Abstract
Background
Habitual moderate alcohol consumption is associated with a lower risk of acute myocardial infarction (MI) whereas heavy (binge) drinking is associated with higher cardiovascular risk. However, less is known about the immediate effects of alcohol consumption on the risk of acute MI and whether any association differs by beverage type or usual drinking patterns.
Methods
We conducted a case-crossover analysis of 3,869 participants from the Determinants of Myocardial Infarction Onset Study who were interviewed during hospitalization for acute MI in one of 64 medical centers across the United States in 1989–1996. We compared the observed number of times that each participant consumed wine, beer or liquor in the hour preceding MI symptom onset with the expected frequency based on each participant’s control information, defined as the number of times the participant consumed alcohol in the past year.
Results
Among 3869 participants, 2119 (55%) reported alcohol consumption in the past year, including 76 within 1 hour before acute MI onset. The incidence rate of acute MI onset was elevated 1.72-fold (95% confidence interval [CI]=1.37–2.16) within 1 hour after alcohol consumption. The association was stronger for liquor than for beer or wine. The higher rate was not apparent for daily drinkers. For the 24 hours after consumption, there was a 14% lower rate (relative risk=0.86 [95% CI=0.79–0.95]) of MI compared with periods with no alcohol consumption.
Conclusions
Alcohol consumption is associated with an acutely higher risk of MI in the subsequent hour among people who do not typically drink alcohol daily.
There is consistent evidence that moderate habitual alcohol consumption is associated with a lower risk of cardiovascular events in subsequent months and years1 and that heavy episodic (binge) drinking is associated with higher cardiovascular risk. In terms of the short-term consequences, previous studies have reported that alcohol is an acute trigger of sudden cardiac death2 and ischemic3–7 and hemorrhagic8, 9 stroke; there are a few discrepant studies on the short-term risk of acute myocardial infarction (MI) in the 12 to 24 hours after intake10–12 However, experimental studies suggest that within 1–2 hours, moderate and high alcohol intake is associated with adverse consequences, including increased blood pressure and heart rate and impaired fibrinolysis.13 None of the prior studies on alcohol as a trigger of acute MI has examined this short period of potentially heightened risk. Furthermore, these studies did not examine whether the association differs by beverage type.
Therefore, we evaluated whether consumption of beer, wine or liquor is associated with a higher risk of acute MI in the following hour and the following 24 hours. In addition, we examined whether usual drinking patterns modify the association – much like regular physical activity blunts the acutely higher risk associated with episodes of physical activity.14
Methods
Study Design
We used the case-crossover design, a design that is appropriate when a transient exposure (alcohol consumption) is associated with an abrupt change in the risk of an acute outcome (nonfatal acute MI).15 We compared a participant’s report of alcohol consumption in the hours prior to MI onset (the case period) with the same participant’s report of alcohol consumption in the prior year (the control period). Because control information for each subject is based on his or her own past exposure experience, self-matching eliminates confounding by risk factors that are constant within individuals over the sampling period but that often differ between study subjects.
Study Population
The Determinants of Myocardial Infarction Onset Study was a multicenter cohort study of participants with confirmed acute MI. In the first phase of the study (August 1989–September 1994), 1,937 participants were enrolled in 45 community hospitals and tertiary care medical centers in the United States. In the second phase (October 1994–September 1996), the study was expanded to 64 medical centers across the United States and an additional 1,949 participants were enrolled. Altogether, 3,886 participants (2,627 men and 1,259 women; mean age, 61.4 years) were interviewed a median of 4 days (range=0 to 30) after sustaining an acute MI. For inclusion in the study, the participants were required to meet all the following criteria: English-speaking, creatine kinase level greater than the upper limit of normal for the clinical laboratory performing the test, positive MB isoenzymes, an identifiable onset of pain or other symptoms typical of infarction, and the ability to complete a structured interview. The institutional review board at each participating center approved the protocol, and each participant provided informed consent.
As previously described,14 detailed chart reviews and participant interviews were conducted by research personnel trained by personal instruction, a training manual and instructional videocassette, and through ongoing feedback the study coordinator. Approximately one third of the interviews were audiotaped for randomly selected quality control of the coding accuracy. To minimize bias in information ascertainment, the interviewers were not told of the duration of the hypothesized hazard. Data were collected on standard demographic variables and risk factors for coronary artery disease. We identified the time, place and quality of the acute MI pain and other symptoms. If the participants reported that they had experienced any chest pain in the week preceding the acute onset, they were classified as having premonitory symptoms. We asked about the timing of their last exposure to several potential triggers and estimated usual frequency of these factors in the year preceding MI onset. For each type of alcoholic beverage (beer, wine and liquor), we asked open-ended questions about usual frequency of intake over the past year, number of servings consumed at each episode and the last time a drink was consumed, and their usual weekly pattern of total alcohol intake (e.g. every Thursday). In addition, we asked participants about alcohol and food intake during each hour in the 36 hours before symptom onset.
Validity and Reliability of the Questionnaire
In a study of 851 patients evaluated in an emergency department,16 the Kappa coefficient between self-reported recent alcohol consumption and blood alcohol concentration was 0.75 (95% confidence interval [CI]=0.68–0.81), suggesting that there was good agreement between self-report and blood alcohol concentration. In a subset of 115 participants in the current study whose HDL-C levels were measured during hospitalization, the correlation between self-reported alcohol consumption and HDL-C levels (Pearson r=0.21)17 was similar to the degree found in the Second National Health and Nutrition Examination Survey.18 In a validation study comparing self-reported alcohol consumption over the prior year to intake from two 1-week dietary records collected ~6 months apart, the Spearman correlation coefficient was 0.90 for women and 0.86 for men.19 There is also high test-retest reliability for self-reported alcohol consumption. In a study of alcohol consumption and ischemic stroke7 that used similar questions about usual and recent alcohol consumption, 25 participants were re-interviewed up to 6 days after their initial interview. There was perfect agreement for reporting alcohol consumption in the hours before stroke onset (K=1.0), and the intraclass correlation for usual frequency of alcohol intake was excellent (0.84).
Statistical Analysis
We applied standard methods for stratified data analysis, where the stratifying variable is the individual participant. For the primary analysis, we calculated the Mantel-Haenszel rate ratio20 as a measure of relative risk (RR) to compare the ratio of the observed exposure frequency in the hazard period to the expected frequency based on control information about alcohol consumption in the previous year. In order to calculate the expected frequency of exposure in the case period, we multiplied the usual annual frequency of alcohol consumption by the hypothesized window of its physiologic effect (1 hour in the primary analysis) to estimate the amount of person-time exposed to alcohol; the unexposed person-time was calculated by subtracting this value from the number of hours in a year. Person-times for all alcohol use were calculated by summing the number of episodes of beer, wine and liquor. The data were analyzed using methods for cohort studies with sparse data in each stratum.20 The Mantel-Haenszel formula is
Where A1i and A0i reflect whether the case consumed alcohol during the hazard period, T1i is the number of hours (person-time) exposed to alcohol, T0i is the number of hours (person-time) unexposed to alcohol and Ti is the total person-time for the individual (i.e., 1 year or 8766 hours).
To estimate the length of time from alcohol consumption to MI onset, we calculated RRs for various hypothesized windows of its physiologic effect. We conducted stratified analyses to assess whether the association differed by drink type (beer, wine, liquor), drinking patterns (every day of the week vs. any other level of drinking frequency), sex, age (<65 vs. ≥65 years), smoking status (current vs. never/former), weekly physical activity (<3 vs. ≥3 times per week), prior coronary artery disease defined as history of acute MI or angina pectoris and jointly by beverage type and smoking status. We compared the RRs by means of a Wald test for homogeneity.20 We examined whether the RR was higher among people who usually consumed more drinks per episode by estimating the RR for 1 to 2, 3 and >3 drinks per episode, and we tested for a linear component of trend using a chi-square test for linear trend.20
In order to address potential confounding by time of day and inaccurate recall of usual alcohol consumption, we used information on consumption in each of the 36 hours prior to MI onset to conduct a sensitivity analysis using conditional logistic regression to compare each person’s exposure during the hazard period with their exposure at the same time on the day before MI onset. Since alcohol absorption is slower when it is consumed with food, we conducted a sensitivity analysis accounting for the fact that some participants consumed alcohol with meals. Since some individuals may consume or abstain from alcohol in response to premonitory symptoms, we conducted a sensitivity analysis restricted to participants who reported no symptoms in the week prior to MI onset. We also conducted a sensitivity analysis restricted to participants interviewed within 1 week of MI onset. All reported p values are 2-sided. In another sensitivity analysis, we excluded participants reporting other potential triggers (anger, physical activity, lifting ≥50 pounds, sexual activity, caffeine, tobacco and illicit drugs) in the proposed case period.
Results
Among 3,886 participants, we excluded 16 participants with a history of alcohol abuse who subsequently abstained from alcohol consumption and 1 participant who did not have information on regular alcohol consumption, leaving 3,869 participants for these analyses. 2,119 (55%) reported alcohol consumption in the previous year. Table 1 summarizes the characteristics of the study population. Compared with nondrinkers, participants who reported alcohol consumption were more likely to be male and to have ever smoked cigarettes. Among those participants who drank alcohol in the prior year, 367 (17%) reported consumption fewer than one time per month, 452 (21%) reported consumption more than once per month, 786 (37%) reported consumption more than once per week, and 514 (24%) reported consumption at least daily. The median number of drinks per drinking episode was 1 serving. Among those who drank alcohol in the prior year, 552 (26%) reported drinking 3 or more drinks within 1–2 hours at least once in the prior year.
Table 1.
Characteristicsa of the Determinants of Myocardial Infarction Onset Study participants.
| Alcohol Consumption in the Past Year | ||
|---|---|---|
| Yes (n=2119) | No (n=1750) | |
| Age (years); mean (SD) | 58.9 (12.3) | 64.6 (12.8) |
| Female | 493 (23) | 764 (4) |
| White race | 1899 (90) | 1506 (86) |
| Married | 1477 (70) | 1009 (58) |
| Income (US$); mean (SD) | 41,1312 (17,616) | 36,726 (16,184) |
| Education | ||
| Less than high school | 351 (17) | 485 (28) |
| Completed high school | 808 (38) | 776 (44) |
| Some college | 924 (44) | 442 (25) |
| Body mass index (kg/m2) | 27 (5) | 28 (6) |
| Smoking status | ||
| Never | 428 (20) | 586 (34) |
| Former | 905 (43) | 653 (37) |
| Current | 776 (37) | 494 (28) |
| History of | ||
| Hypertension | 858 (41) | 832 (48) |
| Diabetes mellitus | 286 (14) | 539 (31) |
| Angina | 451 (21) | 498 (29) |
| Myocardial infarction | 515 (24) | 549 (31) |
| Coronary artery disease | 729 (34) | 753 (43) |
| Regular use of | ||
| ACE inhibitors | 227 (11) | 286 (16) |
| Aspirin | 889 (42) | 652 (37) |
| Beta blockers | 428 (20) | 402 (23) |
| Calcium channel blockers | 449 (21) | 474 (27) |
| Digoxin | 107 (5) | 150 (9) |
| Index Hospitalization | ||
| Thrombolytic use | 954 (45) | 590 (34) |
| Congestive heart failure | 239 (11) | 277 (16) |
| Ventricular tachycardia | 233 (11) | 146 (8) |
| Peak Creatine Kinase level (U/L) | 1621 (1886) | 1380.(1726) |
| Physical Activity (times/week) | ||
| <3 | 1704 (80) | 1545 (88) |
| ≥3 | 414 (20) | 205 (12) |
Missing data on education (n=83), income (n=317), smoking status (n=27)
No. (%) unless otherwise indicated.
Acute MI Incidence within 1 Hour after Consumption
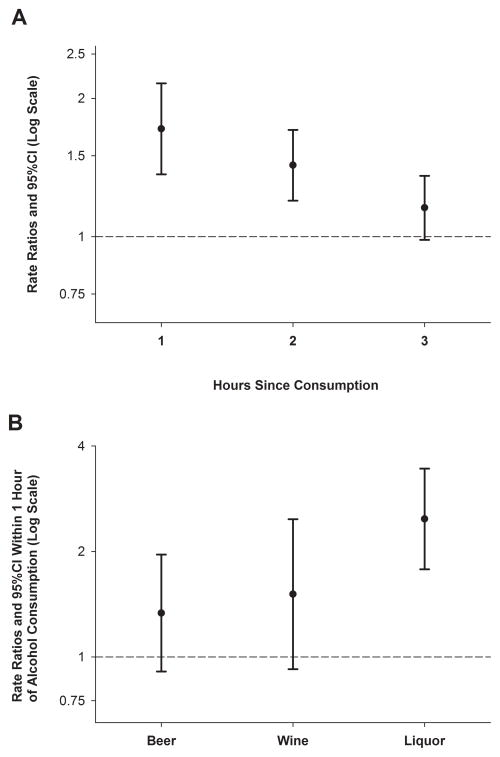
Of the 2,119 participants who drank alcohol in the past year, 76 (3.6%) reported consumption in the hour before acute MI onset. The rate of MI was 1.72 (95% CI=1.37–2.16) times higher in the hour after consumption compared with periods with no alcohol intake. The RR returned to baseline in the third hour after consumption (Figure 1A).
Figure 1.
Association of alcohol consumption with acute MI in the next hour. The error bars indicate the 95% confidence limits. The dashed line indicates the baseline risk. A. Relative risk of acute myocardial infarction after alcohol consumption by time of onset. Each of the hazard periods before MI onset was assessed as independent hazard periods, and each window was compared with exposure during the control period of one year. B. Relative risk of acute myocardial infarction in the hour after consumption of beer, wine or liquor.
Among the 76 participants who consumed alcohol in the hour before MI onset, 26 drank beer, 14 drank wine, 32 drank liquor and 4 drank more than one type of alcoholic beverage. The RR for alcohol consumption in the hour before MI onset was stronger for liquor than for beer or wine (test for homogeneity, P=0.04; Figure 1B). Patterns were similar in analyses restricted to participants reporting only one drink type in the prior year. Among participants who did not drink alcohol daily, there was a 3.29-fold higher MI rate in the hour after consumption (95%CI 2.46–4.41), but there was no association between alcohol consumption and MI among daily alcohol drinkers (RR=0.95 [95% CI=0.65–1.37]; test for homogeneity, P<0.001). We did not have sufficient data to examine the association stratified jointly by beverage type and by drinking patterns. Compared with participants who reported drinking 1–2 servings of alcohol per drinking episode, the RR was higher among people who consumed 2–3 drinks per episode and even higher among participants who reported drinking >3 drinks per episode (test for trend, P<0.001). We did not have sufficient data on the last time participants consumed 3 or more drinks within 1–2 hours, so we could not assess binge drinking as a trigger of MI.
Among 926 participants who completed questions on consumption in each of the 36 hours prior to MI onset, 65 reported alcohol intake only in the hour prior to MI onset, 48 reported intake only during the same hour on the previous day, and 25 reported intake during both periods. The rate of MI was 1.35 (95% CI=0.93–1.97) times higher in the hour after alcohol consumption compared with the same time on the previous day. In a sensitivity analysis accounting for meal consumption, the results were not meaningfully altered (1.45 [0.99–2.12]).
Acute MI Incidence within 24 Hours after Consumption
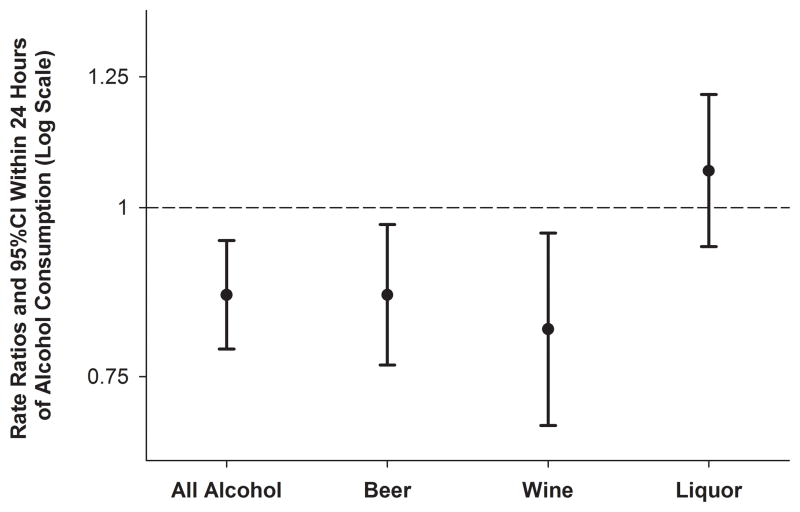
Within 24 hours after consumption, there was a 14% lower rate (RR=0.86 [95% CI=0.79–0.95]) of acute MI compared with 24 hour periods with no alcohol consumption. This protective association was apparent for beer and wine but not for liquor (test for homogeneity, P=0.005; Figure 2). Patterns were similar in analyses restricted to participants reporting only one drink type in the prior year. We combined the first hour with the subsequent 23-hour window to prevent overestimation of a protective effect due to potential error in recalling the exact time of drinking.
Figure 2.
Association of the consumption of beer, wine or liquor with acute myocardial infarction in the subsequent 24 hours. The error bars indicate the 95% confidence limits. The dashed line indicates the baseline risk.
Sensitivity Analyses
The RR in the one hour following alcohol consumption was higher among current smokers (RR=2.35 [95% CI=1.68–3.28]) than among never or former smokers (1.35 [0.98–1.85]; test for homogeneity, P=0.018) and it was higher among participants reporting a history of coronary artery disease (2.34 [1.64–3.35]) compared with those reporting no prior MI or angina (1.44 [1.07–1.94]; test for homogeneity, P=0.04). Estimates stratified jointly by beverage type and smoking status were highest for current smokers who consumed liquor in the hour prior to MI onset. No other differences by any of the other pre-specified potential modifiers were observed (Table 2).
Table 2.
Risk (RR) of acute myocardial infarction within 1 hour after alcohol consumption, according to patient characteristics, among the Determinants of Myocardial Infarction Onset Study participants.
| No. Exposed in Past Hour | No. Exposed in Past Year | RR (95%CI) | Test of Homogeneitya | |
|---|---|---|---|---|
| All Patients | 76 | 2119 | 1.72 (1.37–2.16) | |
| Drink Frequency | ||||
| Daily | 29 | 488 | 0.95 (0.65–1.37) | P<0.001 |
| Not daily | 47 | 1631 | 3.29 (2.46–4.41) | |
| Drink Type in Past Year | ||||
| Beer Only | 12 | 415 | 1.54 (0.88–2.69) | P=0.10 |
| Wine Only | 3 | 258 | 0.98 (0.32–2.98) | |
| Liquor Only | 10 | 276 | 3.14 (1.66–5.94) | |
| Usual Drinks Per Episode | ||||
| 1 – 2 | 19 | 979 | 1.48 (0.95–2.32) | P<0.001b |
| 3 | 15 | 376 | 1.59 (0.94–2.67) | |
| >3 | 38 | 659 | 1.98 (1.43–2.74) | |
| Sex | ||||
| Men | 64 | 1626 | 1.68 (1.31–2.15) | P=0.61 |
| Women | 12 | 493 | 1.97 (1.11–3.53) | |
| Age, y | ||||
| <65 | 55 | 1390 | 1.83 (1.40–2.39) | P=0.41 |
| ≥ 65 | 21 | 729 | 1.48 (0.95–2.29) | |
| Smoking Status | ||||
| Never/Former | 39 | 1333 | 1.35 (0.98–1.85) | P=0.02 |
| Current | 36 | 776 | 2.35 (1.68–3.28) | |
| Beverage Type and Smoking Status | ||||
| Beer | ||||
| Never/Former smoker | 12 | 847 | 1.04 (0.59–1.84) | P=0.26 |
| Current smoker | 13 | 520 | 1.63 (0.95–2.80) | |
| Wine | ||||
| Never/Former smoker 11 | 831 | 1.41 (0.79–2.55) | P=0.65 | |
| Current smoker | 5 | 332 | 1.81 (0.74–4.46) | |
| Liquor | ||||
| Never/Former smoker 17 | 733 | 1.75 (1.09–2.81) | P=0.02 | |
| Current smoker | 19 | 457 | 3.96 (2.49–6.31) | |
| Physical Activity (times/week) | ||||
| <3 | 64 | 1705 | 1.86 (1.45–2.39) | P=0.15 |
| ≥3 | 11 | 391 | 1.17 (0.65–2.10) | |
| History of Coronary Artery Disease | ||||
| No | 44 | 1390 | 1.44 (1.07–1.94) | P=0.04 |
| Yes | 32 | 729 | 2.34 (1.64–3.35) | |
P-value for the χ2 test for homogeneity, except where indicated
P-value for linear trend
The results were not meaningfully altered when we excluded 172 participants who reported any premonitory symptoms (RR=1.78 [1.40–2.26]) or when we excluded 338 participants who were interviewed more than a week after MI onset (1.82 [1.43–2.31]). The association was slightly lower (1.42 [1.06–1.90]) when we excluded 29 participants who reported that, in addition to alcohol consumption, they also had high levels of anger (n=1), physical activity (n=2), lifting ≥50 pounds (n=1), caffeine (n=5), tobacco (n=12), marijuana (n=1), cocaine (n=1) or a combination of several potential triggers (n=6) in the hour prior to MI onset.
Discussion
In this study of 3,869 participants with confirmed acute MI, the rate of MI onset was 72% higher during the hour after drinking alcohol compared with periods with no consumption, and the association was stronger for liquor than for beer or wine. The association was stronger among participants who habitually drank more drinks per episode. The higher risk in the hour following alcohol consumption was not apparent for daily drinkers. The elevated risk in the first hour was followed by reduced risk in the next 23 hours, producing an overall protective association for beer and wine in the 24-hour window before MI. For liquor, an overall association in the 24-window was not observed.
We estimated the proportion of cases whose alcohol consumption was associated with AMI onset in the following hour using the formula for the population attributable fraction.20(p.296) Based on the prevalence of alcohol consumption in the hour before AMI onset and the RR, approximately 1.5% of the cases in this study were associated with recent alcohol consumption, suggesting that the acute effects of alcohol consumption on AMI risk do not have a large public health impact. However, this estimate may be higher in regions with a higher prevalence of alcohol consumption. For instance, the adult per capita alcohol consumption is higher in regions such as Russia, the Czech Republic, the Republic of Moldova and Denmark.21 Additionally, some regions consume mostly liquor, which may incur greater risk than in regions that consume mostly beer or wine.
Despite consistent evidence on the cardiovascular benefits of habitual alcohol consumption and several studies showing that alcohol may be an acute trigger of sudden cardiac death2 and ischemic3–7 and hemorrhagic8, 9 stroke, there have only been a few studies on the short-term risk of acute MI following alcohol consumption. One case-crossover study10 found a higher risk of MI within 12 hours after alcohol consumption, and two case-control studies11, 12 found a protective association between moderate alcohol consumption and MI in the following 24 hours. This is the first study to evaluate whether alcohol consumption triggers an MI in the hour after intake and the first to examine whether there are differences in MI risk according to beverage type.
Results from experimental studies suggest that there are both harmful and protective effects of moderate and high alcohol intake within hours immediately following consumption.13 On the one hand, alcohol intake is associated with increased blood pressure, which may lead to disruption of vulnerable coronary plaques, increased heart rate,22 interarterial electromechanical delay,23 platelet activation and impaired fibrinolysis; on the other hand, there are transient improvements in flow-mediated vasodilatation, endothelial function and fibrinolytic factors. Within weeks, there are also improvements in inflammatory markers,24 lipid profile,25 and adipokines and insulin sensitivity.13 There is some evidence that the acute effect of alcohol intake varies by beverage type. For example, in a randomized trial of 83 healthy young participants,26 red wine and, to a smaller degree, beer, improved endothelial function within 4 hours, whereas whisky had no effect on vascular endothelium. This may at least partially explain why we found a higher acute risk following liquor consumption than following beer or wine and why we found a lower MI risk within 24 hours of beer or wine but not liquor.
In contrast to the acute harmful effects of alcohol, there is consistent evidence that habitual moderate alcohol consumption is associated with a lower risk of cardiovascular disease1 and cardiovascular risk factors,13 whereas binge drinking is associated with a higher risk of cardiovascular disease27–29 and of mortality after MI.13, 30, 31 It is possible that the transiently higher risk of MI in the hour after alcohol consumption is outweighed by the health benefits for the following 24 hours, whereas consuming many drinks at once may result in a spike in risk with potential increased long-term harm as well. Consequently, persons who drink large amounts at each episode may primarily experience the acute harmful effect, whereas persons who regularly drink moderate amounts may experience a transiently higher risk that is at least partially counterbalanced by the subsequent cardioprotective consequences. The higher risk in the hour following alcohol consumption was not apparent for daily drinkers. This may be due to the fact that habitual alcohol intake is associated with up-regulation of enzymes that metabolize alcohol, resulting in a lower physiological response to each drink.32
There are several potential explanations for why we found that the elevated acute MI risk in the hour after alcohol consumption was higher for liquor than for beer or wine and that the lower risk in the 24 hours after consumption was evident for beer and wine but not for liquor. It is possible that, compared with liquor, beer and wine contain more polyphenols and other protective constituents,33–35 but it is also possible that the differences are due to the amount of alcohol consumed or to simultaneous food intake rather than the type of alcoholic drink. Because of the high ethanol concentration of liquor, participants who recently drank liquor may have consumed larger amounts of alcohol at each episode than participants who recently drank beer or wine. In the United States, a standard serving of alcohol is defined as 0.6 fluid ounces (~14 grams) of pure alcohol, which is found in 12 ounces of regular beer (~ 5% alcohol), 5 ounces of wine (~12% alcohol) and 1.5 ounces of distilled spirits (~40% alcohol). These serving sizes may not reflect customary serving sizes; one glass of wine or one can of beer has the same alcohol content as only one shot of whiskey.
Alcohol consumption may have hastened the onset of acute MI for people who would have had an MI in a few hours even in the absence of alcohol intake. Therefore, the susceptible pool would be depleted in the 2–24 hours after the hypothesized hazard of 1 hour after alcohol consumption; this may at least partially explain our finding that alcohol intake is associated with lower MI risk within 24 hours. However, it seems likely that briefly advancing the timing of MI onset would have led to a null association by 24 hours, rather than resulting in estimates indicative of a protective association.
Strengths and limitations
We did not have information on the number of drinks consumed in the hours prior to MI onset, and we had information only on the usual number of drinks per session. Therefore, we cannot directly estimate the dose-response relationship between the amount of alcohol consumed and immediate MI risk. Because some participants may have consumed more than one beverage type during typical drinking episodes, summing across beverage types may have led to an overestimate of the exposed person-time during the preceding year, resulting in an underestimate of the RR. In addition, we did not have data on whether liquor was consumed as a mixed drink. As in other observational studies, information on alcohol consumption was obtained via self-report, and participants may not accurately report consumption. We attempted to minimize recall bias by ensuring participant privacy during the interview and by using a standardized structured interview, and participants were not informed of the duration of the hypothesized hazard period. Additionally, this method mimics clinical care, and assessment of alcohol intake appears reliable7 and valid16 among people seeking medical consultation at a hospital emergency department. Furthermore, our results were similar when we compared each person’s exposure during the hazard period to their exposure at the same time on the day before onset of AMI symptoms, rather than their usual frequency of consumption in the prior year. Finally, our results were similar when we excluded participants who were interviewed more than a week after AMI onset.
Because the case-crossover design uses participants as their own controls, there can be no confounding by measured and unmeasured risk factors that are stable over time, but confounding by factors that change over time within individuals can occur. There is a circadian peak of MI onset in the morning hours, and people may drink more alcohol in the evening and night. However, our results were similar when we compared each person’s exposure during the hazard period to their exposure at the same time on the day before onset of MI symptoms. In a sensitivity analysis excluding individuals also exposed to other potential risk factors, our findings were not materially altered. It is possible that some participants abstained from alcohol as a result of premonitory symptoms that are harbingers of the onset of MI,11, 36 resulting in an underestimate of recent exposure, but the risk of MI remained elevated when we excluded participants who reported premonitory symptoms.
It is possible that, compared with other MI cases, participants who consumed alcohol are more likely to survive37 and to participate in our study, resulting in an overestimate of the RR and the frequency of AMI associated with alcohol consumption. Alternatively, participants who consume alcohol may be less likely to survive,38 resulting in an underestimate of the RR and the frequency with which this occurs. However, it seems unlikely that MI survival is different for cases attributable to different mechanisms. Moreover, we have previously shown in this study population that neither recent nor habitual alcohol consumption affects MI severity,39 and the association between alcohol consumption and MI is similar for fatal and nonfatal events.40 Finally, our results may not be generalizable to people with sociodemographic characteristics different from those of our study participants.
Total alcohol intake and beverage preference cannot fully describe drinking patterns and the complex relationship between alcohol consumption and other lifestyle factors. We did not have enough data to further stratify these subgroups to determine whether other participant characteristics were responsible for the heterogeneity in the observed RRs. Nevertheless, these results suggest that there is a transiently higher risk of MI onset in the hour after alcohol consumption among people who do not drink alcohol every day, and the elevated risk is higher for liquor than beer or wine.
In conclusion, our findings highlight the complex association between alcohol consumption and acute MI onset, including higher MI risk in the hour after intake and lower risk in the following 24 hours. The harmful and protective effects of moderate alcohol consumption are influenced by the amount of alcohol consumed, the time course of consumption (i.e. drinking patterns) and, potentially, by beverage type. Therefore, it is difficult to make simple recommendations about alcohol consumption. In the context of prior research, this study adds to the evidence that, while beer, wine, and, particularly, liquor, may be associated with a transiently higher risk of AMI among people who do not drink alcohol daily, moderate habitual consumption may be protective.
Acknowledgments
Funding/Support: This work was supported by grants HL-120505 and HL-41016 from the National Institutes of Health, United States. Johanna van der Bom was supported by a Stipendium from the Niels Stensen Foundation.
Footnotes
Conflict of Interest Disclosures: None
Contributor Information
Elizabeth Mostofsky, Email: elm225@mail.harvard.edu.
Johanna G. van der Bom, Email: j.g.vanderbom@lumc.nl.
Kenneth J. Mukamal, Email: kmukamal@bidmc.harvard.edu.
Malcolm Maclure, Email: maclure@mail.ubc.ca.
Geoffrey H. Tofler, Email: Geoffrey.Tofler@health.nsw.gov.au.
James E. Muller, Email: jemuller@infraredx.com.
Murray A. Mittleman, Email: mmittlem@bidmc.harvard.edu.
References
- 1.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Šelb Šemerl J, Šelb K. Coffee and alcohol consumption as triggering factors for sudden cardiac death: Case-crossover study. Croat Med J. 2004;45:775–780. [PubMed] [Google Scholar]
- 3.Gorelick PB, Rodin MB, Langenberg P, Hier DB, Costigan J, Gomez I, Spontak S. Is acute alcohol ingestion a risk factor for ischemic stroke? Results of a controlled study in middle-aged and elderly stroke patients at three urban medical centers. Stroke. 1987;18:359–364. doi: 10.1161/01.str.18.2.359. [DOI] [PubMed] [Google Scholar]
- 4.Hillbom M, Haapaniemi H, Juvela S, Palomaki H, Numminen H, Kaste M. Recent alcohol consumption, cigarette smoking, and cerebral infarction in young adults. Stroke. 1995;26:40–45. doi: 10.1161/01.str.26.1.40. [DOI] [PubMed] [Google Scholar]
- 5.Hillbom M, Numminen H, Juvela S. Recent heavy drinking of alcohol and embolic stroke. Stroke. 1999;30:2307–2312. doi: 10.1161/01.str.30.11.2307. [DOI] [PubMed] [Google Scholar]
- 6.Guiraud V, Amor MB, Mas JL, Touze E. Triggers of ischemic stroke: A systematic review. Stroke. 2010;41:2669–2677. doi: 10.1161/STROKEAHA.110.597443. [DOI] [PubMed] [Google Scholar]
- 7.Mostofsky E, Burger MR, Schlaug G, Mukamal KJ, Rosamond WD, Mittleman MA. Alcohol and acute ischemic stroke onset: The stroke onset study. Stroke. 2010;41:1845–1849. doi: 10.1161/STROKEAHA.110.580092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson C, Ni Mhurchu C, Scott D, Bennett D, Jamrozik K, Hankey G. Triggers of subarachnoid hemorrhage: Role of physical exertion, smoking, and alcohol in the australasian cooperative research on subarachnoid hemorrhage study (across) Stroke. 2003;34:1771–1776. doi: 10.1161/01.STR.0000077015.90334.A7. [DOI] [PubMed] [Google Scholar]
- 9.Vlak MH, Rinkel GJ, Greebe P, van der Bom JG, Algra A. Trigger factors and their attributable risk for rupture of intracranial aneurysms: A case-crossover study. Stroke. 2011;42:1878–1882. doi: 10.1161/STROKEAHA.110.606558. [DOI] [PubMed] [Google Scholar]
- 10.Gerlich MG, Kramer A, Gmel G, Maggiorini M, Luscher TF, Rickli H, Kleger GR, Rehm J. Patterns of alcohol consumption and acute myocardial infarction: A case-crossover analysis. Eur Addict Res. 2009;15:143–149. doi: 10.1159/000213641. [DOI] [PubMed] [Google Scholar]
- 11.Jackson R, Scragg R, Beaglehole R. Does recent alcohol consumption reduce the risk of acute myocardial infarction and coronary death in regular drinkers? Am J Epidemiol. 1992;136:819–824. doi: 10.1093/aje/136.7.819. [DOI] [PubMed] [Google Scholar]
- 12.McElduff P, Dobson AJ. How much alcohol and how often? Population based case-control study of alcohol consumption and risk of a major coronary event. BMJ. 1997;314:1159–1164. doi: 10.1136/bmj.314.7088.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of myocardial infarction onset study investigators. N Engl J Med. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 15.Maclure M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 16.Borges G, Cherpitel C, Mittleman M. Risk of injury after alcohol consumption: A case-crossover study in the emergency department. Soc Sci Med. 2004;58:1191–1200. doi: 10.1016/s0277-9536(03)00290-9. [DOI] [PubMed] [Google Scholar]
- 17.Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Prior alcohol consumption and mortality following acute myocardial infarction. JAMA. 2001;285:1965–1970. doi: 10.1001/jama.285.15.1965. [DOI] [PubMed] [Google Scholar]
- 18.Ellison RC, Zhang Y, Qureshi MM, Knox S, Arnett DK, Province MA. Lifestyle determinants of high-density lipoprotein cholesterol: The national heart, lung, and blood institute family heart study. Am Heart J. 2004;147:529–535. doi: 10.1016/j.ahj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Rothman KJ. Introduction to stratified analysis. In: Rothman KJ, Greenland S, Lash T, editors. Modern epidemiology. 3. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 273–279. [Google Scholar]
- 21.World Health Organization. Global status report on alcohol and health. 2011 [Google Scholar]
- 22.Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, Notarius CF, Chan CT, Floras JS. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am J Physiol Heart Circ Physiol. 2008;294:H605–612. doi: 10.1152/ajpheart.01162.2007. [DOI] [PubMed] [Google Scholar]
- 23.Sengul C, Cevik C, Ozveren O, Sunbul A, Oduncu V, Akgun T, Can MM, Semiz E, Dindar I. Acute alcohol consumption is associated with increased interatrial electromechanical delay in healthy men. Cardiol J. 2011;18:682–686. doi: 10.5603/cj.2011.0033. [DOI] [PubMed] [Google Scholar]
- 24.Sacanella E, Vazquez-Agell M, Mena MP, Antunez E, Fernandez-Sola J, Nicolas JM, Lamuela-Raventos RM, Ros E, Estruch R. Down-regulation of adhesion molecules and other inflammatory biomarkers after moderate wine consumption in healthy women: A randomized trial. Am J Clin Nutr. 2007;86:1463–1469. doi: 10.1093/ajcn/86.5.1463. [DOI] [PubMed] [Google Scholar]
- 25.Beulens JW, van den Berg R, Kok FJ, Helander A, Vermunt SH, Hendriks HF. Moderate alcohol consumption and lipoprotein-associated phospholipase a2 activity. Nutr Metab Cardiovasc Dis. 2008;18:539–544. doi: 10.1016/j.numecd.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Tousoulis D, Ntarladimas I, Antoniades C, Vasiliadou C, Tentolouris C, Papageorgiou N, Latsios G, Stefanadis C. Acute effects of different alcoholic beverages on vascular endothelium, inflammatory markers and thrombosis fibrinolysis system. Clin Nutr. 2008;27:594–600. doi: 10.1016/j.clnu.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol consumption, binge drinking, and early coronary calcification: Findings from the coronary artery risk development in young adults (cardia) study. Am J Epidemiol. 2005;161:423–433. doi: 10.1093/aje/kwi062. [DOI] [PubMed] [Google Scholar]
- 28.Ruidavets JB, Ducimetiere P, Evans A, Montaye M, Haas B, Bingham A, Yarnell J, Amouyel P, Arveiler D, Kee F, Bongard V, Ferrieres J. Patterns of alcohol consumption and ischaemic heart disease in culturally divergent countries: The prospective epidemiological study of myocardial infarction (prime) BMJ. 2010;341:c6077. doi: 10.1136/bmj.c6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray RP, Connett JE, Tyas SL, Bond R, Ekuma O, Silversides CK, Barnes GE. Alcohol volume, drinking pattern, and cardiovascular disease morbidity and mortality: Is there a u-shaped function? Am J Epidemiol. 2002;155:242–248. doi: 10.1093/aje/155.3.242. [DOI] [PubMed] [Google Scholar]
- 30.Mukamal KJ, Maclure M, Muller JE, Mittleman MA. Binge drinking and mortality after acute myocardial infarction. Circulation. 2005;112:3839–3845. doi: 10.1161/CIRCULATIONAHA.105.574749. [DOI] [PubMed] [Google Scholar]
- 31.Pai JK, Mukamal KJ, Rimm EB. Long-term alcohol consumption in relation to all-cause and cardiovascular mortality among survivors of myocardial infarction: The health professionals follow-up study. Eur Heart J. 2012;33:1598–1605. doi: 10.1093/eurheartj/ehs047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabakoff B, Cornell N, Hoffman PL. Alcohol tolerance. Ann Emerg Med. 1986;15:1005–1012. doi: 10.1016/s0196-0644(86)80119-6. [DOI] [PubMed] [Google Scholar]
- 33.Koga K, Taguchi A, Koshimizu S, Suwa Y, Yamada Y, Shirasaka N, Yoshizumi H. Reactive oxygen scavenging activity of matured whiskey and its active polyphenols. J Food Sci. 2007;72:S212–217. doi: 10.1111/j.1750-3841.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 34.Arranz S, Chiva-Blanch G, Valderas-Martinez P, Medina-Remon A, Lamuela-Raventos RM, Estruch R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. 2012;4:759–781. doi: 10.3390/nu4070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiva-Blanch G, Arranz S, Lamuela-Raventos RM, Estruch R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: Evidences from human studies. Alcohol Alcohol. 2013;48:270–277. doi: 10.1093/alcalc/agt007. [DOI] [PubMed] [Google Scholar]
- 36.Wouters S, Marshall R, Yee RL, Jackson R. Is the apparent cardioprotective effect of recent alcohol consumption due to confounding by prodromal symptoms? Am J Epidemiol. 2000;151:1189–1193. doi: 10.1093/oxfordjournals.aje.a010169. [DOI] [PubMed] [Google Scholar]
- 37.Wannamethee G, Whincup PH, Shaper AG, Walker M, MacFarlane PW. Factors determining case fatality in myocardial infarction “who dies in a heart attack”? Br Heart J. 1995;74:324–331. doi: 10.1136/hrt.74.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McElduff P, Dobson AJ. Case fatality after an acute cardiac event: The effect of smoking and alcohol consumption. J Clin Epidemiol. 2001;54:58–67. doi: 10.1016/s0895-4356(00)00265-1. [DOI] [PubMed] [Google Scholar]
- 39.Mukamal KJ, Muller JE, Maclure M, Sherwood JB, Mittleman MA. Lack of effect of recent alcohol consumption on the course of acute myocardial infarction. Am Heart J. 1999;138:926–933. doi: 10.1016/s0002-8703(99)70019-0. [DOI] [PubMed] [Google Scholar]
- 40.Mukamal KJ, Conigrave KM, Mittleman MA, Camargo CA, Jr, Stampfer MJ, Willett WC, Rimm EB. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348:109–118. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]