Abstract
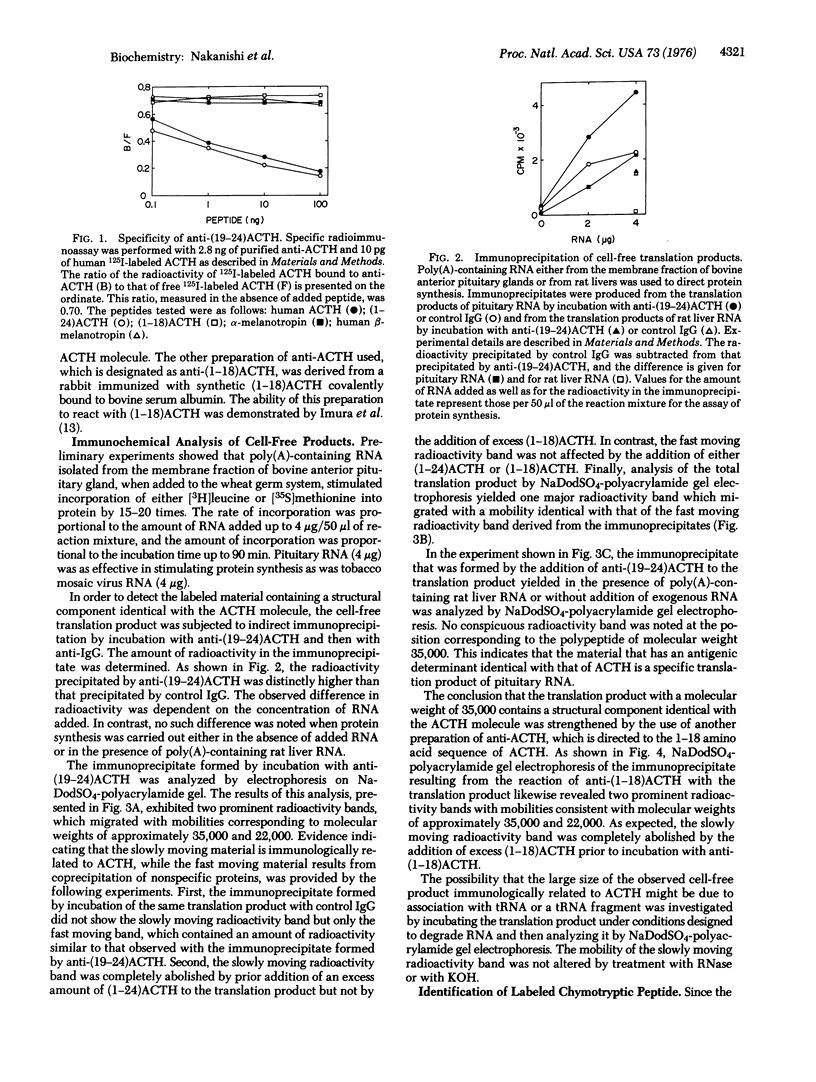
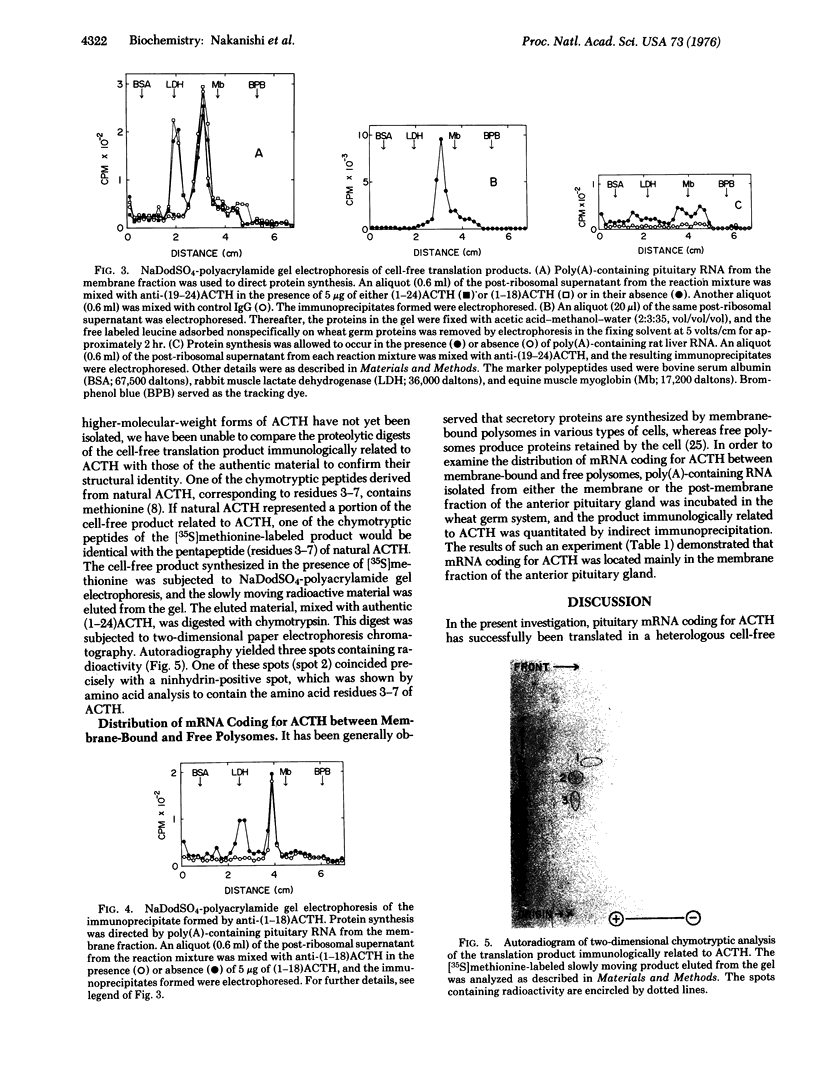
Polyadenylate-containing RNA prepared from the membrane fraction of bovine anterior pituitary gland was shown to direct the synthesis of a large translation product related to corticotropin (adrenocorticotropic hormone) in a cell-free system derived from wheat germ. This product was identified by immunoprecipitation with specific antibody against corticotropin, followed by electrophoretic analysis of the dissociated immunoprecipitate on sodium dodecyl sulfate-polyacrylamide gel. Further evidence for the identity of the translation product was provided by the presence of a common peptide in the chymotryptic digest of [35S]methionine-labeled cell-free product and in that of authentic corticotropin. The molecular weight of the translation product was estimated to be approximately 35,000, based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. These results indicate that corticotropin messenger RNA directs the cell-free synthesis of a product that contains the amino acid sequence of corticotropin but is much larger than this hormone.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Radioimmunoassay of ACTH in plasma. J Clin Invest. 1968 Dec;47(12):2725–2751. doi: 10.1172/JCI105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boime I., Boguslawski S., Caine J. The translation of a human placental lactogen mRNA fraction in heterologous cell-free systems: the synthesis of a possible precursor. Biochem Biophys Res Commun. 1975 Jan 6;62(1):103–109. doi: 10.1016/s0006-291x(75)80411-6. [DOI] [PubMed] [Google Scholar]
- Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus mRNA. 3. Discrete polypeptides translated from a monocistronic messenger in vitro. Arch Biochem Biophys. 1972 Dec;153(2):706–713. doi: 10.1016/0003-9861(72)90389-x. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Eukaryotic messenger RNA. Annu Rev Biochem. 1974;43(0):621–642. doi: 10.1146/annurev.bi.43.070174.003201. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. S., Weintraub B. D., Rosen S. W., Maxwell E. S. Properties of biologically active messenger RNA from human placenta. Cell-free synthesis of two immunoreactive forms of placental lactogen. J Biol Chem. 1976 Mar 25;251(6):1723–1730. [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. High molecular weight forms of adrenocorticotropic hormone in the mouse pituitary and in a mouse pituitary tumor cell line. Biochemistry. 1975 Aug 26;14(17):3836–3844. doi: 10.1021/bi00688a016. [DOI] [PubMed] [Google Scholar]
- Gewirtz G., Schneider B., Krieger D. T., Yalow R. S. Big ACTH: conversion to biologically active ACTH by trypsin. J Clin Endocrinol Metab. 1974 Feb;38(2):227–230. doi: 10.1210/jcem-38-2-227. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Yamamoto H., Matsukura S., Imura H. In vitro release and biosynthesis of tumor ACTH in ectopic ACTH producing tumors. J Clin Endocrinol Metab. 1975 Jul;41(1):106–114. doi: 10.1210/jcem-41-1-106. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Ernst M. D., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: analysis of radioactive tryptic peptides and amino acid sequence. Biochemistry. 1976 Jan 13;15(1):15–19. doi: 10.1021/bi00646a003. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Mulligan R. C., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: a direct translation product of parathyroid messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3731–3735. doi: 10.1073/pnas.71.9.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. H., DIXON J. S., CHUNG D. Adrenocorticotropins. XXI. The amino acid sequence of bovine adrenocorticotropin. Biochim Biophys Acta. 1961 Jan 15;46:324–334. doi: 10.1016/0006-3002(61)90756-9. [DOI] [PubMed] [Google Scholar]
- Maurer R. A., Stone R., Gorski J. Cell-free synthesis of a large translation product of prolactin messenger RNA. J Biol Chem. 1976 May 10;251(9):2801–2807. [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Palmiter R. D., Palacios R., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. II. Quantification and immunoprecipitation of polysomes. J Biol Chem. 1972 May 25;247(10):3296–3304. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman P. M., Tushinski R. J., Bancroft F. C. Pregrowth hormone: product of the translation in vitro of messenger RNA coding for growth hormone. Proc Natl Acad Sci U S A. 1976 Jan;73(1):29–33. doi: 10.1073/pnas.73.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Peptide hormones. Annu Rev Biochem. 1974;43(0):509–538. doi: 10.1146/annurev.bi.43.070174.002453. [DOI] [PubMed] [Google Scholar]
- Tishler P. V., Epstein C. J. A convenient method of preparing polyacrylamide gels for liquid scintillation spectrometry. Anal Biochem. 1968 Jan;22(1):89–98. doi: 10.1016/0003-2697(68)90262-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun. 1971 Jul 16;44(2):439–445. doi: 10.1016/0006-291x(71)90620-6. [DOI] [PubMed] [Google Scholar]