–1227. doi: 10.1039/b807085g. [DOI] [PubMed] [Google Scholar]; b Lin W, Rieter WJ, Taylor KM. Angew Chem Int Ed Engl. 2009;48:650–658. doi: 10.1002/anie.200803387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a Rieter WJ, Pott KM, Taylor KML, Lin W. J. Am. Chem. Soc. 2008;130:11584. doi: 10.1021/ja803383k. [DOI] [PubMed] [Google Scholar]; b Gao PF, Zheng LL, Liang LJ, Yang XX, Li YF, Huang CZ. Journal of Materials Chemistry B. 2013;1:3202–3208. doi: 10.1039/c3tb00026e. [DOI] [PubMed] [Google Scholar]
- 19.Huxford RC, deKrafft KE, Boyle WS, Liu D, Lin W. Chemical Science. 2012;3:198–204. doi: 10.1039/C1SC00499A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu ZD, Hider RC. Coord. Chem. Rev. 2002;232:151–171. [Google Scholar]
- 21.Burgess J, Rangel M. Advances in Inorganic Chemistry, Vol 60. 2008;60:167–243. [Google Scholar]
- 22.Nurchi VM, Crisponi G, Pivetta T, Donatoni M, Remelli M. J. Inorg. Biochem. 2008;102:684–692. doi: 10.1016/j.jinorgbio.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 23.a Szigethy G, Raymond KN. Inorg. Chem. 2010;49:6755–6765. doi: 10.1021/ic1007878. [DOI] [PubMed] [Google Scholar]; b Cho S-H, Gadzikwa T, Afshari M, Nguyen ST, Hupp JT. Eur. J. Inorg. Chem. 2007:4863–4867. [Google Scholar]; c Caulder DL, Bruckner C, Powers RE, Konig S, Parac TN, Leary JA, Raymond KN. J. Am. Chem. Soc. 2001;123:8923–8938. doi: 10.1021/ja0104507. [DOI] [PubMed] [Google Scholar]
- 24.Scarrow RC, Riley PE, Abudari K, White DL, Raymond KN. Inorg. Chem. 1985;24:954–967. [Google Scholar]
- 25.a Menyo MS, Hawker CJ, Waite JH. Soft Matter. 2013;9:10314–10323. doi: 10.1039/C3SM51824H. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Holten-Andersen N, Harrington MJ, Birkedal H, Lee BP, Messersmith PB, Lee KYC, Waite JH. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2651–2655. doi: 10.1073/pnas.1015862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurst SJ, Lytton-Jean AKR, Mirkin CA. Anal. Chem. 2006;78:8313–8318. doi: 10.1021/ac0613582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Note that the gold core is capable of quenching the fluorescence of dye-labelled oligonucleotides, which may lower the apparent fluorescence intensity.
- 28.Zhang K, Hao L, Hurst SJ, Mirkin CA. J. Am. Chem. Soc. 2012;134:16488–16491. doi: 10.1021/ja306854d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.