Abstract
The clinical high risk (CHR) period is a phase denoting a risk for overt psychosis during which subacute symptoms often appear, and cognitive functions may deteriorate. To compare biological indices during this phase with those during first episode schizophrenia, we cross-sectionally examined sex- and age-matched clinical high risk (CHR, n=21), first episode schizophrenia patients (FESZ, n=20) and matched healthy controls (HC, n=25) on oddball and novelty paradigms and assessed the N100, P200, P3a and P3b as indices of perceptual, attentional and working memory processes. To our knowledge, this is the only such comparison using all of these event-related potentials (ERPs) in two paradigms. We hypothesized that the ERPs would differentiate between the three groups and allow prediction of a diagnostic group. The majority of ERPs were significantly affected in CHR and FESZ compared with controls, with similar effect sizes. Nonetheless, in logistic regression, only the P3a and N100 distinguished CHR and FESZ from healthy controls, suggesting that ERPs not associated with an overt task might be more sensitive to prediction of group membership.
Keywords: N100, P200, P3a, P3b
1. Introduction
The clinical high risk (CHR) period is a clinical syndrome denoting a risk for overt psychosis characterized by subthreshold symptoms during which cognitive functions may deteriorate (Yung and McGorry, 1996; Rossler et al., 2011; Giuliano et al., 2012). Conversion to psychosis after CHR diagnosis is in the range of 9-36% between 6 and 36 months (Miller et al., 2002) (Yung et al., 2003; Cannon et al., 2008; Fusar-Poli et al., 2011). Diagnostic criteria such as the COPS (Criteria of Prodromal Syndromes) (Miller et al., 1999) are valuable; however, diagnostic and predictive biological tools are needed to complement them to guide targeted interventions as CHR individuals who progress to psychosis need to be identified and treated (Keshavan et al., 2003). Additionally, CHR individuals who do not progress to psychosis might be vulnerable to other mental conditions (Rossler et al., 2011) and are shown to retain a lower level of functioning than healthy controls with persistent disability at least at 2.5 years after a diagnosis of psychosis risk syndrome (Addington et al., 2011). Event-related brain potentials (ERPs) reflect distinct sensory and cognitive processes, and might offer neurocognitive indices of brain function during the clinical high risk state.
Among ERP components, the P300, or P3b, with typical parietal scalp distribution, is thought to reflect a mechanism involved in the updating of contextual representations in working memory (Donchin, 1981; Polich, 2007). A relative decrease in the amplitude of the P300 component is one of the most replicated findings in schizophrenia in comparison with healthy controls (Jeon and Polich, 2003). Decreased amplitudes have been shown in chronic schizophrenia patients (e.g., Pfefferbaum et al., 1984; McCarley et al., 1991; Ford, 1999; Jeon and Polich, 2003; Javitt et al., 2008), in symptomatically unaffected relatives (Price et al., 2006), in patients recently hospitalized for their first psychotic episode (Salisbury et al., 1998; McCarley et al., 2002), and in individuals at high risk for developing schizophrenia (Bramon et al., 2008; Frommann et al., 2008; van Tricht et al., 2010; Fusar-Poli et al., 2011).
A distinct ERP component, the more fronto-centrally scalp distributed novelty P3 or P3a, which arises ~300 ms after stimulus onset, is also affected in schizophrenia and is thought to represent a mechanism involved in the rapid orienting of attention to events that are unexpected and contextually deviant (Squires et al., 1975; Ranganath and Rainer, 2003; Polich, 2007). Amplitude decrease has been shown in chronic schizophrenia patients (Devrim-Ucok et al., 2006; Mathalon et al., 2010) and in clinical high risk (Jahshan et al., 2012; Mondragon-Maya et al., 2013). In recent onset subjects, amplitude deficits ( Valkonen-Korhonen et al., 2003b; Hermens et al., 2010; Kaur et al., 2011; Jahshan et al., 2012), but also a lack of them, have been reported (Frodl et al., 2001b; Devrim-Ucok et al., 2006; Atkinson et al., 2012).
High heritability of P3b amplitude has been shown in schizophrenia twin and sibling studies (Bramon et al., 2005; Hall et al., 2007; Groom et al., 2008; Bestelmeyer et al., 2009), and the data suggest that P3b might be an endophenotype of an executive control process. The frontal portion of P300, related to novel P300, has also been found to be decreased in unaffected siblings of patients with schizophrenia (Turetsky et al., 2007; Turetsky et al., 2000). A longitudinal study, aimed at tracking changes in P3b and P3a amplitude in schizophrenia patients, suggests auditory P3b and P3a as trait markers of schizophrenia (Mathalon et al., 2000).
According to Polich (2007), “it is reasonable to infer that stimulus evaluation engages focal attention (P3a) to facilitate context maintenance (P3b), which is associated with memory operations,” indicating that assessing the two processes in the same subjects might be the essential step in dissecting and defining the extent of contribution of the two processes to clinical diagnosis. Only a few studies have investigated both the P3b and the P3a components in the same subjects (Kirihara et al., 2009; Mathalon et al., 2010), and to the best of our knowledge not in clinical high risk or recent onset individuals. The aim of the present investigation was thus to assess the P3a and the P3b, in addition to the mid-latency N100 and P200 components, in clinical high risk (HR) subjects, in first episode schizophrenia (FESZ) subjects and in age-matched healthy controls (HC) cross-sectionally, where all subjects including CHR, FESZ and HC were assessed on auditory classical and novelty oddball tasks. Both tasks were administered during the same visit, reducing the possibility of variations in the subject physical and/or mental status (Ford, 1999). Thus, the ERP components were used in a preliminary logistic model to assess their ability to predict group membership.
We note that since complex deviants elicited both a P3a and a P3b (del Re et al., 2014), the P3b to complex deviants will be refered to as P3bn to distinguish it from the P3b elicited by target sounds.
2. Methods
2.1. Participants
Participants comprised 21 CHR (8 females), 20 FESZ (6 females), and 25 HC (13 females), recruited via the Boston Center for Intervention Development and Applied Research (www.bostoncidar.org). HC were recruited from the general community via Internet advertisements. CHR and FESZ were recruited from outpatient clinics affiliated with Harvard Medical School, or through referrals from clinicians. The study was approved by the local IRB committees at Harvard Medical School, Beth Israel Deaconess Medical Center, Massachusetts General Hospital, Brigham and Women's Hospital, and the Veterans’ Affairs Boston Healthcare System (Brockton campus). All study participants, or legal guardians for those under 18 years of age, gave written informed consent and received payment for participation.
Inclusion criteria for all subjects were as follows: no mental retardation (IQ<70), right-handedness, no history of electroconvulsive shock treatment (ECT) ever for HC and within the past 5 years for FESZ, no history of neurological illness, no alcohol/drug dependence in the last 5 years, and no abuse in the past month. The HC participants were drawn from the same geographic bases as the FESZ patients, with comparable age,gender, race and ethnicity, handedness, and parental socioeconomic status (see Table 1). No HC subject met criteria for any current major DSM-IV-TR Axis I disorders or had a history of psychosis, major depression (recurrent), bipolar disorder, obsessive-compulsive disorder, posttraumatic stress disorder, or developmental disorders. HC subjects were also excluded if they had a history of psychiatric hospitalizations, prodromal symptoms, schizotypal or other Cluster A personality disorders, first degree relatives with psychosis, or any current or past use of antipsychotics.
Table 1.
Socio-demographic and clinical information.
| HC (n=25) | CHR (n=21) | FESZ (n=20) | ||
|---|---|---|---|---|
| Mean age (SD) | 21.9 (2.4) | 20.6 (3.7) | 22.4 (4.9) | N.S. |
| Gender (male/female) | 12/13 | 13/8 | 14/6 | N.S. |
| Pre-morbid IQ (oral reading) | 117.7 (14.9) | 115.4 (12.9) | 114.9 (14.9) | N.S. |
| Current IQ | 121.4 (14.5) | 121.4 (11.0) | 106.3 (15.9) | p<0.01 |
| Education (years) | 14.6 (2.0) | 12.9 (2.8) | 13.5 (2.6) | N.S. |
| PSES | 1.7 (1.0) | 1.9 (0.9) | 2.2 (0.95) | N.S. |
| GAF | 84.7 (7.5) | 49.2 (9.8) | 51.7 (13.1) | p<0.01 |
| Time between Ist admission-EEG (years) | N.A. | N.A. | 1.36 (0.6) | N.A. |
| SOPS Total | N.A. | 26 (8.9) | N.A. | N.A. |
| SOPS Positive | N.A. | 11.8 (5.1) | N.A. | N.A. |
| SOPS Negative | N.A. | 14.2 (7.7) | N.A. | N.A. |
| BPRS Total | N.A. | N.A. | 96.0 (12.6) | N.A. |
| BPRS Positive | N.A. | N.A. | 6.9 (3.3) | N.A. |
| BPRS Negative | N.A. | N.A. | 6.1 (2.9) | N.A. |
| Medicated/unmedicated | N.A. | 7/14 | 18/2 | p<0.01 |
| CPZ equivalents | N.A. | 68.6 (51.0) | 334.4 (350.4) | p<0.01 |
| Targets | 35.8 (1.7) | 36.6 (1.9) | 37.6 (1.9) | N.S. |
Values are mean (SD); HC, Healthy controls; CHR, Clinical high risk individuals; FESZ, First episode schizophrenia patients; CPZ, Chlorpromazine; PSES, Parental Socioeconomic Status; N.A., not applicable; N.S., not significant, p >0.05. CPZ equivalents were calculated for subjects on medication (CHR, n=7; FESZ, n=18) (according to Stoll, 2009, and Woods, 2003); Targets, number of targets counted across the classic and novelty oddball tasks.
Exclusion criteria for all subjects were sensory-motor handicaps; neurological disorders; medical illnesses that significantly impair neurocognitive function; education less than 5th grade if under 18 years of age and less than 9th grade if over 18 years of age, not fluent in English; DSM-IV substance abuse in the past month; DSM-IV substance dependence, excluding nicotine, in the past 3 months; current suicidality; or study participation by another family member.
In the CHR group, prodromal phase COPS (Criteria of Prodromal Symptoms) criteria were assessed using the Structured Interview for Prodromal Symptoms (SIPS) (Miller et al., 1999), and the presence of personality disorders was determined using the Diagnostic Interview of Personality Disorders (DIPD) (Zanarini et al., 1987). The 10 items from the Bonn Scale for the Assessment of Basic Symptoms (BSABS) identified as having high predictive validity for the development of psychosis (Klosterkotter et al., 2001) and together termed Cognitive-Perceptive Basic Symptoms (COPER) (Schultze-Lutter F, 2007b) were also used. These symptoms are implemented in the Schizophrenia Proneness Instrument, Adult Version (SPI-A) (Schultze-Lutter, 2007a). No CHR subject had converted to schizophrenia within the 1-year follow-up used by the study. The following additional diagnoses existed in the CHR group: major depressive disorder (1); major depressive disorder and posttraumatic stress disorder (1); major depressive disorder and phobia (3); major depressive disorder and generalized anxiety (1); bipolar disorder II and anxiety disorder (2), bipolar disorder II and posttraumatic stress disorder (1); dysthymic disorder and anxiety (1); attention deficit-hyperactivity disorder (1); conduct disorder (1); and generalized anxiety (5).
FESZ patients met DSM-IV-TR criteria for either schizophrenia, schizoaffective disorder or schizophreniform disorder. FESZ diagnoses were based on interviews with the Structured Clinical Interview for DSM-IV-TR (SCID), Research Version (First, 2002) and information from medical records. FESZ clinical symptoms were rated using the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962) and with the Scale for Asssessment of Positive (SAPS) and Negative (SANS) symptoms (Andreasen 1984, 1983). For FESZ the average time between first hospitalization and the electroencephalographic (EEG) session was 1.36 ± 0.63 years (range 0.5-2.34 years). Three FESZ patients had additional diagnoses as follows: posttraumatic stress disorder (1); major depressive disorder (1); and specific phobia (1).
All participants were evaluated with the Global Assessment of Functioning scale (GAF) (Jones et al., 1995).
Handedness was assessed using the Annett Handedness Questionnaire (Annett, 1970). Subjects' education years and parents' socioeconomic status (PSES), assessed with the Hollingshead two-factor index ( Hollingshead, 1965; Hollingshead, 1975), determined the social status of the study sample. Premorbid IQ was assessed using the reading scale of the Wide Range Achievement Test-4 (WRAT-4) (Wilkinson and Robertson, 2006) and current IQ was assessed using the vocabulary and block design T-score of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). Seven CHR and 18 FESZ subjects were medicated at the time of testing. Medications were as follows: for CHR subjects, aripripazole 2, risperidone 2, quetiapine 1, olanzapine 1, aripripazole + olanzepine 1; for FESZ subjects, aripripazole 3, quetiapine 2, olanzepine 1, risperidone 2, paliperidone 1, clozapine 1; fluphenazine decanoate +fluphenazine HCl 1; fluphenazine HCl + ziprasidone 1; quetiapine + aripripazole 1; quetiapine + olanzepine 1; risperidone + aripripazole 1; risperidone + ziprasidone 1; risperidone + aripripazole + risperidone 1. Medication dosage was estimated using chlorpromazine (CPZ) equivalents (Table 1).
All demographic and clinical data are summarized in Table 1.
2.2. Stimuli and tasks
Subjects performed a classic two-stimulus oddball task and a three-stimulus novelty oddball task, each 4 min in duration. In the classic oddball, stimuli were tones (82 ms in duration, 75 dB SPL), with 20% (36) infrequent target tones (1.5 kHz) and 80% (144) frequent standard tones (1 kHz) presented with a stimulus onset asynchrony of 976 ms (onset-to-onset). The subjects’ task was to silently count the targets. In the novelty oddball task, infrequent non-target complex deviants were also included. Complex deviants consisted of complex environmental stimuli (300-320 ms in duration, 75 dB SPL) such as dog barking ot door slamming. Six environmental sounds were presented six times each during the task. Novel auditory oddball sequences consisted of 20% (36) target (1.5 kHz), 20% (36) novel, and 60% (108) standard tones (1 kHz).
2.3. EEG recording and processing
The EEG was recorded with a Biosemi Active-Two system using sintered Ag/Ag-Cl electrodes in an electrode cap at 71 standard scalp sites, as described elsewhere (Oribe et al., 2013). The EEG data were processed using BrainVision Analyzer 2.0. The bipolar vertical electro-oculogram (EOG) was derived from electrode Fp1 and an electrode below the left eye. The horizontal EOG was derived from electrodes on the left and right outer canthi. The continuous EEG recordings were segmented from -100 to 900 ms relative to stimulus onset and re-referenced to the algebraic average of the left and right mastoids. Ocular artifact correction was performed using an earlier described method (Gratton et al., 1983). Epochs with artifacts exceeding ± 100 μV with a maximal allowed difference of 200 μV were excluded. Average ERPs were computed from the artifact-free epochs and baseline-corrected with a pre-stimulus baseline of -100 to 0 ms following digital filtering (0-16 Hz).
2.4. ERP analyses
The P3a was measured at frontal (electrodes F1/Fz/F2) and central (C1/Cz/C2) regions; the P3b, elicited by targets in the oddball paradigm, and the P3bn, elicited by complex environmental sounds in the novelty paradigm, were measured at central and parietal (electrodes P1/Pz/P2) regions. The P3a and P3bn in the novelty waveform were analyzed as previously described (del Re et al., 2014). The N100 and the P200, measured in response to standard stimuli, were assessed at frontal (F1/Fz/F2) and central (C1/Cz/C2) regions. The latency ranges for selecting ERP component peaks in individual subject data were determined by visual inspection of the grand average ERPs. All electrodes of interest were inspected to ensure that the same component was being selected at each site. In the classic oddball data, the P3b peak was selected as the most positive point between 250 and 400 ms (Fig. 1). In the case of a bimodal peak, the later parietal maximum peak was manually selected. In the novelty oddball data, the P3a/b waveform was biphasic (Fig. 2). The P3a and P3bn peaks were selected as the most positive peaks between 215-285 ms and 315-390 ms, respectively. In both tasks, the N100 peak was selected as the most negative point between 80 and 150 ms, and the P200 peak was selected as the most positive point between 150 and 300 ms.
Fig. 1.
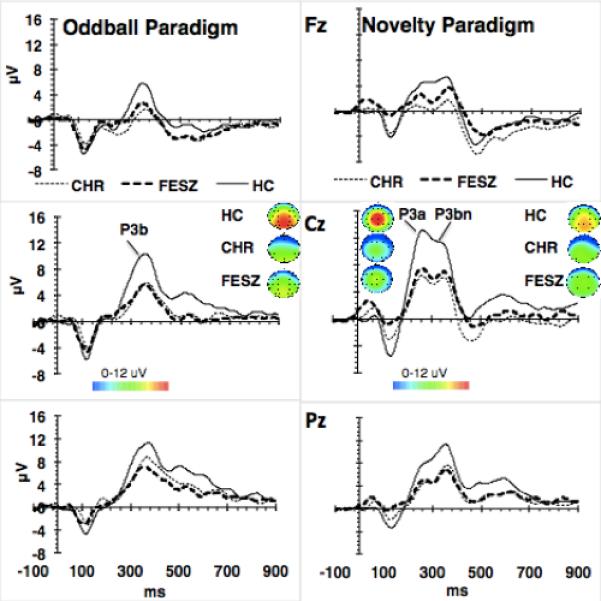
P3a/b. Grand average ERPs to targets in the oddball task, and complex environmental sounds in the novelty oddball paradigm. HC, healthy controls; CHR, clinical high risk; FESZ, first episode schizophrenia patients. Topographical maps indicate scalp distribution of P3a/b in the 3 groups. P3b and P3bn were measured between 315 and 390 ms; P3a between 215 and 285 ms. P3b and P3bn amplitudes measured at Cz and Pz were not different in CHR and FESZ, and both were significantly lower than in HC; P3a amplitude measured at Fz and Cz was also not different in CHR and FESZ but significantly lower than in HC.
Fig. 2.
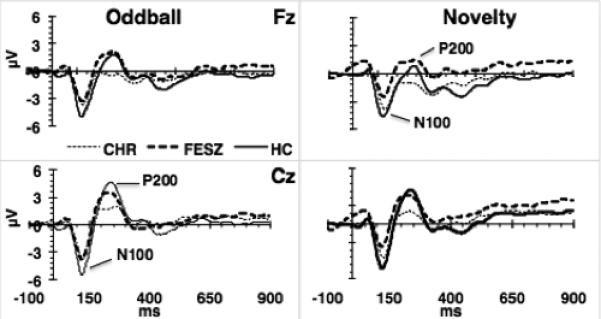
N100-P200. Grand average ERPs of standards in the oddball and novelty paradigms. P200 amplitude was significantly lower in CHR compared with both FESZ and HC, whereas P200 amplitude of these two latter groups did not differ. N100 amplitude was decreased and not significantly different across tasks in CHR and FESZ compared with HC.
The number of epochs included in an average by paradigm and group did not differ significantly. Details are presented in Supplementary Table 3.
2.5. Statistical analyses
All statistical analyses were performed with SPSS v. 22 except effect sizes, which were calculated with G*Power 3.1, 2009 (Faul et al., 2007). Accuracy was assessed using the number of counted targets and tested in an analysis of variance (ANOVA) with Group (HC/CHR/FESZ) as the between-subjects factor and Task (classic/novelty oddball) as the within-subjects factor. ERP peak amplitude and latency were analyzed using multivariate analysis of variance (MANOVA) models. For P3a the model included Group as a between-subjects factor and Region as a within-subjects factor (frontal: F1/2,Fz; central: C1/2,Cz). For P3b and P3bn, the model included Group as a between-subjects factor and Region as a within-subjects factor (central: C1/2,Cz; parietal P1/2, Pz). N100 and P200 were analysed with Group as a between-subjects factor and Task (classic and novel) and Region (frontal/central) as within-subject factors. Where significant group differences were found in the MANOVA, effect sizes are reported as Cohen's f for three-group comparisons and as Cohen's d for two-group comparisons. Tukey's test was applied for post hoc comparisons of groups. Correlations between ERP and clinical/demographic measures were computed using Spearman's rho and were Bonferroni-corrected, i.e., the p value was corrected for the number of variables included in the correlation analysis. The Chi-square test was used to compare categorical data. The impact of ERPs and demographic variables on diagnostic group was assessed with logistic regression.
3. Results
3.1. Subject group characteristics and task performance
The groups did not differ by age (F[2,63] = 1.33, p = 0.272), years of education (F[2,63] = 2.7, p = 0.08), or premorbid IQ (F[2,61] = 0.228, p = 0.798). There were significant differences in current IQ (F[2,61] = 6.7, p < 0.01), which did not differ between CHR and HC (t[42] = 0.4113, p = 0.68), but was lower in FESZ compared with HC (t[41] = -3.2, p < 0.01) and CHR (t[37] = 3.4, p < 0.01). The GAF score differed among the groups (F[2,61] = 7.7, p < 0.01), with equivalent scores in CHR and FESZ (t[39] = 0.68 p = 0.49), which were both lower than in HC (CHR vs. HC: t[42] = −13.5, p < 0.001; FESZ vs. HC: t[41] = −10.33, p < 0.001). PSES did not differ among groups (F[2,63] = 1.8, p = 0.179). The proportion of males and females did not differ between groups (χ2[2] = 2.33; p = 0.33). CPZ-equivalent medication dosages were lower in medicated CHR than FESZ (t[18.6] = 3.1, p < 0.01 with t calculated for unequal variances). There were no significant differences in accuracy among the groups (F[2,63] = 0.33), p = 0.72). These data are summarized in Table 1.
3.2. Classic oddball task: P3b
The P3b elicited by target stimuli in the classic oddball task had a central-parietal scalp distribution (see Fig. 1), and no significant Group × Region interaction (F[1,63]=2.43; p=0.096) was found. There was an effect of Group on P3b amplitude (F[2,63] = 5.9, p < 0.01, f = 0.36). Tukey's test post-hoc comparisons indicated that P3b amplitude was equivalent in CHR and FESZ (p =0.782) and reduced in each group compared with HC (CHR vs. HC, p<0.05; FESZ vs. HC, p < 0.01). P3b latency did not differ between groups (F[2,63] = 2.8, p = 0.068) (see Supplementary Table 1).
3.3. Novelty oddball task: P3a, P3bn and P3b
Complex environmental auditory stimuli elicited a biphasic late positive waveform (Fig. 1). The P3a peak had a central distribution, while the P3bn peak had a central-parietal distribution. For the P3a there was no significant interaction of Region × Group, but there was an effect of Group on peak amplitude (F[2,63] = 8.3, p < 0.01, f = 0.51). Tukey's post-hoc comparisons found that P3a amplitudes in CHR and FESZ did not differ (p = 0.812), but were reduced compared with HC (CHR vs. HC, p < 0.01; FESZ vs. HC, p < 0.05). P3a latency did not differ between groups (F[2,63] = 0.15, p = 0.859). There was also a Group effect on P3bn amplitude (F[2,63] = 8.9, p < 0.01, f = 0.5). P3bn amplitudes, in CHR and FESZ, according to Tukey's tests, did not differ (p = 0.9), and were reduced compared with HC (CHR vs. HC, p < 0.01; FESZ vs. HC, p < 0.01). P3bn latency did not differ between groups (F[2,63] = 2.7, p = 0.073).
The analysis of P3b amplitude elicited by targets in the novelty oddball task did not show a significant effect of Group (F[2,63]=2.43; p=0.1). The analysis of P3b latency elicited by targets in the novelty oddball task demonstrated a main effect of Group (F[2,63]=3.5; p<0.05). Tukey's post hoc analysis showed longer latency in CHR compared with FESZ (p<0.05), but equivalent latencies between CHR and HC (p=0.14) and between FESZ and HC (p=0.7).
To investigate further the effect of three- versus two-stimulus paradigms on the amplitude of targets, the P3b elicited by targets in the oddball paradigms at the Pz electrode was correlated with the P3b elicited by targets in the novelty oddball task, also measured at the Pz electrode. Spearman's analysis demonstrated a positive correlation between the two measures. In the control group, the rho was 0.47, p<0.05; in the CHR group, the rho was 0.71, p<0.01; while in FESZ, the rho was 0.5, p<0.05.
3.4. N100 and P200
The N100 and P200 evoked by standard stimuli in the classic and novelty oddball tasks are presented in Fig. 2. For N100 amplitude there was an effect of Group (F[2,63] = 5.8, p<0.01, f = 0.43). Post hoc Tukey's tests showed that N100 amplitudes did not differ between CHR and FESZ (p =0.93) and were reduced compared with HC (CHR vs. HC, p < 0.05; FESZ vs. HC, p < 0.01). The effect of Task was not significant (F[1,63] = 0.13, p=0.72), nor was the Task × Group interaction (F[2,63] = 2.9, p = 0.061). N100 latency did not differ between groups (F[2,63] = 0.78, p = 0.464), but was significantly shorter (F[1,63] = 7.1, p < 0.05) in the classic oddball (121.9 ± 14.6 ms) than in the novelty oddball task (126.7 ± 14.8 ms).
For P200 amplitude there was an effect of Group (F[2,63] = 4.57, p < 0.05, f = 0.38). P200 amplitude was lower in CHR compared with HC (p < 0.05) and FESZ (p <0.05) but was not significantly different in FESZ compared twith HC (p=0.9). The effect of Task was significant, with P200 amplitude being greater in the classic oddball than the novelty oddball task (F[1,63] = 19.7, p < 0.01, f = 0.56). For P200 latency, there were no significant effects of Group (F[2,63] = 2.2; p = n.s.) or Task (F[1,63] = 2.2, p = 0.14), and the Group × Task interaction was not significant (F[2,63] = 0.28, p = 0.75).
3.5. Effect of medication in FESZ and CHR
There were no significant correlations between CPZ-equivalent medication dosages and ERP latency or amplitude in medicated FESZ or CHR. In addition, effects of medication were evaluated in CHR subjects by comparing unmedicated (14/21) and medicated (7/21) CHR subgroups on clinical, demographic, and ERP measures. No differences were found (Supplementary Table 2 and Fig. 3).
Fig. 3.
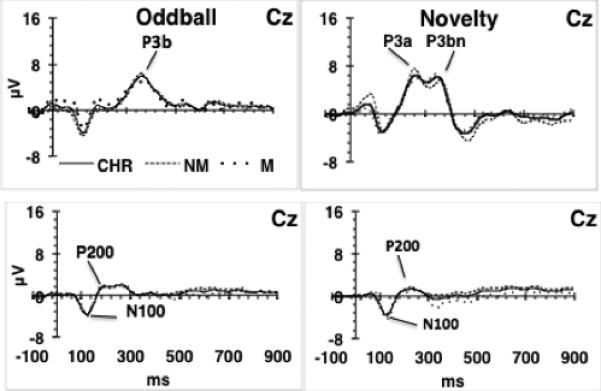
Medicated and unmedicated CHR subjects’ grand averages. Comparison of grand average ERPs in medicated (M) vs. not medicated (NM) CHR subjects. CHR, medicated + not medicated; NM, not medicated, n=14; M, medicated, n=7. No significant differences were found between M and NM for any of the ERPs assessed.
3.6. Logistic regression analysis
The impact of ERPs and demographic variables on diagnostic group membership was assessed by direct logistic regression. The independent variables were entered simultaneously, and included the classic oddball P3b at Pz, novelty oddball P3a at Fz, novelty oddball P3b at Pz, and N100 and P200 amplitudes averaged at frontal and central electrodes across tasks, in addition to age, years of education and current IQ. The dependent variable was diagnosis.
Model 1 compared CHR and FESZ. The significant model (χ2[8, n=39] = 26.9, p = 0.001; Hosmer and Lemeshow Goodness of Fit p > 0.05) explained between 49.8% (Cox and Snell R2) and 66.6% (Nagelkerke R2) of the variance with 84.6% of the cases correctly classified. Current IQ (B = 0.151, p < 0.05) and P200 amplitude (B−1.48, p < 0.05) uniquely contributed to the model, indicating that higher IQ and lower P200 amplitude distinguished CHR from FESZ (Table 2A).
Table 2 A, B and C.
Regression models
| A. CHR vs. FESZ | B | S.E. | Sig. | 95% C.I. | Exp (B) |
|---|---|---|---|---|---|
| IQ | 0.151 | 0.071 | 0.034 | 1.012 | 1.338 |
| Age | 0.171 | 0.275 | 0.535 | 0.692 | 2.036 |
| Education (years) | −0.505 | 0.451 | 0.263 | 0.249 | 1.461 |
| Target P3b | 0.188 | 0.135 | 0.164 | 0.926 | 1.571 |
| Novel P3b | 0.150 | 0.142 | 0.289 | 0.880 | 1.535 |
| Novel P3a | 0.026 | 0.144 | 0.854 | 0.775 | 1.361 |
| N100 | −0.034 | 0.374 | 0.927 | 0.464 | 2.010 |
| P200 | −1.481 | 0.676 | 0.028 | 0.060 | 0.855 |
| Constant | −13.78 | 7.532 | 0.067 | ||
| B. CHR vs. Controls | |||||
| IQ | 0.028 | 0.044 | 0.524 | 0.943 | 1.122 |
| Age | −0.319 | 0.297 | 0.282 | 0.406 | 1.300 |
| Education (years) | 0.786 | 0.419 | 0.061 | 0.965 | 4.992 |
| Target P3b | 0.028 | 0.096 | 0.769 | 0.853 | 1.240 |
| Novel P3b | −0.006 | 0.118 | 0.958 | 0.789 | 1.252 |
| Novel P3a | 0.411 | 0.157 | 0.009 | 1.108 | 2.052 |
| N100 | −0.800 | 0.347 | 0.021 | 0.228 | 0.886 |
| P200 | 0.252 | 0.252 | 0.317 | 0.785 | 2.109 |
| Constant | −14.27 | 7.611 | 0.061 | ||
| C. FESZ vs. Controls | |||||
| IQ | 0.181 | 0.084 | 0.031 | 1.017 | 1.412 |
| Age | −0.115 | 0.231 | 0.617 | 0.567 | 1.400 |
| Education (years) | 0.133 | 0.403 | 0.742 | 0.518 | 2.518 |
| Target P3b | 0.034 | 0.140 | 0.808 | 0.786 | 1.363 |
| Novel P3b | 0.065 | 0.160 | 0.685 | 0.780 | 1.459 |
| Novel P3a | 0.608 | 0.243 | 0.012 | 1.142 | 2.955 |
| N100 | −1.216 | 0.578 | 0.035 | 0.095 | 0.921 |
| P200 | −0.280 | 0.333 | 0.400 | 0.393 | 1.452 |
| Constant | −29.678 | 12.705 | 0.019 | ||
A. The indicated ERPs and demographic variables were investigated for their ability to predict high risk vs. first episode schizophrenia status; B. high risk vs. control or C. first episode vs. control status.
The CHR and HC groups and the FESZ and HC groups were compared separately, with the same model. The significant model comparing CHR with controls (Table 2B; χ2[8, n=46] = 31.6, p < 0.001; Hosmer and Lemeshow Goodness of Fit p > 0.05) explained between 49.6% (Cox and Snell R2) and 66.4 % (Nagelkerke R2) of the variance with 87.0% of the cases correctly classified. N100 (B = −0.8, p < 0.05) and P3a amplitudes (B = 0.41, p < 0.01) uniquely contributed to the model's predictive ability, indicating that decreased N100 and P3a amplitude increased the probability of a subject belonging to the CHR group. Regression of variables to compare FESZ and HC (Table 2C) also produced a significant model (χ2[8, n=43] = 32.7, p < 0.001; Hosmer and Lemeshow Goodness of Fit p > 0.05), explaining between 53.2% (Cox and Snell R2) and 71.6% (Nagelkerke R2 square) of the variance with 83.7% of the cases correctly classified. Lower current IQ (B=0.18, p<0.05), lower amplitude of P3a (B=0.61, p<0.05) and decreased N100 amplitude (B=-01.22, p<0.05) predicted FESZ status.
3.7. Relationships between ERP variables and clinical scales
Correlations between the ERP measures entered into the regression models and BPRS total, positive and negative scale scores; SANS total and SAPS total scores; and SOPS total, positive, and negative scale scores; the GAF scale was also explored. None of the probability values survived Bonferroni correction. Nonetheless, an inverse correlation between P3b amplitude at Pz and the SAPS psychotic index (rho=−0.57, p<0.01) and the BPRS total positive score (rho=−0.44, p=0.05) was detected at trend level. An additional trend level inverse correlation was found between P3b at Pz and the BPRS total score for anxiety symptoms (rho=-0.53, p<0.05).
4. Discussion
Although the groups did not differ behaviorally, electrophysiological evidence of “deficits of both attention and memory” was observed in CHR and FESZ compared with age-matched controls. Similarity of effect sizes, an index of the magnitude of abnormalities, suggests that deficits in brain mechanisms that underlie P3a and P3b exist at both the CHR stage and at the onset of psychosis.
The data are in line with the findings of P3b deficits in individuals at risk of psychosis (van der Stelt et al., 2004; Frommann et al., 2008; Fusar-Poli et al., 2011) and first episode patients (Salisbury et al., 1998; McCarley et al., 2002), and indicate reduced P3a amplitude in both CHR and first episode individuals. P3a deficits in the schizophrenia spectrum have not been consistently reported. For example, two recent cross-sectional studies have shown comparable and significantly reduced P3a amplitude in prodromal and recent onset patients with respect to controls (Jahshan et al., 2012; Mondragon-Maya et al., 2013). In contrast, Atkinson et al. (Atkinson et al., 2012) have reported reduced P3a amplitude in prodromal but not first episode patients, and Devrim-Ucok et al. (2006) reported normal P3a amplitude in first episode patients.
In chronic schizophrenia patients, in addition to P3a deficits (Grillon et al., 1990; Devrim-Ucok et al., 2006; Mathalon et al., 2010), unchanged or even increased P3a amplitudes have been reported (Grillon et al., 1990; Schall et al., 1999; Frodl et al., 2001a). Differences in paradigms might partly explain differences in outcome. Most recent studies have assessed P3a in a passive two-stimulus paradigm, while older studies, and our own, have used an active novelty paradigm, where novel stimuli are imbedded in an oddball paradigm. Although the P3a components elicited in both types of paradigms are most probably variants of the same ERP, the morphology of P3a components is strongly influenced by attention and task demands (Polich, 2007).
Our data on N100 measured to standard stimuli indicate comparable effect sizes of amplitude decreases in both CHR and FESZ relative to HC. The data confirm findings of reduced N100 amplitude in FESZ (Salisbury et al., 2010; Foxe et al., 2011) and indicate similar deficits in CHR individuals. Although no N100 group differences to standard stimuli in an oddball paradigm in at-risk individuals were reported in other studies (Bramon et al., 2008; van Tricht et al., 2010), gating studies have shown S1 and S1-S2 N100 deficits in prodromes but not in at-risk individuals (Brockhaus-Dumke et al., 2008). Longitudinally, a Time × Group interaction for N100 and P200 has been reported with deepening deficits in high risk individuals transitioning to psychosis (van Tricht et al., 2010; van Tricht et al., 2011; van Tricht et al., 2012). Several studies have shown that N100 abnormalities are present in unaffected relatives/twins of schizophrenia patients (Blackwood et al., 1991; Frangou et al., 1997; Ahveninen et al., 2006; Anokhin et al., 2006; Foxe et al., 2011), and magnetic resonance imaging (MRI) studies of gray matter have shown reduction of superior temporal gyrus at illness onset (Shenton et al., 1992; McCarley et al., 1999; Shenton et al., 2001) in young of schizophrenia patients (Rajarethinam et al., 2004) and in clinical high risk individuals whether they later develop schizophrenia or not (Borgwardt et al., 2007; Takahashi et al., 2010; Mechelli et al., 2011).
We note that in another study with similar subject samples, the N100 evoked by standard stimuli in a visual oddball task was decreased in FESZ but not in CHR (Oribe et al., 2013). Comparison of those findings with the present findings suggests that early auditory processing might be more affected than early visual processing in CHR individuals.
An attempt to distinguish CHR and FESZ using a logistic regression model generated a prediction model confirming results of the MANOVA, where.the amplitude of the P200 was an independent predictor of CHR versus FESZ status. The same model aimed at distinguishing CHR from HC or FESZ from HC in separate analyses demonstrated that the N100 and the P3a distinguished both CHR and FESZ from HC. Combined EEG-functional MRI studies indicate the P3a to be a part of the salience network involving the anterior cingulate and the insula (Crottaz-Herbette and Menon, 2006), regions repeatedly found to be affected in schizophrenia, and in a variety of other disorders such as mania, depression and anxiety (Devinsky et al., 1995; Hatton et al., 2012). As the N100 in Näätänen's model (Näätänen, 1990) is also considered an important aspect of for the redirection of attention to salient stimuli, our data indicate that mechanisms that are supported by the salience network might be indicative of CHR and FESZ states. Considering that the present study investigated cross-sectional data, and considering that there were no conversions to overt psychosis among those at clinical high risk at the 1-year time point, it might be hypothesized that the P3a and the N100 are indexing brain processing characteristics shared by CHR and FESZ and marked by functional disability (Cornblatt et al., 2003; Addington et al., 2011).
Recent studies of non-converting individuals in fact indicate that CHR might be prone to a variety of mental disorders ( Rossler et al., 2011; Carrion et al., 2013). Accordingly, Rosburg and collegues (Rosburg et al., 2008) demonstrated that “A reduced N100 amplitude is found in first degree relatives of schizophrenia patients, but the risk of developing schizophrenia is not reflected in the N100 amplitude reduction.” All in all, N100 amplitude deficits seem to reflect underlying abnormalities of brain functioning that do not necessarily overlap with developing schizophrenia.
The P3a and P3b have been shown to be trait markers of schizophrenia (Mathalon et al., 2000). Nonetheless, we propose that because P3a and P3b abnormalities are also present in CHR and show a lack of specificity (Ford, 1999), P3a and P3b might indicate trait marker status of a core deficit that is common to, but not specific to, schizophrenia.
The GAF was equivalent in CHR and FESZ and significant lower compared with controls, indicating that impaired functioning as measured on the GAF scale, might represent a common thread between both CHR and FESZ that may be mediated by neurocognitive impairments indexed with ERPs.
Although we cannot exclude the effects of medication on our results as the majority of FESZ in this study were medicated, none of the ERP amplitude or latency findings in FESZ correlated with chlorpromazine-equivalent dosage. In addition, comparison of ERPs in medication-naïve and medicated CHR individuals demonstrated a lack of ERP differences (Fig. 3). Although Turetsky (2009) reported reduced P3a amplitude with increased CPZ-equivalent dosage in chronic schizophrenia patients, other reports indicate that medication might have a very minor effect on P3a (Valkonen-Korhonen et al., 2003a; Mondragon-Maya et al., 2013) and N100 (Foxe et al., 2011).
In summary, if we keep in mind the limited sample size, this study indicates that deficits of memory and attention in CHR, as indexed by ERPs in classical and novelty oddball tasks, are as profound as those of first episode schizophrenia patients, suggesting that these two groups are characterized by similar deficits of middle and late latency ERPs, irrespective of whether CHR and FESZ diagnoses represent a continuum or two different conditions. In addition, in regression analysis, the N100 and P3a amplitudes were the most sensitive ERP measures in discriminating between healthy and afflicted individuals. We suggest that these specific ERPs might be indices of a core disability independent of psychosis that distinguishes healthy controls from individuals at clinical high risk and individuals by schizophrenia of recent onset.
Supplementary Material
Acknowledgments
Support was provided by Department of Veterans’ Affairs Medical Research Awards (Schizophrenia Center, Merit Awards to R.W.M. and M.E.S.) and National Institute of Mental Health (K05MH070047 and R01MH50747 to M.E.S., R01MH40799 and R01MH052807 to R.W.M., CIDAR P50MH080272 to R.W.M. and M.E.S.).
Footnotes
Contributors
E.C. del Re collected EEG data, analyzed the data, and wrote the manuscript. K.M. Spencer designed the study, contributed to data analysis, and edited the manuscript. N. Oribe collected EEG data and was involved with data analysis. L.J. Seidman and R. Mesholam-Gately collected clinical data and edited the manuscript. T. Petryshen, M. Shenton, and J. Goldstein edited the manuscript. R.W. McCarley designed the study, contributed to data analysis, and edited the manuscript. M. Niznikiewicz was involved with EEG data analysis and contributed to manuscript editing. All authors approved the final manuscript
Conflict of interest
All authors declare that they do not have conflicts of interest.
References
- Addington J, Cornblatt BA, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Heinssen R. At clinical high risk for psychosis: outcome for nonconverters. American Journal of Psychiatry. 2011;168(8):800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahveninen J, Jaaskelainen IP, Osipova D, Huttunen MO, Ilmoniemi RJ, Kaprio J, Lonnqvist J, Manninen M, Pakarinen S, Therman S, Naatanen R, Cannon TD. Inherited auditory-cortical dysfunction in twin pairs discordant for schizophrenia. Biological Psychiatry. 2006;60(6):612–620. doi: 10.1016/j.biopsych.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City: 1983. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms. University of Iowa; Iowa City: 1984. [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Myers E. Genetic and environmental influences on frontal EEG asymmetry: a twin study. Biological Psychology. 2006;71(3):289–295. doi: 10.1016/j.biopsycho.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biological Psychiatry. 2012;71(2):98–104. doi: 10.1016/j.biopsych.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Bestelmeyer PE, Phillips LH, Crombie C, Benson P, St Clair D. The P300 as a possible endophenotype for schizophrenia and bipolar disorder: evidence from twin and patient studies. Psychiatry Research. 2009;169(3):212–219. doi: 10.1016/j.psychres.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, St Clair DM, Muir WJ, Duffy JC. Auditory P300 and eye tracking dysfunction in schizophrenic pedigrees. Archives of General Psychiatry. 1991;48(10):899–909. doi: 10.1001/archpsyc.1991.01810340031004. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, McGuire PK, Aston J, Berger G, Dazzan P, Gschwandtner U, Pfluger M, D'Souza M, Radue EW, Riecher-Rossler A. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. The British Journal of Psychiatry. 2007;51(Suppl.):s69–75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- Bramon E, McDonald C, Croft RJ, Landau S, Filbey F, Gruzelier JH, Sham PC, Frangou S, Murray RM. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27(4):960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Bramon E, Shaikh M, Broome M, Lappin J, Berge D, Day F, Woolley J, Tabraham P, Madre M, Johns L, Howes O, Valmaggia L, Perez V, Sham P, Murray RM, McGuire P. Abnormal P300 in people with high risk of developing psychosis. Neuroimage. 2008;41(2):553–560. doi: 10.1016/j.neuroimage.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophrenia Research. 2008;99(1-3):238–249. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Archives of General Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion RE, McLaughlin D, Goldberg TE, Auther AM, Olsen RH, Olvet DM, Correll CU, Cornblatt BA. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2013;70(11):1133–1142. doi: 10.1001/jamapsychiatry.2013.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. Journal of Cognitive Neuroscience. 2006;18(5):766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- del Re EC, Bergen SE, Mesholam-Gately RI, Niznikiewicz MA, Goldstein JM, Woo TU, Shenton ME, Seidman LJ, McCarley RW, Petryshen TL. Analysis of schizophrenia-related genes and electrophysiological measures reveals ZNF804A association with amplitude of P300b elicited by novel sounds. Translational Psychiatry. 2014;4:e346. doi: 10.1038/tp.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Devrim-Ucok M, Keskin-Ergen HY, Ucok A. Novelty P3 and P3b in first-episode schizophrenia and chronic schizophrenia. Progress in Neuropsychopharmacology & Biological Psychiatry. 2006;30(8):1426–1434. doi: 10.1016/j.pnpbp.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Donchin E. Presidential address, 1980. Surprise!...Surprise? Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSMIV-TR Axis I Disorders, Research Version. Biometrics Research. New York State Psychiatric Institute; New York, NY.: 2002. [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(6):667–682. [PubMed] [Google Scholar]
- Foxe JJ, Yeap S, Snyder AC, Kelly SP, Thakore JH, Molholm S. The N1 auditory evoked potential component as an endophenotype for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives, first-episode, and chronic schizophrenia patients. European Archives of Psychiatry Clinical Neuroscience. 2011;261(5):331–339. doi: 10.1007/s00406-010-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S, Sharma T, Alarcon G, Sigmudsson T, Takei N, Binnie C, Murray RM. The Maudsley Family Study, II: Endogenous event-related potentials in familial schizophrenia. Schizophrenia Research. 1997;23(1):45–53. doi: 10.1016/S0920-9964(96)00089-8. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Muller D, Greiner J, Juckel G, Leinsinger G, Hahn H, Moller HJ, Hegerl U. Corpus callosum and P300 in schizophrenia. Schizophrenia Research. 2001a;49(1-2):107–119. doi: 10.1016/s0920-9964(00)00123-7. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Muller D, Leinsinger G, Juckel G, Hahn K, Moller HJ, Hegerl U. The effect of the skull on event-related P300. Clinical Neurophysiology. 2001b;112(9):1773–1776. doi: 10.1016/s1388-2457(01)00587-9. [DOI] [PubMed] [Google Scholar]
- Frommann I, Brinkmeyer J, Ruhrmann S, Hack E, Brockhaus-Dumke A, Bechdolf A, Wolwer W, Klosterkotter J, Maier W, Wagner M. Auditory P300 in individuals clinically at risk for psychosis. International Journal of Psychophysiology. 2008;70(3):192–205. doi: 10.1016/j.ijpsycho.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crossley N, Woolley J, Carletti F, Perez-Iglesias R, Broome M, Johns L, Tabraham P, Bramon E, McGuire P. Gray matter alterations related to P300 abnormalities in subjects at high risk for psychosis: longitudinal MRI-EEG study. Neuroimage. 55(1):320–328. doi: 10.1016/j.neuroimage.2010.11.075. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crossley N, Woolley J, Carletti F, Perez-Iglesias R, Broome M, Johns L, Tabraham P, Bramon E, McGuire P. Gray matter alterations related to P300 abnormalities in subjects at high risk for psychosis: longitudinal MRI-EEG study. Neuroimage. 2011;55(1):320–328. doi: 10.1016/j.neuroimage.2010.11.075. [DOI] [PubMed] [Google Scholar]
- Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Current Pharmaceutical Design. 2012;18(4):399–415. doi: 10.2174/138161212799316019. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grillon C, Courchesne E, Ameli R, Geyer MA, Braff DL. Increased distractibility in schizophrenic patients. Electrophysiologic and behavioral evidence. Archives of General Psychiatry. 1990;47(2):171–179. doi: 10.1001/archpsyc.1990.01810140071010. [DOI] [PubMed] [Google Scholar]
- Groom MJ, Bates AT, Jackson GM, Calton TG, Liddle PF, Hollis C. Event-related potentials in adolescents with schizophrenia and their siblings: a comparison with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;63(8):784–792. doi: 10.1016/j.biopsych.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Hall MH, Rijsdijk F, Picchioni M, Schulze K, Ettinger U, Toulopoulou T, Bramon E, Murray RM, Sham P. Substantial shared genetic influences on schizophrenia and event-related potentials. American Journal of Psychiatry. 2007;164(5):804–812. doi: 10.1176/ajp.2007.164.5.804. [DOI] [PubMed] [Google Scholar]
- Hatton SN, Lagopoulos J, Hermens DF, Naismith SL, Bennett MR, Hickie IB. Correlating anterior insula gray matter volume changes in young people with clinical and neurocognitive outcomes: an MRI study. BMC Psychiatry. 2012;12:45. doi: 10.1186/1471-244X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Progress in Neuropsychopharmacology & Biological Psychiatry. 2010;34(6):822–829. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-Factor Index of Social Position. Yale University Press; New Haven, CT.: 1975. [Google Scholar]
- Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychological Medicine. 2012;42(1):85–97. doi: 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nature Reviews. Drug Discovery. 2008;7(1):68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40(5):684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). The British Journal of Psychiatry : the Journal of Mental Science. 1995;166(5):654–659. doi: 10.1192/bjp.166.5.654. [DOI] [PubMed] [Google Scholar]
- Kaur M, Battisti RA, Ward PB, Ahmed A, Hickie IB, Hermens DF. MMN/P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophrenia Research. 2011;130(1-3):203–209. doi: 10.1016/j.schres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Haas G, Miewald J, Montrose DM, Reddy R, Schooler NR, Sweeney JA. Prolonged untreated illness duration from prodromal onset predicts outcome in first episode psychoses. Schizophrenia Bullettin. 2003;29(4):757–769. doi: 10.1093/oxfordjournals.schbul.a007045. [DOI] [PubMed] [Google Scholar]
- Kirihara K, Araki T, Uetsuki M, Yamasue H, Hata A, Rogers MA, Iwanami A, Kasai K. Association study between auditory P3a/P3b event-related potentials and thought disorder in schizophrenia. Brain Imaging and Behavior. 2009;3(3):277–283. doi: 10.1007/s11682-009-9069-0. [DOI] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Archives of General Psychiatry. 2001;58(2):158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biological Psychiatry. 2000;47(5):434–449. doi: 10.1016/s0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Hoffman RE, Watson TD, Miller RM, Roach BJ, Ford JM. Neurophysiological distinction between schizophrenia and schizoaffective disorder. Frontiers in Human Neuroscience. 2010;3:70. doi: 10.3389/neuro.09.070.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Faux SF, Shenton ME, Nestor PG, Adams J. Event-related potentials in schizophrenia: their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schizophrenia Research. 1991;4(2):209–231. doi: 10.1016/0920-9964(91)90034-o. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, Kikinis R, Jolesz FA, Shenton ME. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Archives of General Psychiatry. 2002;59(4):321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biological Psychiatry. 1999;45(9):1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Riecher-Rossler A, Meisenzahl EM, Tognin S, Wood SJ, Borgwardt SJ, Koutsouleris N, Yung AR, Stone JM, Phillips LJ, McGorry PD, Valli I, Velakoulis D, Woolley J, Pantelis C, McGuire P. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Archives of General Psychiatry. 2011;68(5):489–495. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. American Journal of Psychiatry. 2002;159(5):863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. The Psychiatric Quarterly. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Mondragon-Maya A, Solis-Vivanco R, Leon-Ortiz P, Rodriguez-Agudelo Y, Yanez-Tellez G, Bernal-Hernandez J, Cadenhead KS, de la Fuente-Sandoval C. Reduced P3a amplitudes in antipsychotic naive first-episode psychosis patients and individuals at clinical high-risk for psychosis. Journal of Psychiatric Reserch. 2013;47(6):755–761. doi: 10.1016/j.jpsychires.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials ans other brain measures of cognitive function. Behavioral and Brain Sciences. 1990;13:201–288. [Google Scholar]
- Oribe N, Hirano Y, Kanba S, del Re EC, Seidman LJ, Mesholam-Gately R, Spencer KM, McCarley RW, Niznikiewicz MA. Early and late stages of visual processing in individuals in prodromal state and first episode schizophrenia: an ERP study. Schizophrenia Research. 2013;146(1-3):95–102. doi: 10.1016/j.schres.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Pfefferbaum A, Wenegrat BG, Ford JM, Roth WT, Kopell BS. Clinical application of the P3 component of event-related potentials. II. Dementia, depression and schizophrenia. Electroencephalography and Clinical Neurophysiology. 1984;59(2):104–124. doi: 10.1016/0168-5597(84)90027-3. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GW, Michie PT, Johnston J, Innes-Brown H, Kent A, Clissa P, Jablensky AV. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian family study of schizophrenia. Biological Psychiatry. 2006;60(1):1–10. doi: 10.1016/j.biopsych.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Rajarethinam R, Sahni S, Rosenberg DR, Keshavan MS. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. American Journal of Psychiatry. 2004;161(6):1121–1124. doi: 10.1176/appi.ajp.161.6.1121. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nature Reviews. Neuroscience. 2003;4(3):193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia-- a critical review. Psychiatry Research. 2008;161(3):259–274. doi: 10.1016/j.psychres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Rossler W, Hengartner MP, Ajdacic-Gross V, Haker H, Gamma A, Angst J. Sub-clinical psychosis symptoms in young adults are risk factors for subsequent common mental disorders. Schizophrenia Research. 2011;131(1-3):18–23. doi: 10.1016/j.schres.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophrenia Bullettin. 2010;36(5):991–1000. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Archives of General Psychiatry. 1998;55(2):173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall U, Catts SV, Karayanidis F, Ward PB. Auditory event-related potential indices of fronto-temporal information processing in schizophrenia syndromes: valid outcome prediction of clozapine therapy in a three-year follow-up. International Journal of Neuropsychopharmacology. 1999;2(2):83–93. doi: 10.1017/S1461145799001418. [DOI] [PubMed] [Google Scholar]
- Schultze-Lutter F AJ, Ruhrmann S, Klosterkotter J. Giovanni Fioriti Editore s.r.l. Rome: 2007a. The Schizophrenia Proneness Instrument, Adult version (SPI-A). [Google Scholar]
- Schultze-Lutter F KJ, Picker H, Steinmeyer E-M, Ruhrmann S. Predicting first-episode psychosis by basic symptom criteria. Clinical Neuropsychiatry. 2007b;4(1):11–22. [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49(1-2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. New England Journal of Medicine. 1992;327(9):604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology. 1975;38(4):387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Stoll AL. The Psychopharmacology Reference Card, 1989-2009. McLean Hospital; Belmont, MA.: 2009. [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Walterfang M, Phillips LJ, Soulsby B, Kawasaki Y, McGorry PD, Suzuki M, Velakoulis D, Pantelis C. Superior temporal gyrus volume in antipsychotic-naive people at risk of psychosis. The British Journal of Psychiatry. 2010;196(3):206–211. doi: 10.1192/bjp.bp.109.069732. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Research. 2009;165:27–37. doi: 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkonen-Korhonen M, Laukkanen E, Tarkka IM, Partanen J, Lehtonen J. Enhanced frontal processing of auditory stimuli in siblings with acute psychosis. Journal of Nervous and Mental Disease. 2003a;191(1):60–62. doi: 10.1097/00005053-200301000-00012. [DOI] [PubMed] [Google Scholar]
- Valkonen-Korhonen M, Purhonen M, Tarkka IM, Sipila P, Partanen J, Karhu J, Lehtonen J. Altered auditory processing in acutely psychotic never-medicated first-episode patients. Brain research. Cognitive Brain Research. 2003b;17(3):747–758. doi: 10.1016/s0926-6410(03)00199-x. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Frye J, Lieberman JA, Belger A. Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Archives of General Psychiatry. 2004;61(3):237–248. doi: 10.1001/archpsyc.61.3.237. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, Koelman JH, Bour LJ, van der Meer JN, van Amelsvoort TA, Linszen DH, de Haan L. Auditory ERP components before and after transition to a first psychotic episode. Biological Psychology. 2011;87(3):350–357. doi: 10.1016/j.biopsycho.2011.04.005. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, K.J., Mensink AJ, Bour LJ, van der Meer JN, van Amelsvoort TA, Linszen DH, de Haan L. Sensory gating in subjects at ultra high risk for developing a psychosis before and after a first psychotic episode. The World Journal of Biological Psychiatry. 2012 doi: 10.3109/15622975.2012.680911. electronic publication 26 June 26; doi:10.3109/15622975.2012.680911. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, Koelman JH, van der Meer JN, Bour LJ, de Haan L, Linszen DH. Reduced parietal P300 amplitude is associated with an increased risk for a first psychotic episode. Biological Psychiatry. 2010;68(7):642–648. doi: 10.1016/j.biopsych.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; Harcourt Brace, New York.: 1999. [Google Scholar]
- Wilkinson GS, Robertson GJ. Psychological Assessment Resources. Lutz; FL.: 2006. Wide Range Achievement Test-Fourth Edition. [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. Australian and New Zealand Journal of Psychiatry. 1996;30(5):587–599. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, Francey SM, McFarlane CA, Hallgren M, McGorry PD. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophrenia Research. 2003;60(1):21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Chauncey DL, Gunderson JG. The Diagnostic Interview for Personality Disorders: interrater and test-retest reliability. Comprehensive Psychiatry. 1987;28(6):467–480. doi: 10.1016/0010-440x(87)90012-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


