Abstract
Novel dual vaccine, WSN-Aβ1–10, based on the recombinant influenza virus, expressing immunodominant B-cell epitope of β-amyloid, simultaneously induced therapeutically potent anti-Aβ and anti-influenza antibodies. In this study we showed that boosting of WSN-WT primed mice with WSN-Aβ1–10 enhances anti-viral, but fails to induce anti-Aβ antibody responses. This inhibition is associated to expression of Aβ1–10 within the context of an inactivated influenza virus vaccine. These results demonstrate that the use of an inactivated influenza virus as a carrier for AD vaccine may not be applicable due to the possible inhibition of anti-Aβ antibody response in individuals previously vaccinated or infected with influenza.
Keywords: Alzheimer’s disease, influenza, dual vaccine, immune responses, memory T cells
1. Introduction
Alzheimer’s disease (AD) is the most frequent cause of dementia, which is characterized clinically by a progressive cognitive decline eventually resulting in death, usually within 10 years of diagnosis. Although there have been numerous advances in treatment options currently there are no effective treatments that can halt or reverse AD.
The neuropathological features of AD include neurofibrillary tangles (NFT), deposition of Aβ soluble (monomeric, oligomeric) and insoluble (senile plaques) forms, and neuronal loss in affected brain regions (Price and Sisodia, 1994). During the last decade, much effort have been done on targeting clearance of Aβ from the brain of AD patients via the administration of anti-Aβ antibodies (passive vaccination) or Aβ antigens (active vaccination). While several clinical trials using active vaccines based on small B cell epitope of Aβ are ongoing, two major clinical trials on passive vaccination performed in patients with mild to moderate AD have been completed. Based on data from these passive vaccination trials, the scientific community and medical doctors have suggested that treatment must be initiated earlier on, at the prodromal stage, or even earlier. We assume that active immunization is the most feasible approach for the prevention or treatment at the early stage AD, if candidate vaccines are safe, fairly immunogenic in the elderly, and does not activate the potentially harmful autoreactive Th cells in vaccinated subjects. Previously, we reported on an AD epitope vaccines based on the conventional influenza vaccine (Davtyan et al., 2011). Specifically, two chimeric flu viruses expressing either 7 or 10 aa of Aβ42 (WSN-Aβ1–7 or WSN-Aβ1–10) were generated and tested in mice as inactivated vaccines. We demonstrated that this dual vaccine induced therapeutically potent anti-Aβ antibodies and anti-influenza antibodies in mice. This strategy might be beneficial for treatment of AD patients as well as for prevention of development of AD pathology in pre-symptomatic individuals while concurrently boosting immunity against influenza.
It is well known that although yearly influenza vaccines are different due to genetic drift in circulating viruses, they are conserved T and B cell antigenic epitopes in these vaccines. Specifically, the population of conserved memory CD4+ Th cells generated by vaccination and/or previous infection can be boosted by next year vaccination. Based on these observations, as well as on the results generated with other chimeric viruses (Gonzalo et al., 1999, Zheng et al., 2000), including our AD epitope influenza vaccine results (Davtyan et al., 2013) we investigated whether pre-existing memory CD4+T cells generated by influenza virus vaccination could impact the induction of anti-Aβ antibodies by B cells after one, two or three injections with recombinant flu-Aβ1–10. Contrary to our expectations, this study demonstrates that anti-Aβ humoral responses are compromised in WSN-WT primed mice immunized with WSN-Aβ1–10. These findings are suggestive of the occurrence of carrier induced epitopic suppression (CIES) seen in several conjugate vaccines (Pobre et al., 2014, Jegerlehner et al., 2010, Herzenberg and Tokuhisa, 1980, Herzenberg and Tokuhisa, 1982) or so called original antigenic sin, whereby immune memory is biased toward specific antigenic determinants encountered previously and renders immune responses less effective during sequential exposure to virus variants (Kim et al., 2009).
2. Materials and methods
2.1. Mice
Female, 5–6 week-old C57Bl/6 mice were obtained from the Jackson Laboratory (CA). All animals were housed in a temperature- and light cycle-controlled animal facility at the University of California, Irvine (UCI). Animal use protocols were approved by the Institutional Animal Care and Use Committee of UCI and were in accordance with the guidelines of the National Institutes of Health.
2.2. Preparation of vaccines
Generation and purification of A/WSN/33 (H1N1) wild-type (WSN-WT) virus as well as chimeric influenza virus expressing B cell epitope Aβ1–10 (WSN-Aβ1–10) from Aβ42 was described previously (Davtyan et al., 2011). Protein concentration in purified virus samples was determined using BCA Protein Assay Kit (Pierce, IL). Epitope vaccine was prepared to comprise two copies of the same Aβ B cell epitope fused with universal, promiscuous Th epitope PADRE and synthesized as multiple antigenic peptide, 2Aβ11-PADRE-MAP (Invitrogen, IL) have been described previously (Petrushina et al., 2007).
2.3. Study design
2.3.1. Study 1
In Study 1 mice were immunized subcutaneously (s.c.) with 50µg of inactivated WSN-WT formulated in QuilA adjuvant (Fig. 1). Control mice were injected with QuilA adjuvant. Both groups of mice were injected three times at biweekly intervals and have been boosted three times biweekly with 50µg inactivated chimeric virus, WSN-Aβ1–10 after three months of resting period. Sera were collected 12 days after each prime and booster immunizations except the last booster injection when experiment was terminated and blood and spleens were collected at day 7 after injection. Sera were used to measure anti-Aβ and anti-viral antibody responses. Splenocytes cultures were used to detect cellular immune responses and to analyze myeloid-derived suppressor cell (MDSC) and regulatory T cell (Treg) populations.
Fig. 1.
Design of immunization Study 1. C57Bl/6 mice were primed (3 injections) with inactivated WSN-WT formulated in QuilA adjuvant (n=11) or injected with QuilA adjuvant only (n=11). Four mice from each group (n=4) were terminated after 3 months of resting period and cellular immune responses were analyzed. The remaining animals from both groups (n=7 per group) were boosted three times with inactivated WSN-Aβ1–10.
2.3.2. Study 2
In Study 2 we studied the effect of immunization after switching from WSN-WT to different vaccines without resting period (Fig. 2). After immunizations of mice with inactivated WSN-WT formulated in QuilA, mice were vaccinated with inactivated WSN-Aβ1–10 (Gr.1) or 2Aβ11-PADRE-MAP (50µg per mouse; Gr.2) both formulated in QuilA. Appropriate control groups of mice injected three times with adjuvant were immunized with WSN-Aβ1–10 (Gr.3), 2Aβ11-PADRE-MAP (Gr.4), or WSN-WT (Gr.5) formulated in QuilA. Finally, one group of mice was injected only with adjuvant six times (Gr.6). All experiments were repeated twice.
Fig. 2.
Design of immunization Study 2. C57Bl/6 mice (n=6 per group) were primed (3 injections) with inactivated WSN-WT or injected with QuilA only and switched to inactivated WSN-Aβ1–10, 2Aβ11-PADRE-MAP (3 immunization). Two additional control groups injected with QuilA have been switched to inactivated WSN-WT or continued to be injected with QuilA. In both studies blood was collected after each immunization, on 7th day after last injection mice were terminated and T cell responses were analyzed in splenocytes.
2.4. Detection of cellular immune responses
Analysis of T cell proliferation was performed in splenocyte cultures from individual animals using a [3H]-thymidine incorporation assay, as we described repeatedly (Cribbs et al., 2003, Agadjanyan et al., 2005). The same splenocytes used to assess T cell proliferation were utilized in ELISPOT assay (BD Pharmingen, CA) for detection of cells producing IFN-γ cytokine (Agadjanyan et al., 2005, Petrushina et al., 2007). The level of T cell proliferation and the number of cells producing IFN-γ were detected in splenocyte cultures after their re-stimulation with 10 µg/ml Aβ40 peptide and WSN-WT. Of note, in Study 1 four mice from experimental and control groups were terminated prior to the first booster injection with WSN-Aβ1–10 and cellular immune responses to flu or Aβ were measured in splenocytes cultures obtained from individual animals. In the remaining mice from each group (n=7) cellular immune responses were evaluated at the end of Study 1 on day 155 (Fig. 1). In Study 2 we analyzed cellular immune responses specific to WSN-WT or Aβ in experimental and control mice after termination of whole study (Fig. 2). In addition, cellular immune responses specific to PADRE (re-stimulation with 10 µg/ml peptide) were evaluated in mice from Groups 2, 4 and 6.
2.5. Detection of anti-Aβ and anti-influenza antibody responses
2.5.1. ELISA
Concentration of anti-Aβ and anti-flu antibodies in sera of immunized and control mice was measured by ELISA as described previously (Cribbs et al., 2003, Davtyan et al., 2011). Briefly, 96-well plates (Immulon II; Dynax Laboratories, VA) were coated with 2.5 µM soluble Aβ42 (pH 9.7, o/n, and 4°C) or 10 µg/ml protein from inactivated WSN-WT virus. Immune and control sera were added to the wells at indicated dilutions and binding of mouse antibodies to Aβ and virus were detected by HRP-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, ME). The reaction was visualized by 3,3′,5,5′tetramethylbenzidine (TMB) (Pierce, IL) substrate solution. The optical density (OD) was read at 450 nm (Biotek, Synergy HT, VT), and anti-Aβ antibody concentrations were calculated using a calibration curve generated with 6E10 monoclonal antibody (Covance, CA). For measurement of antiviral antibodies, half maximal antibody titers (HMAT) were obtained by dividing the highest OD450 value in the dilution range of each serum sample by two (Davtyan et al., 2011).
2.5.2. Hemagglutination inhibition assay
In addition, we detected virus neutralizing antibodies by hemagglutination inhibition (HI) assay, as described earlier (Davtyan et al., 2011). Briefly, two fold dilutions of RDE-treated serum from immunized and control mice were prepared in saline solution. Then diluted sera were incubated with eight hemagglutination assay (HA) units of WSN-WT. After incubation chicken red blood cells (RBC) were added to each well and HI titer was expressed as the reciprocal of the highest dilution of serum able to inhibit hemagglutination.
2.6. Analysis of MDSC and TREG cells
Flow cytometry (using a MacsQuant cytometer, Miltenyi Biotec, CA) was used to determine and quantify the percentage of two most common populations of suppressor cells, MDSCs and Tregs exactly as we described (Ghochikyan et al., 2014). Briefly, for detection of MDSCs in population of splenocytes we used APC-conjugated anti-CD11b and FITC-conjugated anti-Gr-1 antibodies (Miltenyi Biotec, CA). For detection of Treg cells, splenocytes were first stained with VioBlue-conjugated anti-CD4 antibody (eBioscience, CA), then fixed, permeabilized and stained with FITC-conjugated anti-FoxP3 antibody and control antibody (rat IgG2a). FlowJo software (Treestar, OR) was used to analyze data obtained by flow cytometer.
2.7. Statistical analysis
Statistical parameters (mean, standard deviation (SD), significant difference, etc.) were calculated using Prism 6 software (GraphPad Software, Inc., CA). Statistically significant differences were examined using a t-test or analysis of variance (ANOVA) and Tukey's multiple comparisons post-test (a P value of less than 0.05 was considered significant).
3. Results
3.1. Anti-Aβ humoral responses induced by inactivated chimeric virus WSN-Aβ1–10 are compromised in mice with pre-existing memory against wild-type virus
We first tested the generation of memory Th cells specific to virus in response to vaccination with inactivated WSN-WT (Fig. 1). As expected from earlier studies (Davtyan et al., 2013), after a threemonth resting period, mice vaccinated with WSN-WT possessed significantly higher number of influenza specific splenocytes (P<0.001) producing IFN-γ compared to control animals injected with QuilA only (Fig. 3A). Recall cellular immune responses in immunized mice with pre-existing memory Th cells were confirmed by measuring of proliferation of splenocytes (Fig. 3B). However, both groups of mice responded equally well to three booster injections with inactivated WSN-Aβ1–10 regardless of presence of pre-existing memory Th cells: no differences were observed in numbers of IFN-γ producing cells and the level of splenocytes proliferation (Fig. 3C, D). Importantly, booster injections with WSN-A β1–10 did not induce potentially harmful autoreactive Th cells (no cellular responses were detected after re-stimulation of splenocytes with Aβ40 peptide), that was observed in Phase IIa trial evaluating AN-1792 vaccine consisted of fibrillar Aβ42 as the immunogen and a strong Th1-type adjuvant, QS21. The trial had been halted because approximately 6% of the volunteers developed some degree of meningoencephalitis (infiltration of T cells and macrophages) likely due to activation of autoreactive T cell specific to self-epitopes within the Aβ42 peptide (Nicoll et al., 2003, Ferrer et al., 2004, Lobello et al., 2012, Orgogozo et al., 2003).
Fig. 3.
Mice immunized with inactivated WSN-Aβ1–10 generated equally strong virus-specific T cell response regardless of presence of virus-specific memory T cells. (A, B) Number of IFNγ producing T cells (A) and proliferation rate of T cells (B) detected in splenocyte cultures of mice primed with inactivated WSN-WT and then rested for three months. (C, D) Number of IFNγ producing T cells (C) and proliferation rate of T cells (D) detected in splenocyte cultures of mice primed with inactivated WSN-WT and then boosted with inactivated chimeric virus after three months of resting period. Error bars represent average ± SD (n=4/group for A and B, and n=7/group for C and D, ***P<0.001).
No measurable concentration of anti-Aβ antibody was detected in sera of mice primed with inactivated WSN-WT formulated in QuilA or injected with adjuvant alone (Fig. 4A left and middle panel). Previously, we demonstrated that a single injection with recombinant protein vaccine, Lu AF20513 formulated in QuilA, lead to induction of robust anti-Aβ antibody responses only in mice with pre-existing memory Th cells (Davtyan et al., 2013). Accordingly, we expected that mice with pre-existing anti-flu memory Th cells will respond identically to a single boost with WSN-Aβ10. However, in contrast to our previously published results with Lu AF20513 booster, injection with chimeric virus did not induce robust humoral immune responses specific to amyloid. Furthermore, we detected significantly lower (P<0.05) concentrations of anti-Aβ antibodies in sera of mice primed with WSN-WT/QuilA and boosted with chimeric virus compared with mice injected with adjuvant alone and boosted with WSN-Aβ1–10 (Fig. 4A right panel). Moreover, additional booster injections not only were unable to maintain humoral immune responses specific to the amyloid, but exacerbated this inhibition (after 2nd and 3rd booster injections; P<0.01). Thus, instead of enhancing humoral immune responses, pre-existing anti-flu responses decreased anti-Aβ antibody responses after vaccination of mice with inactivated WSN-Aβ1–10.
Fig. 4.
Anti-Aβ, but not anti-WSN humoral response is inhibited in mice possessing pre-existing virus-specific memory T cells after booster injections with inactivated chimeric virus WSN-Aβ1–10. Concentrations of anti-Aβ antibodies (A) and half-maximal titers of anti-WSN antibodies (B) detected in sera of individual mice by ELISA. Error bars represent average ± SD for n=7 per group (*P<0.05; **P<0.01; ***P<0.001; **** P<0.0001)
To see if this inhibition is specific to Aβ or humoral immune responses specific to viral antigens are also suppressed in mice pre-immunized with inactivated WSN-WT we measured titers of anti-WSN-WT antibodies. Priming of mice with WSN-WT formulated in QuilA generated high titers of anti-flu antibodies that persisted during 3 mo of resting period (Fig. 4B left and middle panel). Evidently, control animals injected with adjuvant did not generate anti-viral antibodies. Importantly, the presence of pre-existing anti-viral Th cells appeared to facilitate anti-viral antibody responses after boosting of mice with WSN-Aβ1–10 (Fig. 4B, right panel): 1, 2 and 3 booster injections significantly enhanced (P<0.05, P<0.001 and P<0.0001, respectively) titers of anti-WSN-WT antibody responses (Fig. 4B, compare data in right and middle panels). At the same time, our results showed that WSN-WT and WSN-Aβ1–10 are equally immunogenic in mice, because three injections with either of these vaccines induce similar levels of anti-viral humoral responses (Fig. 4B, compare data in left and right panels). In sum, while anti-Aβ antibody response was inhibited, anti-viral humoral response was increased in mice primed with WSN-WT.
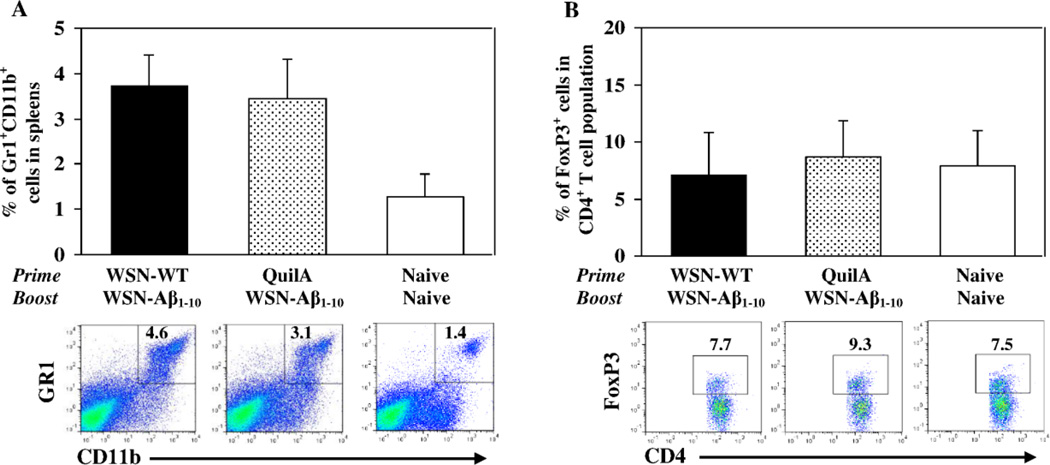
To evaluate the possible role of two major populations of suppressor cells in inhibition effect of anti-Aβ antibody response we measured the percentage of myeloid derived suppressor cells (MDSCs) and regulatory Th cells (Treg) in spleens of experimental and control animals. The level of GR1+CD11b+ MDSCs detected in spleens isolated from mice primed with inactivated WSN-WT and boosted after 3 months of resting period with inactivated WSN-Aβ1–10 was equal to that detected in spleens of mice injected with QuilA and immunized with WSN-Aβ1–10 (Fig. 5A). Equal numbers of CD4+FoxP3+Treg cells were also detected in the spleens isolated from mice injected with WSN-WT formulated in QuilA or QuilA alone and boosted with WSN-Aβ1–10 (Fig. 5B). These data suggest that this anatomic compartment did not experience any changes in levels of MDSC and Treg cells due to the administration of WSN-WT, QuilA or chimeric vaccine, WSN-Aβ1–10.
Fig. 5.
Inhibition of anti-Aβ antibody responses in mice primed with inactivated WSN-WT and boosted with inactivated WSN-Aβ1–10 is not associated with changes in levels of major subsets of immunosuppressor cells, MDSC and Treg cells in spleens of mice. (A) Depicts the percent of MDSC (Gr1+CD11b+ cells) within all nucleated spleen cells analyzed by flow cytometry (n=7 per group). Representative plots for each group are presented. (B) Depicts the percentage of Treg cells (FoxP3+ cells in CD4+ T cell population) in the spleens (n=7 per group). Representative plots for each group are presented.
3.2. Pre-existing anti-viral memory Th cells are not responsible for inhibition of anti-Aβ antibody responses
To understand if the inhibition effect of anti-Aβ antibodies is connected with generation of anti-viral memory Th cells, we designed another experiment where mice immunized 3 times with WSN-WT/QuilA or QuilA alone were then immunized with WSN-Aβ1–10 without resting period (Fig. 2). Several control groups have been included into the experimental design as outlined in Fig. 2 table. First we analyzed the cellular immune responses in spleens of mice from experimental and control groups and showed that all mice that have been administrated either inactivated WSN-WT or WSN-Aβ1–10, but not QuilA or 2Aβ11-PADRE-MAP generated Th cells specific to virus (Fig. 6A, C). Aβ40-specific cellular response have not been detected in any experimental or control group (Fig. 6A, C). At the same time, as shown previously (Petrushina et al., 2007) 2Aβ11-PADRE-MAP formulated in QuilA induced strong Th cell responses specific to PADRE (Fig. 6B, D).
Fig. 6.
Immunization with inactivated WSN-WT followed by switching to inactivated WSN-Aβ1–10 did not affect the virus specific T cell response. Numbers of IFNγ-producing T cells (A, B) and proliferation of T cells (C, D) was analyzed in splenocyte cultures by ELISPOT assay and [3H] thymidine incorporation assay, respectively. Splenocytes were re-stimulated in vitro with the 10µg/ml WSN-WT antigen (■), Aβ40 (□) or PADRE (▥) peptides. Bars represent average ± SD for n=6/group.
Analysis of antibody responses generated in these mice revealed significant inhibition of anti-Aβ antibody responses in mice immunized with WSN-WT and then immunized with WSN-Aβ1–10 compared with mice that have been injected with adjuvant only and then switched to immunization with chimeric virus. The significant inhibition of anti-Aβ antibody responses was observed after each injection with WSN-Aβ1–10 (Fig. 7A; P<0.001, P<0.05 and P<0.01 after 1st, 2nd and 3rd immunizations, respectively). In contrast, when we switched immunizations from WSN-WT to WSN-Aβ1–10 significantly higher titers of antibodies against virus were detected in comparison to mice injected with adjuvant only and immunized with chimeric virus (Fig. 7B; P<0.001, P<0.0001 and P<0.001 after 1st, 2nd and 3rd immunizations, respectively). These differences in the levels of anti-flu antibody responses are associated with number of immunizations (mice injected with both WSN-WT and WSN-Aβ1–10 obtained 3 additional doses of flu antigens than animals injected with QuilA and WSN-Aβ1–10) as is evident from correlation of results presented in Fig. 7B (R2=0.9979 and R2=0.9985 for WSN-WT/WSN-Aβ1–10 and QuilA/WSN-Aβ1–10, respectively). These ELISA data have been supported by titers of neutralizing antibodies. Again in mice that have been primed by WSN-WT anti-viral humoral immune response was not suppressed after boosting with WSN-Aβ1–10 (Fig. 7C). Again, the differences between titers of virus neutralizing antibodies detected between WSN-WT/WSN-Aβ1–10 and QuilA/WSN-Aβ1–10 groups (Fig. 7C) likely related to the doses of viral antigens (R2=1 for these groups). Of note, no changes in numbers of suppressor cells (MDSCs and Tregs) in splenocyte cultures obtained from mice primed with WSN-WT or QuilA and vaccinated with WSN-Aβ1–10 were detected (Fig. 8).
Fig. 7.
Anti-Aβ, but not anti-WSN humoral response is inhibited in mice immunized with inactivated WSN-WT followed by switching to inactivated WSN-Aβ1–10. (A) Significantly lower concentrations of anti-Aβ antibodies were detected in mice immunized with WSN-WT, then switched to WSN-Aβ1–10 compared with control group injected with QuilA then switched to WSN-Aβ1–10 (B) Significantly higher titers of anti-WSN antibodies were detected in mice immunized with WSN-WT, then switched to WSN-Aβ1–10. compared with control group obtained three QuilA and WSN-WT injections. (C) Slightly different titers of virus-neutralizing antibodies were generated in both groups probably reflecting differences in the number of immunizations. (D) No inhibition of anti-Aβ responses were observed in mice immunized with WSN-WT then switched to 2Aβ11-PADRE-MAP, which contains the same epitope of amyloid, but does not possess viral antigens. Humoral immune responses to Aβ and WSN virus were measured in individual sera by ELISA. Titers of virus-neutralizing antibodies were measured by HI assay. Error bars represent average ± SD for n=6 per group (*P<0.05; **P<0.01; ***P<0.001; ****P<0.0001).
Fig. 8.
Inhibition of anti-Aβ antibody responses in mice immunized with inactivated WSN-WT followed by switching to inactivated WSN-Aβ1–10 is not associated with changes in levels of major subsets of immunosuppressor cells, MDSC and Treg cells in spleens of mice. (A) Depicts the percent of MDSC (Gr1+CD11b+ cells) within all nucleated spleen cells analyzed by flow cytometry (n=6 per group). Representative plots for each group are presented. (B) Depicts the percentage of Treg cells (FoxP3+ cells in CD4+ T cell population) in the spleens (n=6 per group). Representative plots for each group are presented.
To understand the specificity of this inhibition we analyzed anti-Aβ antibody responses in mice primed with WSN-WT and boosted with 2Aβ11-PADRE-MAP, the AD vaccine that has the similar epitope of amyloid, but do not possessed any viral antigens. As shown in Fig. 7D these mice generated anti-Aβ antibody responses similar to that generated in animals primed with QuilA and boosted with the same 2Aβ11-PADRE-MAP vaccine. In other words, priming with WSN-WT did not immunosuppress anti-Aβ specific B cells. These results suggested that the inhibition of anti-Aβ antibody responses in mice immunized with WSN-WT and switched to WSN-Aβ1–10 is associated with presentation of the non-flu epitope Aβ1–10 in the context of an inactivated WSN virus.
4. Discussion
Current data suggest that the most promising strategy for treatment/prevention of AD is immunotherapy, which via Aβ- and/or tau-specific antibodies could facilitate the clearance of the most toxic forms of β-amyloid and abnormal tau deposits from the brain (Wisniewski and Konietzko, 2008, Schenk et al., 2004, Agadjanyan et al., 2009, Agadjanyan et al., 2005, Cribbs and Agadjanyan, 2005, Weiner and Frenkel, 2006, Sigurdsson, 2009, Ubhi and Masliah, 2011, Gotz et al., 2012). One active vaccine trial based on Aβ42 peptide formulated in QS21 adjuvant (AN1792) and two passive vaccination trials targeting β- amyloid are already completed. From AN1792 clinical trials we learned that to be effective and safe an active vaccine should induce therapeutically relevant titers of anti-Aβ antibodies without activation of autoreactive T-cells (Nicoll et al., 2003, Ferrer et al., 2004, Holmes et al., 2008, Gilman et al., 2005). To eliminate the harmful effect of autoreactive Th cells and have therapeutically relevant concentrations of anti-Aβ antibodies, several companies decided to use passive vaccination strategy. Alternatively, several clinical trials testing active vaccines targeting N-terminal region of β-amyloid linked to carrier are currently ongoing including CAD106 (Novartis), ACC-001 (Pfizer/Elan/Janssen), V950 (Merk), UB311 (United Biomedical), ACI-24 (AC Immune) and AD01/02 (GlaxoSmithKline using technology of AFFITOPE from AFFiRiS) that are already being tested in various Phase I-III trials (Delrieu et al., 2012, Lobello et al., 2012, Lemere and Masliah, 2010, AC Immune, Clinical Pipeline).
During the last decade our group generated various DNA and peptide/recombinant protein based epitope vaccines also targeting small immunodominant B cell epitope of Aβ42. These vaccines induce high levels of therapeutically potent anti-Aβ42 antibodies without activation of potentially harmful autoreactive Th cells in mice, rabbits and monkeys (Agadjanyan et al., 2005, Ghochikyan et al., 2006, Movsesyan et al., 2008, Petrushina et al., 2007, Davtyan et al., 2010, Davtyan et al., 2011, Davtyan et al., 2013, Evans et al., 2014, Ghochikyan et al., 2013, Davtyan et al., 2014). Given the fact that the immune system in elderly people poorly responds to new vaccines due to immunosenescence, we tried to enhance the immunogenicity of epitope vaccine in this population by taking an advantage of yearly vaccinations with seasonal flu vaccine. We designed a dual chimeric vaccine based on flu virus and Aβ1–10 B cell epitope. Multiple copies of the antigen incorporated into the HA protein are displayed in an ordered way on the surface of virus, providing optimal presentation of the antigen to the immune system. We hypothesized that the advantage of such AD vaccine can be associated with quick activation of pre-existing memory Th cells generated after seasonal flu vaccination and/or infection with flu of general population and stimulation of appropriate B cells specific to flu or Aβ1–10. Unexpectedly, data from this study revealed that anti-flu memory T cells generated by immunizations with WSN-WT (Fig. 3) inhibited anti-Aβ antibody response to vaccination with chimeric virus, instead of enhancing this humoral immune response (Fig. 4A). At the same time priming with WSN-WT and generation of anti-viral specific memory T cells did not influence the level of anti-flu antibody responses at all (Fig. 4B). This inhibition of anti-Aβ, but not anti-WSN antibody responses is not correlated with changes of two major subpopulations of suppressor cells (MDSC and Treg) in the spleens of vaccinated mice too (Fig. 5).
To reveal the role of pre-existing anti-viral specific memory Th cells in inhibition of anti-Aβ antibody responses, we tested anti-Aβ and anti-WSN humoral immune responses after continuous immunizations with WSN-WT to WSN-Aβ1–10 vaccines (Fig. 2). In these studies, switching of WSN-WT to chimeric virus again induced significant inhibition of anti-Aβ (Fig. 7A), but not anti-flu (Fig. 7B, C) specific antibody responses. This suppression of humoral responses is not associated with the Aβ1–10 epitope itself, since switching WSN-WT vaccine to the AD vaccine composed of the similar amyloid epitope, Aβ1–11, but irrelevant to flu carrier (2Aβ1–11-PADRE-MAP) did not suppress the production of anti-Aβ antibodies (Fig. 7D). Thus, this phenomenon of anti-Aβ antibody inhibition is not associated with pre-existing anti-viral memory Th cells and/or the level of MDSCs and Tregs (Fig. 8).
We previously reported that pre-existing memory Th cells specific to T cell epitope from tetanus toxoid can dramatically enhance anti-Aβ antibody responses in wild-type (Davtyan et al., 2013) and APP/Tg (paper submitted) mice vaccinated with recombinant protein-based AD vaccine, Lu AF20513. These data, along with results presented in this study, indicated that inhibition of anti-Aβ antibody responses is linked to expression of Aβ1–10 within the context of an influenza virus inactivated vaccine. Based on these data, we suggest that inhibition of anti-Aβ antibody responses in WSN-WT primed mice may reflect two similar mechanisms: original antigenic sin (OAS) or carrier induced epitopic suppression (CIES). OAS phenomenon observed in several viruses frequently drifting. It is well known that infection with influenza virus leads to generation of lifelong memory cells and neutralizing antibodies (Kim et al., 2009). To escape the host immunesurveillance, viruses adapt and evolve via antigenic drift that prevents the neutralization of virus with these antibodies. When the immune host is exposed to the drifted virus, the response to the latter variant is not induced, whereas antibodies to former virus can not neutralize the drifted one. OAS is more pronounced when drift is minor and strains are closely related whereas antigenically distant and dissimilar strains failed to induce OAS (Virelizier et al., 1974, Webster, 1966). In our study, WSN-WT and chimeric WSN-Aβ1–10 differ only by incorporation of small non-viral peptide, Aβ1–10 into the HA simulating the new epitope in drifted strain. Another similar phenomenon called CIES, has been observed after immunization of carrier-primed animals with conjugated vaccines (Pobre et al., 2014, Jegerlehner et al., 2010, Herzenberg and Tokuhisa, 1980, Herzenberg and Tokuhisa, 1982). CIES is explained by competition of carrier-specific memory B cells with naive B cells specific to new antigen for Th cells. It is shown that high density of conjugated antigen (~300 copies) or consequent immunizations may overcome CIES. In our chimeric vaccine Aβ epitope is incorporated into the HA molecule in a genetic level, so the density is coincides with the density of HA in a virion (~ 500 copies). However we observed constant suppression of anti-Aβ antibody response after three immunizations. Is it true that the inhibition of the antibody response to Aβ epitope in a chimeric virus occurs through a mechanism identical to the OAS or CIES, as well as the exact mechanism of this suppression remains to be determined. Nevertheless, our studies indicate an interesting phenomenon, the understanding of which is very important for the further development of new effective dual vaccines based on flu or other viral particles.
Highlights.
Boosting of WSN-WT primed mice with WSN-Aβ1–10 inhibit anti-Aβ antibody responses.
Boosting of WSN-WT primed mice with WSN-Aβ1–10 enhance anti-viral antibody responses.
Boosting of WSN-WT primed mice with WSN-Aβ1–10 enhance anti-viral T cell responses.
Inhibition is associated to expression of Aβ1–10 within the context of an influenza virus.
Acknowledgements
This work was supported by funding from NIH [R01-NS057395 (MGA), R01-AG020241 (MGA) and R01-NS050895 (MGA)] and Alzheimer's Association [IIRG 12-239626 (MGA) and NIRG 13-281227 (HD)]. This work was also partly supported by the Center for Research on Influenza Pathogenesis (CRIP), an NIAID funded Center of Excellence for Influenza Research and Surveillance [CEIRS, contract # HHSN266200700010C (AGS)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts interests
Author(s) declare no financial and commercial conflict of interests. Dr. García-Sastre is named inventor of a patent filed through Mount Sinai School of Medicine that is related to the generation of recombinant influenza A viruses from plasmid DNA.
References
- Agadjanyan M, Cribbs DG, editors. Active and Passive Aβ-Immunotherapy: Positive and Negative Outcomes from Pre-Clinical and Clinical Trials and Future Directions. CNS Neurol Disord Drug Targets. 2009;8 doi: 10.2174/187152709787601849. [DOI] [PubMed] [Google Scholar]
- Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, Saing T, Cribbs DH. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Agadjanyan MG. Immunotherapy for Alzheimer's Disease: Potential Problems and Possible Solutions. Current Immunology Reviews. 2005;1:139–155. [Google Scholar]
- Cribbs DH, Ghochikyan A, Tran M, Vasilevko V, Petrushina I, Sadzikava N, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int. Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtyan H, Ghochikyan A, Cadagan R, Zamarin D, Petrushina I, Movsesyan N, Martinez-Sobrido L, Albrecht RA, Garcia-Sastre A, Agadjanyan MG. The immunological potency and therapeutic potential of a prototype dual vaccine against influenza and Alzheimer's disease. J Transl Med. 2011;9:127. doi: 10.1186/1479-5876-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Cribbs DH, Agadjanyan MG. The MultiTEP platform-based Alzheimer's disease epitope vaccine activates a broad repertoire of T helper cells in nonhuman primates. Alzheimers Dement. 2014;10:271–283. doi: 10.1016/j.jalz.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Poghosyan A, Marleau AM, Movsesyan N, Kiyatkin A, Rasool S, Larsen AK, Madsen PJ, Wegener KM, Ditlevsen DK, Cribbs DH, Pedersen LO, Agadjanyan MG. Immunogenicity, Efficacy, Safety, and Mechanism of Action of Epitope Vaccine (Lu AF20513) for Alzheimer's Disease: Prelude to a Clinical Trial. J Neurosci. 2013;33:4923–4934. doi: 10.1523/JNEUROSCI.4672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtyan H, Mkrtichyan M, Movsesyan N, Petrushina I, Mamikonyan G, Cribbs DH, Agadjanyan MG, Ghochikyan A. DNA prime-protein boost increased the titer, avidity and persistence of anti-Abeta antibodies in wild-type mice. Gene Ther. 2010;17:261–271. doi: 10.1038/gt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrieu J, Ousset PJ, Caillaud C, Vellas B. 'Clinical trials in Alzheimer's disease': immunotherapy approaches. J Neurochem. 2012;120(Suppl 1):186–193. doi: 10.1111/j.1471-4159.2011.07458.x. [DOI] [PubMed] [Google Scholar]
- Evans CF, Davtyan H, Petrushina I, Hovakimyan A, Davtyan A, Hannaman D, Cribbs DH, Agadjanyan MG, Ghochikyan A. Epitope-based DNA vaccine for Alzheimer's disease: Translational study in macaques. Alzheimers Dement. 2014;10:284–295. doi: 10.1016/j.jalz.2013.04.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Rovira MB, Guerra MLS, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghochikyan A, Davtyan A, Hovakimyan A, Davtyan H, Poghosyan A, Bagaev A, Ataullakhanov RI, Nelson EL, Agadjanyan MG. Primary 4T1 tumor resection provides critical "window of opportunity" for immunotherapy. Clin Exp Metastasis. 2014;31:185–198. doi: 10.1007/s10585-013-9619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghochikyan A, Davtyan H, Petrushina I, Hovakimyan A, Movsesyan N, Davtyan A, Kiyatkin A, Cribbs DH, Agadjanyan MG. Refinement of a DNA based Alzheimer's disease epitope vaccine in rabbits. Hum Vaccin Immunother. 2013;9:1002–1010. doi: 10.4161/hv.23875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghochikyan A, Mkrtichyan M, Petrushina I, Movsesyan N, Karapetyan A, Cribbs DH, Agadjanyan MG. Prototype Alzheimer's disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine. 2006;24:2275–2282. doi: 10.1016/j.vaccine.2005.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Gonzalo RM, Rodriguez D, Garcia-Sastre A, Rodriguez JR, Palese P, Esteban M. Enhanced CD8+ T cell response to HIV-1 env by combined immunization with influenza and vaccinia virus recombinants. Vaccine. 1999;17:887–892. doi: 10.1016/s0264-410x(98)00274-6. [DOI] [PubMed] [Google Scholar]
- Gotz J, Ittner A, Ittner LM. Tau-targeted treatment strategies in Alzheimer's disease. Br J Pharmacol. 2012;165:1246–1259. doi: 10.1111/j.1476-5381.2011.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg LA, Tokuhisa T. Carrier-priming leads to hapten-specific suppression. Nature. 1980;285:664–667. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- Herzenberg LA, Tokuhisa T. Epitope-specific regulation. I. Carrier-specific induction of suppression for IgG anti-hapten antibody responses. J Exp Med. 1982;155:1730–1740. doi: 10.1084/jem.155.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunization in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- AC Immune. Clinical Pipeline. http://www.acimmune.com/content/?p=41. [Google Scholar]
- Jegerlehner A, Wiesel M, Dietmeier K, Zabel F, Gatto D, Saudan P, Bachmann MF. Carrier induced epitopic suppression of antibody responses induced by virus-like particles is a dynamic phenomenon caused by carrier-specific antibodies. Vaccine. 2010;28:5503–5512. doi: 10.1016/j.vaccine.2010.02.103. [DOI] [PubMed] [Google Scholar]
- Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol. 2009;183:3294–3301. doi: 10.4049/jimmunol.0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobello K, Ryan JM, Liu E, Rippon G, Black R. Targeting Beta amyloid: a clinical review of immunotherapeutic approaches in Alzheimer's disease. Int J Alzheimers Dis. 2012;2012:628070. doi: 10.1155/2012/628070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, Head E, Biragyn A, Cribbs DH, Agadjanyan MG. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine- a novel immunotherapeutic strategy. PLos ONE. 2008;3:e21–e24. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JM, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, Patel A, Head E, Cribbs DH, Agadjanyan MG. Alzheimer's Disease Peptide Epitope Vaccine Reduces Insoluble But Not Soluble/Oligomeric A{beta} Species in Amyloid Precursor Protein Transgenic Mice. J Neurosci. 2007;27:12721–12731. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobre K, Tashani M, Ridda I, Rashid H, Wong M, Booy R. Carrier priming or suppression: understanding carrier priming enhancement of anti-polysaccharide antibody response to conjugate vaccines. Vaccine. 2014;32:1423–1430. doi: 10.1016/j.vaccine.2014.01.047. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS. Cellular and molecular biology of Alzheimer's disease and animal models. Annu Rev Med. 1994;45:435–446. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- Schenk D, Hagen M, Seubert P. Current progress in beta-amyloid immunotherapy. Curr Opin Immunol. 2004;16:599–606. doi: 10.1016/j.coi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM. Tau-focused immunotherapy for Alzheimer's disease and related tauopathies. Curr Alzheimer Res. 2009;6:446–450. doi: 10.2174/156720509789207930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Masliah E. Recent advances in the development of immunotherapies for tauopathies. Exp Neurol. 2011;230:157–161. doi: 10.1016/j.expneurol.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier JL, Allison AC, Schild GC. Antibody responses to antigenic determinants of influenza virus hemagglutinin. II. Original antigenic sin: a bone marrow-derived lymphocyte memory phenomenon modulated by thymus-derived lymphocytes. J Exp Med. 1974;140:1571–1578. doi: 10.1084/jem.140.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG. Original antigenic sin in ferrets: the response to sequential infections with influenza viruses. J Immunol. 1966;97:177–183. [PubMed] [Google Scholar]
- Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer's disease. Nat Rev Immunol. 2006;6:404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- Wisniewski T, Konietzko U. Amyloid-beta immunisation for Alzheimer's disease. Lancet Neurol. 2008;7:805–811. doi: 10.1016/S1474-4422(08)70170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Palese P, Garcia-Sastre A. Antitumor properties of influenza virus vectors. Cancer Res. 2000;60:6972–6976. [PubMed] [Google Scholar]