Abstract
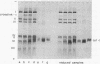
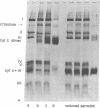
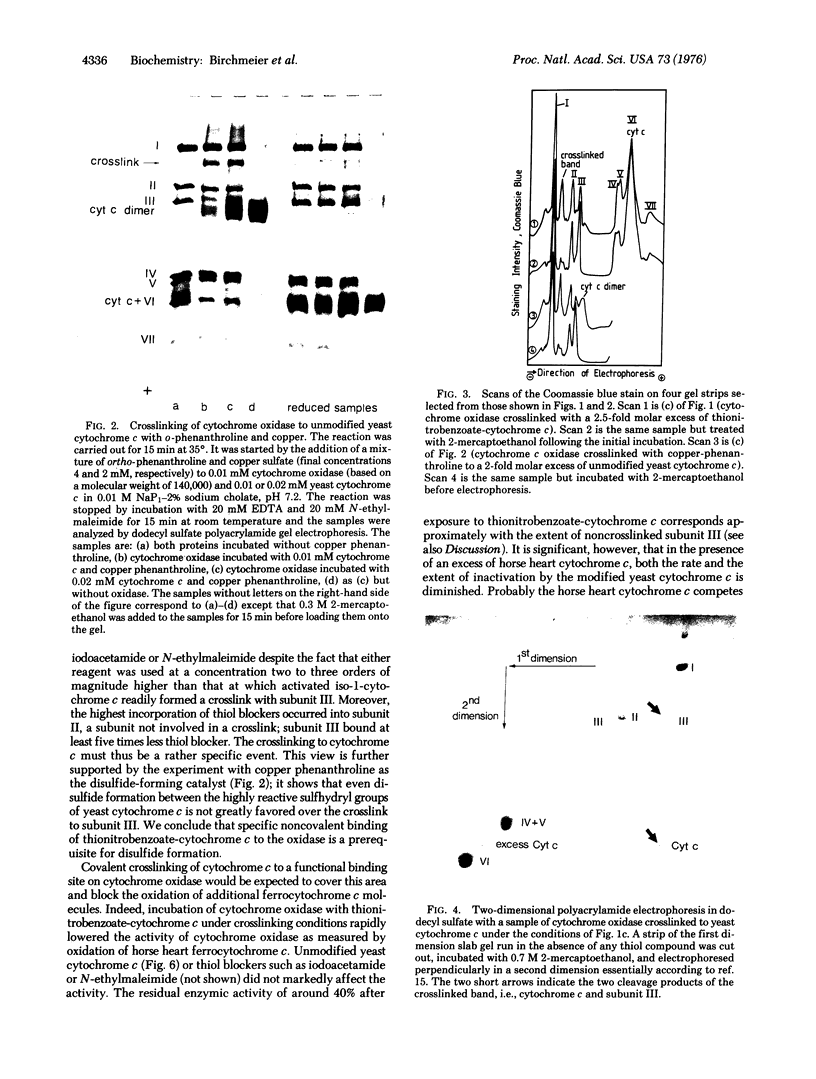
To identify possible substrate-binding subunit(s) of yeast cytochrome c oxidase (ferrocytochrome c:oxygen oxidoreductase, EC 1-9-3-1), the purified enzyme was reacted with yeast iso-1-cytochrome c whose single free sulfhydryl group at position 107 had been activated with 5,5'-dithiobis(2-nitrobenzoate). The resulting cytochrome c derivative appeared to function as an "affinity-label" of cytochrome oxidase, since it rapidly inactivated the enzyme. Inactivation was competitively prevented by underivatized cytochrome c. When the "affinity-labeled" oxidase was analyzed by two-dimensional polyacrylamide electrophoresis in dodecyl sulfate (separation in the second dimension being carried out in the presence of excess sulfhydryl compound), it was found that the derivatized cytochrome c had specifically formed a mixed disulfide with the mitochondrially made subunit III (apparent molecular weight 24,000) of the oxidase. Similar results were obtained when underivatized iso-I-cytochrome c was crosslinked to the oxidase by oxidative disulfide bridge formation in the presence of ortho-phenanthroline and Cu++. These data indicate that the hydrophobic mitochondrially made subunit III of yeast cytochrome c oxidase is in close proximity to the cytochrome c binding site on the enzyme. Since cytochrome c and the mitochondrially made cytochrome oxidase subunit III are typical peripheral and integral membrane proteins, respectively, the present study suggests a useful approach for analyzing specific interactions between these different classes of membrane proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birchmeier W., Tuchshmid P. E., Winterhalter K. H. Comparison of human hemoglobin A carrying glutathione as a mixed disulfide with the naturally occurring human hemoglobin A. Biochemistry. 1973 Sep 11;12(19):3667–3672. doi: 10.1021/bi00743a015. [DOI] [PubMed] [Google Scholar]
- Birchmeier W., Wilson K. J., Christen P. Cytoplasmic aspartate aminotransferase: syncatalytic sulfhydryl group modification. J Biol Chem. 1973 Mar 10;248(5):1751–1759. [PubMed] [Google Scholar]
- DAVIES H. C., SMITH L., WASSERMAN A. R. THE INFLUENCE OF IONIC STRENGTH AND POLYCATIONS ON THE OXIDATION OF FERROCYTOCHROME C BY CYTOCHROME C OXIDASE. Biochim Biophys Acta. 1964 May 4;85:238–246. doi: 10.1016/0926-6569(64)90244-5. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Dickerson R. E. The structure and history of an ancient protein. Sci Am. 1972 Apr;226(4):58–passim. doi: 10.1038/scientificamerican0472-58. [DOI] [PubMed] [Google Scholar]
- Downer N. W., Robinson N. C. Characterization of a seventh different subunit of beef heart cytochrome c oxidase. Similarities between the beef heart enzyme and that from other species. Biochemistry. 1976 Jun 29;15(13):2930–2936. doi: 10.1021/bi00658a036. [DOI] [PubMed] [Google Scholar]
- Errede B., Haight G. P., Jr, Kamen M. D. Oxidation of ferrocytochrome c by mitochondrial cytochrome c oxidase. Proc Natl Acad Sci U S A. 1976 Jan;73(1):113–117. doi: 10.1073/pnas.73.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eytan G. D., Carroll R. C., Schatz G., Racker E. Arrangement of the subunits in solubilized and membrane-bound cytochrome c oxidase from bovine heart. J Biol Chem. 1975 Nov 25;250(22):8598–8603. [PubMed] [Google Scholar]
- Eytan G. D., Schatz G. Cytochrome c oxidase from bakers' yeast. V. Arrangement of the subunits in the isolated and membrane-bound enzyme. J Biol Chem. 1975 Jan 25;250(2):767–774. [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J Biol Chem. 1976 Feb 25;251(4):1104–1115. [PubMed] [Google Scholar]
- Margoliash E., Ferguson-Miller S., Tulloss J., Kang C. H., Feinberg B. A., Brautigan D. L., Morrison M. Separate intramolecular pathways for reduction and oxidation of cytochrome c in electron transport chain reactions. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3245–3249. doi: 10.1073/pnas.70.11.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T. L., Poyton R. O., Wharton D. C., Schatz G. Cytochrome c oxidase from bakers' yeast. I. Isolation and properties. J Biol Chem. 1973 Feb 25;248(4):1346–1354. [PubMed] [Google Scholar]
- Mason T. L., Schatz G. Cytochrome c oxidase from bakers' yeast. II. Site of translation of the protein components. J Biol Chem. 1973 Feb 25;248(4):1355–1360. [PubMed] [Google Scholar]
- Nicholls P. Cytochrome c binding to enzymes and membranes. Biochim Biophys Acta. 1974 Dec 30;346(3-4):261–310. doi: 10.1016/0304-4173(74)90003-2. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Mahler H. R. Studies on cytochrome oxidase. Partial resolution of enzymes containing seven or six subunits, from yeast and beef heart, respectively. J Biol Chem. 1976 Jan 25;251(2):257–269. [PubMed] [Google Scholar]
- Phan S. H., Mahler H. R. Studies on cytochrome oxidase. Preliminary characterization of an enzyme containing only four subunits. J Biol Chem. 1976 Jan 25;251(2):270–276. [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. III. Physical characterization of isolated subunits and chemical evidence for two different classes of polypeptides. J Biol Chem. 1975 Jan 25;250(2):752–761. [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. IV. Immunological evidence for the participation of a mitochondrially synthesized subunit in enzymatic activity. J Biol Chem. 1975 Jan 25;250(2):762–766. [PubMed] [Google Scholar]
- Sebald W., Machleidt W., Otto J. Products of mitochondrial protein synthesis in Neurospora crassa. Determination of equimolar amounts of three products in cytochrome oxidase on the basis of amino-acid analysis. Eur J Biochem. 1973 Oct 5;38(2):311–324. doi: 10.1111/j.1432-1033.1973.tb03064.x. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J., Erecińska M., Chance B. Cytochrome c interaction with membranes. II. Comparative study of the interaction of c cytochromes with the mitochondrial membrane. Arch Biochem Biophys. 1973 Aug;157(2):531–540. doi: 10.1016/0003-9861(73)90672-3. [DOI] [PubMed] [Google Scholar]
- Wada K., Okunuki K. Studies on chemically modified cytochrome c. II. The trinitrophenylated cytochrome c. J Biochem. 1969 Aug;66(2):249–262. doi: 10.1093/oxfordjournals.jbchem.a129141. [DOI] [PubMed] [Google Scholar]
- Wang K., Richards F. M. An approach to nearest neighbor analysis of membrane proteins. Application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem. 1974 Dec 25;249(24):8005–8018. [PubMed] [Google Scholar]