Abstract
Imaging with Ca2+-sensitive fluorescent dye has provided a wealth of insight into the dynamics of cellular Ca2+ signaling. The spatiotemporal evolution of intracellular free Ca2+ observed in imaging experiments is shaped by binding and unbinding to cytoplasmic Ca2+ buffers, as well as the fluorescent indicator used for imaging. These factors must be taken into account in the interpretation of Ca2+ imaging data, and can be exploited to investigate endogenous Ca2+ buffer properties. Here we extended the use of Ca2+ fluorometry in the characterization of Ca2+ binding molecules within cells, building on a method of titration of intracellular Ca2+ binding sites in situ with measured amounts of Ca2+ entering through voltage-gated Ca2+ channels. We developed a systematic procedure for fitting fluorescence data acquired during a series of voltage steps to models with multiple Ca2+ binding sites. The method was tested on simulated data, and then applied to 2-photon fluorescence imaging data from rat posterior pituitary nerve terminals patch clamp-loaded with the Ca2+ indicator fluo-8. Focusing on data sets well described by a single endogenous Ca2+ buffer and dye, this method yielded estimates of the endogenous buffer concentration and Kd, the dye Kd, and the fraction of Ca2+ inaccessible cellular volume. The in situ Kd of fluo-8 thus obtained was indistinguishable from that measured in vitro. This method of calibrating Ca2+-sensitive fluorescent dyes in situ has significant advantages over previous methods. Our analysis of Ca2+ titration fluorometric data makes more effective use of the experimental data, and provides a rigorous treatment of multivariate errors and multiple Ca2+ binding species. This method offers a versatile approach to the study of endogenous Ca2+ binding molecules in their physiological milieu.
Keywords: Ca2+ imaging, 2-photon microscopy, neurosecretion, fluorescent Ca2+ indicators, Ca2+ binding proteins
1. Introduction
Fluorometric Ca2+ indicators have served as powerful tools in the study of Ca2+ in living cells [1–5]. Photometric experiments with these probes provide measurements of cytoplasmic free Ca2+ concentration ([Ca2+]Free), and imaging experiments in real time have provided insight into the spatiotemporal dynamics of [Ca2+]Free as it rises and falls during physiological processes. Changes in [Ca2+]Free are modified in amplitude, time course, and spatial spread by molecules that bind Ca2+, including both fluorescent indicators and endogenous Ca2+ buffers. Quantitative Ca2+ fluorometry can provide useful information about endogenous Ca2+ buffers, provided that care is taken to resolve the contributions to Ca2+ binding by the various relevant species. By evaluating the effects of different dye concentrations on Ca2+ signals one can estimate the endogenous buffering capacity [4,6–9].
Most studies of cytoplasmic Ca2+ buffering simply measure buffering capacity without determining the Kd and concentration of endogenous Ca2+ binding molecules. Furthermore, relatively few studies have used measurements of amount of added Ca2+ and cellular volume to estimate the change in total Ca2+ concentration ([Ca2+]Total). Measurements of radiolabeled Ca2+ [10], photoreleased Ca2+ [11,12], and Ca2+ current (ICa) [13] have all been used to estimate the added Ca2+ in conjunction with fluorometric [Ca2+]Free in various cell types. Loss of cellular Ca2+ can also be determined from the current of the Ca2+/Na+ exchanger [14] or from changes in extracellular Ca2+ [15], and used to estimate buffer capacity. By titrating Ca2+ with multiple steps using ICa [16] or added Ca2+ [17], one can follow the saturation of cytoplasmic binding sites as a function of [Ca2+]Free. The buffering capacity decreases as [Ca2+]Free rises and this saturation provides a determination of the Kd and concentration of endogenous Ca2+ buffer [16]. A variation of this approach treated the Ca2+ entry per pulse as a parameter and analyzed the rise in [Ca2+]Free with a two-binding site model to determine the endogenous buffer properties [18].
Previous studies of in situ Ca2+ titration used the average [Ca2+]Free for each step rather than the initial and final [Ca2+]Free [16,18], and relied on a morphological estimate of the available cellular volume [16]. In the case of cardiac myocytes, optical signals were quite large and the buffering contribution of the Ca2+ indicator was relatively small [16]. In an effort to improve the utility of Ca2+ titration and apply it more broadly to different preparations, we developed a more robust method of analyzing the titration of cellular Ca2+ binding sites. Starting with the mass balance relation between initial and final [Ca2+]Free for each pulse together with ICa, we developed a procedure for fitting to a model with multiple binding sites that accounts for the errors in all three relevant measurements. We applied this method to patch clamp/imaging experiments in peptidergic nerve terminals of the rat posterior pituitary [19], estimated the concentration and Kd of an endogenous buffer, the Kd of the Ca2+-sensitive dye fluo-8, and the fraction of Ca2+ inaccessible volume.
2. Materials and Methods
2.1 Posterior Pituitary Slice preparation
Sprague-Dawley rats (aged 6–9 weeks) were anesthetized with isoflurane and decapitated according to procedures approved by the Animal Care and Use Committee at the University of Wisconsin. The posterior pituitary gland was removed and sliced at 70 μm with a VT1200S vibratome (Leica Biosystems) in ice cold artificial cerebrospinal fluid (aCSF) consisting of 125 mM NaCl, 4 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 2 mM CaCl2, and 1 mM MgCl2 saturated with 95% O2/5% CO2. Slices were used immediately or stored in aCSF at room temperature for up to four hours. The posterior pituitary consists primarily of peptidergic nerve terminals, many of which are over 10 μm in diameter [20], and patch clamp recordings provide estimates of capacitance consistent with this size [21,22]. Morphological studies of patch clamped structures in posterior pituitary slices revealed fine appended axons [23]. These axons have ~20-fold smaller diameters than the swellings and should have minimal effects on Ca2+, but as an added precaution we discarded recordings when axons were visible in order to minimize the effects of Ca2+ diffusion into axons on fluorescence measurements. We also discarded recordings in which ICa recordings showed notches or humps that indicate poor space clamp.
2.2 Electrophysiology
Large nerve terminals with diameters of ~10 μm were located at the surface of a slice under visual guidance using laser scanning gradient contrast microscopy [24,25]. An Axopatch 200B patch clamp amplifier (Molecular Devices) was used to record from nerve terminals in the whole-terminal configuration. Signals were digitized by a digidata 1322A interface connected to a computer running pClamp 8. Slices were perfused with a solution consisting of 121 mM NaCl, 4 mM CsCl, 10 mM CaCl2, 1 mM MgCl2, 20 mM tetraethylammonium chloride, 2 mM 4-aminopyridine, 10 mM glucose, 3 μM tetrodotoxin, and 10 mM HEPES, pH 7.3. Patch pipettes fabricated from borosilicate glass capillaries (King Precision Glass) had resistances of 2–5 MΩ when filled with a solution consisting of 130 mM CsCl, 10 mM Na phosphocreatine, 15 mM tetraethylammonium chloride, 4 mM Mg-ATP, 0.3 mM GTP, and 10 mM HEPES, pH 7.3. These solutions blocked voltage-gated Na+ and K+ channels to isolate ICa [26]. In addition, the pipette solution contained 50 or 100 μM of the Ca2+ indicator dye Fluo-8 (Teflabs). Leak current was subtracted by a P/4 protocol.
2.3 Calcium imaging and two-photon microscopy
Fluorescence was imaged with a Prairie Ultima two-photon microscope using galvanometer based scanning (Bruker Corporation). A chameleon Ti-sapphire laser (Coherent) tuned to λ = 920 nm delivered sub-100 fs pulses to the sample. Epifluorescence and transfluorescence were collected by a 60x, NA 0.9 water immersion objective and an oil immersion, NA 1.4 condenser (Olympus), respectively.
We used the dynamic range Ca2+ indicator fluo-8. At equilibrium the fluorescence of such a dye can be used to calculate [Ca2+]Free as:
| (1) |
F is the ratio of fluorescence to the maximal fluorescence when the dye is saturated with Ca2+ (f/fmax). RF is the dynamic range, the ratio of bound/free dye fluorescence (fmax/fmin). According to the manufacturer (Teflabs), RF ~150 in vitro. We determined a somewhat lower dynamic range of ~100, but this difference may be due to limitations in the dynamic range of our system. In general, for very large values, errors in RF introduce only small errors in calculated [Ca2+]Free [27]. This uncertainty between RF = 100 versus 150 contributed an error to our determination of [Ca2+]Free of only ~0.7% for [Ca2+]Free near Kd, which is well below the other sources of error.
Dye loading after break-in was monitored by observing the rise in resting fluorescence. Fluorescence stabilized within about one minute of break-in. Experiments began only after this stable state had been reached. Nerve terminals retained their fluorescence for up to an hour after the termination of a recording, making it unlikely that dye efflux pathways could create a steady state with lower dye concentration in a nerve terminal than in the pipette. We therefore took the intracellular dye concentration as equal to that in the pipette.
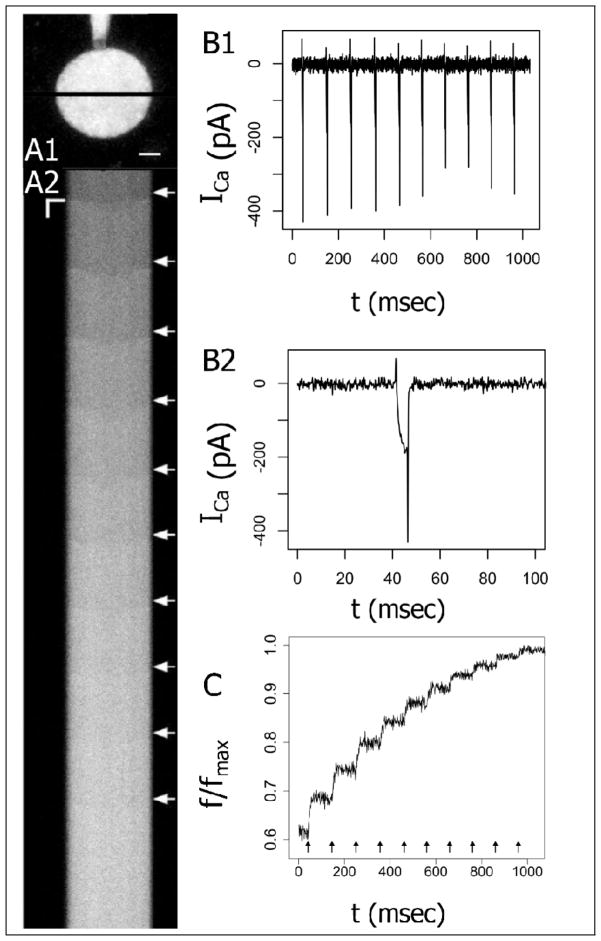
Fluorescence was acquired in line-scan mode with a temporal resolution of ~1 msec per line and diffraction limited spatial resolution (Fig. 1A). Immediately after the application of a voltage step, a spatial fluorescence gradient appeared across the nerve terminal and collapsed over the course of several milliseconds. This is indicated by the delayed rise in the center of the scanned lines (Fig. 1A2). Subsequent to the collapse of the gradient, [Ca2+]Free was taken as spatially uniform. Fluorescence averaged over the scanned line through the nerve terminal (Fig. 1A1) increased (Fig. 1C) as voltage pulses evoked ICa (Figs. 1B1 and 1B2). For each 5 msec pulse, the fluorescence trace was averaged off-line over the inter-stimulus time interval (during which it is nearly constant) to produce measurements of initial fluorescence fI before the pulse, and final fluorescence fF after the pulse.
Fig 1.
A1. Two-photon fluorescence micrograph of a posterior pituitary nerve terminal loaded with 100 μM fluo-8. The dye-filled patch electrode is visible above. The black line indicates the segment selected for line-scanning. Scale bar = 2 μm. A2. Line scans show fluorescence versus time. Fluorescence increases were evoked by a train of 5 msec voltage steps from −80 mV to 10 mV at 100 msec intervals delivered at the white arrows. The horizontal axis corresponds to the spatial dimension along the black line shown in A1 (scale bar = 2 μm). The vertical axis corresponds to time (scale bar = 25 msec). B1. Ca2+ current (ICa) was recorded during the train of 10 pulses. B2. ICa from the first pulse is shown on an expanded time scale. C. Fluorescence was averaged over the scanned line through the nerve terminal (indicated in 1A1) and plotted versus time.
2.4 Ca2+ binding Model
We treat a volume, V, determined from a z-series of optical sections. Nerve terminals with visible axons [23] were not used, in order to avoid their contributions to volume, although as noted above the small diameters of axons should minimize their effects on our measurements. A fraction of the volume that excludes Ca2+ (VE) will reduce the effective volume. VE will be estimated in the course of our analysis.
Our model cell has a concentration BT,D of Ca2+-sensitive fluorescent dye (with dissociation constant, Kd,D) as well as endogenous Ca2+ buffers. Each species has a buffering capacity, κj:
| (2) |
where BJ denotes the Ca2+ bound form of the Jth Ca2+-binding species. The total buffering capacity of the cytoplasm then becomes a sum of the contributions from each species:
| (3) |
This sum includes contributions from both the dye and endogenous Ca2+ buffers (Kd,E1, Kd,E2, etc.). Each species saturates as Ca2+ concentration rises, leading to the following concentration dependence for κj[6,16,18]:
| (4) |
Each voltage pulse gives us a pair of fluorescence measurements, the initial value before the pulse, FI (=fI/fmax) and the final value immediately after, FF (=fF/fmax). We can calculate the corresponding [Ca2+]Free,I and [Ca2+]Free,F, from Eq. 1. For a single buffer (with BT and Kd), the change in [Ca2+]Total is the sum of the change in [Ca2+]Free and the change in the concentration of Ca2+-buffer complex:
| (5) |
Assuming that ICa is the sole source of Ca2+ during a voltage step, we can determine Δ[Ca2+]Total in Eq. 5 from the integral of ICa over one voltage pulse extended to include the ICa tail current. This leads to the following expression:
| (6) |
In dividing the integrated ICa by volume, we must correct the total volume, V, measured from microscopy by the fraction of volume that excludes Ca2+, VE. Q is Faraday’s constant. We used Eq. 1 to replace [Ca2+]Free in Eq. 5 with F and then substituted to simplify the notation.
Eq. 6 does not have a unique solution. Changes in the factor 1-VE can be compensated by proportional changes in Kd and BT. Thus, Eq. 6 defines a family of solutions of the form for any positive value of α. This will be discussed further below.
Eq. 6 can be extended to an arbitrary number of Ca2+ binding species. For a fluorescent dye and one endogenous buffer (dissociation constant Kd,E1 and concentration BT,E1) we have:
| (7) |
The terms containing x1 and x2 have been rearranged to make the expression simpler.
Eqs. 6 and 7 represent models that relate three measured quantities, Δ[Ca2+]Total (as determined from ICa), FI, and FF. Each voltage step provides one triplet data point and each train of pulses provides 10–20 such triplets. The strategy of the present work is to vary the parameters in the model to fit these triplets of data. This work extends previous applications of Ca2+ titration [16,18] by using the initial and final fluorescence rather than a time average, by incorporating multiple binding sites, and by incorporating VE as a free parameter. Furthermore, our use of line scans provides data in which uncertainty due to spatial variations can be avoided.
As noted following Eq. 6, Eq. 7 also defines a family of solutions , and a scaling factor, α, must be determined. We can take α = 1 in the present scenario by equating the cytoplasmic dye concentration with the concentration in the pipette (2.3 Methods). Scenarios with α not equal to one include cases where the dye fails to equilibrate between the pipette and cell, or with dye loading as an AM ester such that its concentration is unknown. If the dye concentration in the cell cannot be equated with that in the pipette then independent knowledge of another parameter is needed. This extends the sphere of problems to which this method can be applied, as will be expanded upon in the discussion. Simulations indicated that with realistic noise levels, the estimation of the parameters Kd,D and Kd,E1 remained robust, even under the condition of strong Ca2+ buffering (Supplemental Material). Examination of Eq. 7 makes the important point that parameter determination requires measurements over a wide range of [Ca2+]Free. κ must change in order for the different terms in parentheses to make their presence felt.
2.5 Simulations and data analysis
Simulated and experimental data were fitted to Eq. 7 by varying some or all of the parameters VE, Kd,D, BT,D, Kd,E1, and BT,E1 and using the method of total least squares (TLS) to evaluate the quality of the fit. (Supplemental Material develops this method in detail). Briefly, the standard method of minimizing the sum of the squared residuals, or ordinary least squares (OLS), relies on the assumption that errors in the independent variables are negligibly small and errors in the dependent variable are independent, identically distributed random normal variables. Our data set consists of triplet measurements: FI, FF, and integrated ICa. Although the error in ICa is very small compared to the errors in FI and FF, we cannot choose two independent variables that satisfy the condition of negligibly small errors. The OLS estimator yielded unreliable estimates of the parameters with simulated data. TLS accounted for the errors in the independent variables and its application to simulated data yielded unbiased parameter estimates with small variance. Supplemental Material presents simulations with data generated by taking an experimental FI value and integrated ICa from a single nerve terminal to calculate FF from Eq. 7. We fitted models to data with a stochastic gradient descent algorithm that converged with high probability to the same set of parameters from any starting estimates. Goodness of fit was assessed with the F-test using a cutoff of P = 0.05. Data analysis was performed in the statistical computing environment R [28]. The code and a sample data set are available as Supplemental Material. Model fits for single data sets are reported as the TLS estimator with standard errors determined by bootstrap resampling with 10000 resampled data sets [29]. Parameter errors were also estimated with the block jackknife method [29], and the differences between the results of the two procedures were not statistically significant. Parameter estimates from the population of nerve terminals in this study are presented as mean ± s.e.m. The standard deviations calculated by averaging over nerve terminals were found to be similar to the uncertainty estimated by the bootstrap and jackknife estimates for errors in the individual fits.
3. Results
3.1 Saturation of dye and determination of fmax
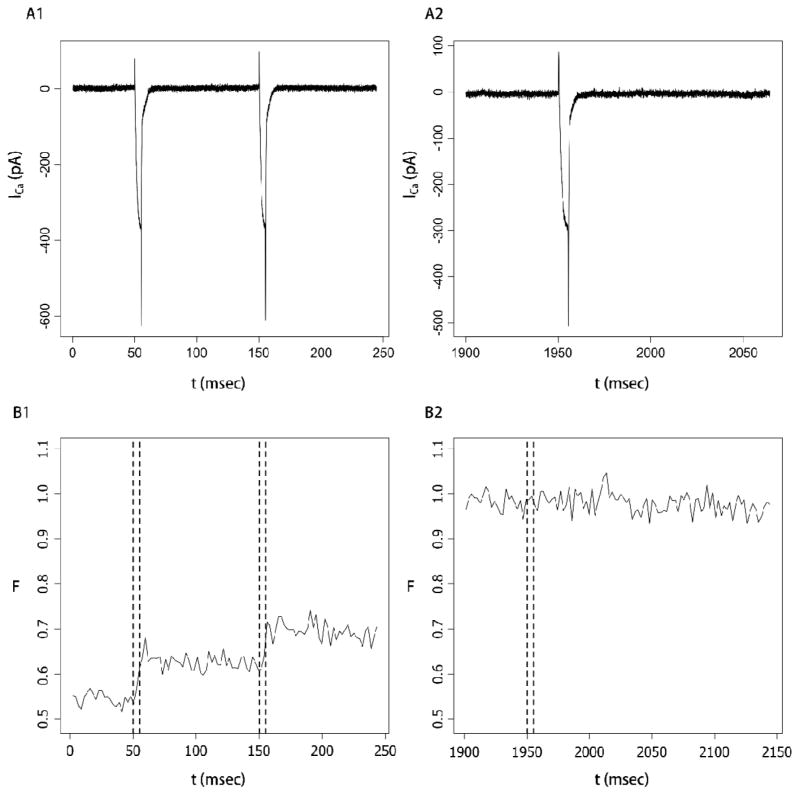
Nerve terminals were held at −80 mV and depolarized in trains of 10 or 20 steps of 5 msec to +10 mV at 100 msec intervals. ICa and fluorescence were recorded simultaneously. This stimulus protocol has been shown to be effective in elevating [Ca2+]Free in this preparation [19,26]. Initial fluorescence before the start of a train was generally slightly above 0.5, consistent with a resting [Ca2+]Free of pituitary nerve terminals that is close to the Kd of fluo-8 [7,26]. Voltage pulses opened Ca2+ channels, and early in a train these pulses produced clear increases in fluorescence in register with ICa (Figs. 2A1 and 2B1). These increments became smaller as the train continued, and the last pulse produced little or no fluorescence increase (Figs. 1C and Fig. 2B2). Because ICa varied by less than 30% throughout the train of voltage steps we can conclude that pulses continued to allow substantial amounts of Ca2+ to enter as the fluorescence leveled off. Fluorescence remained high following the final pulse with no visible decay for at least 100 msec (Fig. 2B2). By contrast, after early pulses the fluorescence showed a modest but clear decay starting immediately (Fig. 2B1). This decay reflects the activity of Ca2+ extrusion and sequestration processes. These processes presumably extruded Ca2+ at the end of the last pulse as well, but when [Ca2+]Free was high the fraction of dye bound to Ca2+ did not fall. Thus, the plateau in fluorescence at the end of a train reflected saturation of the dye rather than a balance between Ca2+ entry and removal.
Fig. 2.
A. Ca2+ current for the first two pulses (A1) and final pulse (A2) of a train of 20 steps from −80 to +10 mV. B. Simultaneous fluorescence recordings during the first two (B1) and last (B2) pulse. The dashed vertical lines in B1 and B2 indicated the times of the voltage pulses in A1 and A2. Fluorescence increased during the first two pulses but not during the final pulse. Fluorescence remained constant for at least 200 msec after the final pulse. Note that different scales and offsets were used for ICa and F in A2 and B2 in order to display a longer time in fluorescence with no decay. The nerve terminal contained 100 μM fluo-8.
The saturation of dye during trains thus provided an estimate of fmax, the fluorescence when all of the fluo-8 is bound to Ca2+. We evaluated the fluorescence before and after the final pulse and considered saturation to have occurred when there was no statistically significant difference. When p < 0.1 we concluded that dye saturation had not been reached and these data sets were not analyzed. Because of the uncertainty of [Ca2+]Free inherent in Eq. 1 when f approaches fmax, we only used f measurements more than 2 standard deviations below fmax. Our analysis of Ca2+ binding depends on this measurement, but more generally, the calculation of [Ca2+]Free using Eq. 1 also depends on knowledge of fmax [27].
3.2 Saturation of endogenous buffers
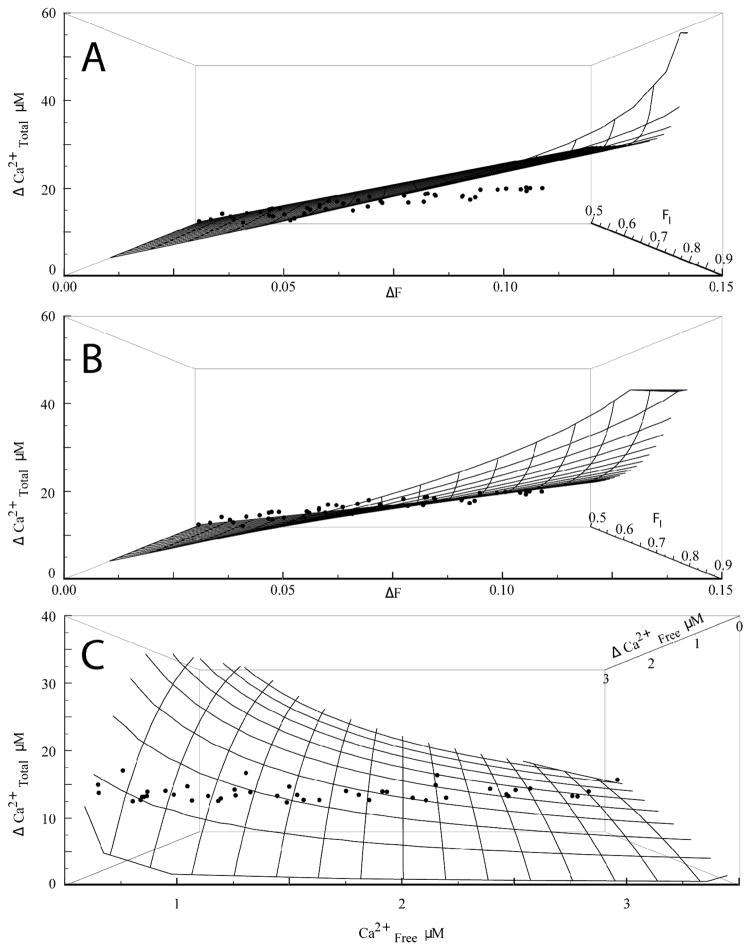
With fmax determined as described above from the fluorescence at the end of a train, we converted initial and final fluorescence readings to normalized values FI and FF that appear in Eq. 6 or 7. Initial tests modeling the relationship between FI, FF, and ICa showed that a model with only one Ca2+ binding species (dye) failed to fit our data. This is illustrated in a 3-dimensional surface plot with Δ[Ca2+]Total, FI, and ΔF=FF−FI (Fig. 3A). The model defines a surface in this coordinate system, represented as a wire mesh in the figure. With only one species at 100 μM (the concentration of fluo-8) the points fell well below the wire mesh computed from Eq. 6 for the best fitting parameter values. Furthermore, the fit returned an unrealistic value for VE of −0.52. The failure is most pronounced for small values of FI (low initial [Ca2+]Free), where ΔF (and Δ[Ca2+]Free) was high. Adding a second buffer improved the fit (Fig. 3B) and yielded highly significant p-values for the parameters. We were thus able to estimate Kd,D = 424 nM, BT,E1 = 110 μM, Kd,E1 = 2.79 μM, and VE = 0.33, from the experiment used to create Fig. 3.
Fig. 3.
A. and B. Δ[Ca2+]Total was plotted as a function of initial fluorescence (FI) and change in fluorescence ΔF. Points represent data from a single nerve terminal stimulated with a train of 20 pulses, and the train was repeated 10 times. The wire mesh shows the surface calculated from Eq. 7 with parameters determined by fitting to a model with one buffer (A, Kd=278±11 nM, VE = −0.52±0.08) or two (B, Kd,D =424±5 nM, Kd,E1 = 2.79±46 μM, BT,E1 =110±1.6 μM, VE =0.33±0.01). Adding a second buffer improved the fit significantly over the simpler model (p < 10−150 by the F-test). C. Δ[Ca2+]Total is plotted as a function of [Ca2+]Free,I and Δ[Ca2+]Free, calculated from the fluorescence in A–B, and the wire mesh is the model prediction as in B for two buffers.
We performed the analysis described for Fig. 3 on data from 21 nerve terminals that were significantly better fitted by one endogenous buffer plus dye (Eq. 7) than by dye alone (Eq. 6), but for which the addition of a second endogenous buffer did not significantly improve the fit. The parameter values obtained are presented in Table 1. In much of our data, a second endogenous buffer significantly improved the fit when we fixed Kd,D, and these results will be published separately. We also displayed the 3-dimensional surface plot for computed [Ca2+]Free (Fig. 3C). Although the fitting was performed based on fluorescence, this plot illustrates the larger increments Δ[Ca2+]Free for lower [Ca2+]Free, and again shows how the data points fell on a path within the surface defined by Eq. 7.
Table 1.
Parameters obtained from fits of Eq. 7 to data from 21 nerve terminals are presented as mean ± s.e.m.
| Kd,D | VE | Kd,endo | BT,endo |
|---|---|---|---|
| 0.38±.045 μM | 0.38±.04 | 2.2±.27 μM | 100±18μM |
We can evaluate the model with respect to [Ca2+]Free in more accessible 2-dimensional plots by noting that variations in ICa (and Δ[Ca2+]Total) were generally less than 30% (~15% in the example in Fig. 3). This variation was much less than that of [Ca2+]Free and Δ[Ca2+]Free. Thus, a simple plot of [Ca2+]Free,I versus Δ[Ca2+]Free illustrated the important features of the 3-dimensional plots in Fig. 3. With one buffer the model clearly failed to fit the data and the points deviated from the model over the entire range (Fig. 4A). The deviation at high [Ca2+]Free reflected the large uncertainty resulting from the nonlinearity of Eq. 1. However, the deviation at low [Ca2+]Free was also clear and systematic. This 2-dimensional plot illustrated once again that incorporating the endogenous buffer yielded a model that predicted the relation between [Ca2+]Free,I and Δ[Ca2+]Free more accurately (Fig. 4B).
Fig. 4.
Data from Fig. 3 were projected into the Δ[Ca2+]Free,I, [Ca2+]Free plane. A. The solid curve is Eq. 7 with parameters from in Fig. 3A. B. The solid curve is Eq. 7 with parameters from Fig. 3B. As in Fig. 3, the addition of a second buffer improved the fit. Note that the data in the two panels are scaled differently according to the respective Kd,D values from Fig. 3A versus Fig. 3B.
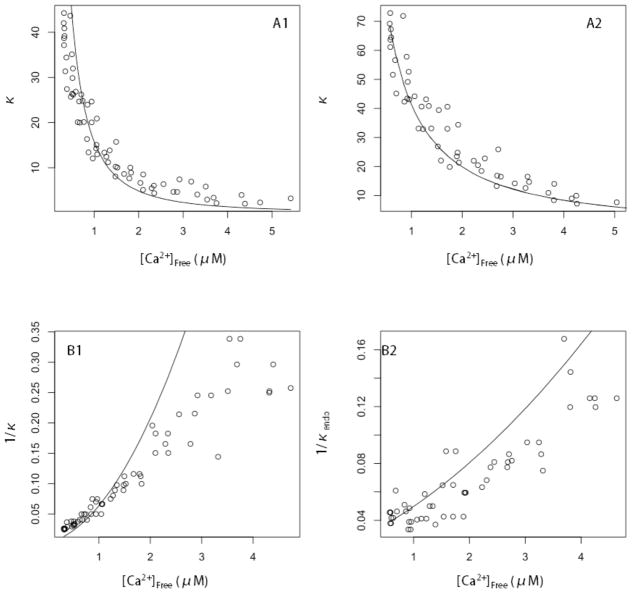
To illustrate the properties of the endogenous buffer, and its saturation as [Ca2+]Free rises, we plotted the total Ca2+ buffering capacity versus [Ca2+]Free (Fig. 5A1 and 5A2). Previous studies have used these plots to illustrate the saturation of Ca2+ binding sites in situ [16,18]. Eqs. 3 and 4 provide the predicted behavior. With one buffer the curve fell below the data points (Fig. 5A1) at high [Ca2+]free and above them at low [Ca2+]Free, and with both dye and endogenous buffer the fitted curve was very close to the data (Fig. 5A2). We also plotted 1/κ versus [Ca2+]Free as these plots have a simple parabolic shape for one Ca2+ binding species (Figs. 5B1 and 5B2). Fig. 5B1 was simply a transform of Fig. 5A1 and shows the disagreement between the model and data more clearly. Fig. 5B2 shows the results with both dye and endogenous buffer included in the fit, and with κendo of the endogenous buffer plotted rather than total κ. Fig. 5 clearly demonstrated the saturation of Ca2+ binding sites in pituitary nerve terminals, and this saturation reflected Ca2+ binding to both the fluorescent dye and an endogenous buffer.
Fig. 5.
A. The total Ca2+ buffering capacity, κ, calculated from data as Δ[Ca2+]Total/Δ[Ca2+]Free plotted versus Δ[Ca2+]Free. A1. For exogenous dye alone, the solid curve is κ calculated from Eq. 3. Without endogenous buffer, the fit yielded VE=−0.52 (as already noted) and the resulting curve fell below the data points. Including dye plus one endogenous buffer (A2) yielded VE=0.33 and a better fit to the data. B1. 1/κ was plotted versus Δ[Ca2+]Free for the same fit in A1. The solid curve was obtained by inverting Eq. 4. B2. κendo was calculated as Δ[Ca2+]Total/Δ[Ca2+]Free−κdye, with κdye from Eq 4. 1/κendo was plotted as a function of Δ[Ca2+]Free. The solid curve is 1/κendo from Eq. 4 with Kd,E1 and BT,E1 from Fig. 3B. Note that in A1 and B1 different values for the dye Kd (278 nM vs. 424 nM) altered the concentration scales from those in A2 and B2.
3.3 Kd of fluo-8
Quantitative interpretation of Ca2+ fluorometry experiments depends on accurate measurements of the Kd of the Ca2+ indicator, but this quantity can vary between in vitro solutions where it is typically determined versus cellular environments where it is used [4–6,26,30–35]. The difference presumably reflects the influence of the local chemical environment on the Ca2+ binding equilibrium; the Kd is sensitive to pH, ionic strength, temperature, and physiological concentrations of protein [35] (see the Table 19.2 of Molecular Probes Handbook for a number of examples; http://www.lifetechnologies.com). The Kd of Ca2+ indicators in different cellular environments nearly always exceeds in vitro values, sometimes by as much as 8-fold [31,32,36], and in the few cases where the in vitro Kd exceeds the in situ Kd, the difference is very small. Kd values also vary between cell type, subcellular compartment, and laboratory.
Calibrating a dye Kd in situ is difficult and the methods have significant limitations [30,33,37–39]. One approach entails loading cells through a patch pipette with calibration solutions in which Ca2+ concentration has been fixed by a chelator such as EGTA or BAPTA [40]. However, Ca2+ binding to these chelators should have a similar dependence on environment, as they are structurally closely related to the Ca2+ indicators. Environment effects on the Kd of BAPTA have been reported [17]. This will give rise to errors in the calculated [Ca2+]Free of the calibration solutions that are comparable to those based on the in vitro Kd of a fluorescent dye. A second calibration method employs solutions with chelators to control [Ca2+]Free outside a cell. Dye is then loaded into the cell, and a Ca2+ ionophore (such as ionomycin) is used to allow extracellular Ca2+ to enter and equilibrate [30,41]. This approach places the chelator in an environment where its Kd is known precisely, but it requires complete blockade of cellular Ca2+ extrusion and sequestration mechanisms. Cells have different Ca2+ handling machinery and sensitivities to drugs, so that intracellular [Ca2+]Free may not equal extracellular [Ca2+]Free.
When we allowed Kd,D in Eq. 7 to vary, the fit yielded a value for the Kd,D for fluo-8 in situ of 380 ± 45 nM, which is not significantly different from the in vitro value of 390 nM provided by the manufacturer. Our simulations indicated that this estimate is robust and insensitive to errors in the estimate of fmax on the order of typical noise levels (Supplemental Material).
4. Discussion
We have investigated intracellular Ca2+ binding molecules by in situ titration with Ca2+ supplied through voltage-gated Ca2+ channels [16,18]. This method entailed fitting a model with multiple binding sites to sets of 3-tuple data consisting of FI, FF, and ICa, measured over a train of voltage steps. We have systematically explored the information contained in this form of data, provided a framework for fitting models, and explored the efficiency of parameter determination under different conditions. This method builds on earlier work in which Ca2+ entry could not be measured directly [18]. That study estimated values of ~500 nM and ~130 μM for the Kd and concentration, respectively, of the endogenous Ca2+ buffer in dentate gyrus granule cell nerve terminals. The major endogenous buffer of these neurons, calbindin D28K was subsequently shown by patch clamp loading and immunohistochemistry to be present in the cell bodies of these neurons at a concentration of 33 μM, which with four binding sites per molecule gives a binding site concentration of 152 μM [42]. This agreement may seem surprising considering the washout of cytoplasmic proteins during patch clamp recording, but diffusion out of nerve terminals hundreds of microns down an axon presumably takes more time than diffusion out of a nerve cell body. Washout might be expected to have a greater impact in the present study because here the nerve terminals were patch clamped directly. However, a previous patch clamp study of posterior pituitary nerve terminals showed little if any buffer washout for up to 5 minutes [7].
Although we developed our method for dynamic range indicators, simple modifications to the equations would render the method applicable to ratiometric Ca2+ indicators [43,44], which would obviate the need for saturation of the dye with Ca2+ for measurement of fmax. This method depends on Ca2+ channels as the only source of cytoplasmic Ca2+, and cannot be applied to cells with Ca2+-induced Ca2+ release. The posterior pituitary nerve terminals studied here are insensitive to IP3 receptor and ryanodine receptor antagonists, and appear to lack Ca2+-operated stores [7] in the absence of μ-opioid receptor activation [45]. Use of this method in cells with mobile Ca2+ stores would require eliminating this source of Ca2+.
Fitting Eq. 7 to data such as ours defines a family of solutions related by a factor α. Here we took α=1 by setting the cellular dye concentration equal to that in the patch pipette. Other experimental designs may dictate alternative approaches. For example, one could determine α from the Kd of the indicator, if one had a reliable in situ measurement, or from an independent measurement of VE. With a ratiometric dye for which an in situ calibration is available it is possible to show that the fluorescence intensity is proportional to the product (1−VE)BT, and with a calibrated photometric system this measurement can also be used to determine α. Knowledge of any one of the parameters of Eq. 7 enables fitting to extract the remaining parameters. In experiments with dye loaded as an AM-ester or with a genetically-encoded sensor, a Ca2+ ionophore could be used to control Ca2+ and estimate Kd,D [30]. In experiments with caged Ca2+ the amount of Ca2+ liberated by a pulse of light may be specified and serve as a unit of measure, with other parameters determined in these units. Experimental designs along these lines could also be applied to diffusionally isolated subcellular compartments which are difficult to access directly, such as dendritic spines.
In situ Ca2+ titration depends on knowledge of VE in order to convert the integral of ICa into Δ[Ca2+]Total. We estimated a value for VE of 0.38. Ultrastructural studies of posterior pituitary provide measurements of vesicle numbers and average diameter [20] from which we estimated VE=0.6. Given the difference between these two experimental methods, these values should be viewed as qualitatively similar. We can be confident that both Ca2+ and dye are excluded from the same compartments, as both are charged and should not readily cross intracellular membranes. As already noted, intracellular Ca2+ stores would present a problem for this method. Rapid movement of Ca2+ into dye-inaccessible compartments would reduce VE for Ca2+ below that for dye. [Ca2+]Free calculated from Eq. 1 would apply only to the dye-accessible compartment, but [Ca2+]Total would apply to a larger volume, so Eq. 7 would no longer be valid. A dye with access to compartments that exclude Ca2+ would be a problem not only for titration, but more fundamentally for calculating [Ca2+]Free with Eq. 1. Dye in a Ca2+-inaccessible compartment would increase the background and reduce the effective dynamic range of the dye. For a large dynamic range this effect has little impact, as noted in 2.3 Methods.
In this study, we made a preliminary estimate of the Kd and concentration of the endogenous buffer in pituitary nerve terminals. With our values for BT.E1 and Kd,E1 from Table 1 Eq. 4 gives k~35, which is clearly much lower than the value of 175 measured by the classical analysis of the relation between Ca2+ rise and dye buffering capacity [7]. This may reflect differences between dissociated nerve terminals and intact slices, or it may reflect errors in the classical method arising from the failure to address endogenous buffer saturation [18]. In fact, it is likely that nerve terminals contain more than one Ca2+ buffer and their concentrations may vary from terminal to terminal. Here, to illustrate the method we focused on nerve terminals that were adequately described by a single endogenous buffer. Although in these data sets adding a second endogenous buffer did not significantly improve the fit, additional species would be missed if the fluorescence noise was high. Although Muller et al [42] raised the concern that low affinity species could increase the Ca2+ buffering capacity above that estimated in Ca2+ imaging experiments, Eq. 4 indicated that low affinity molecules would need to have implausibly high concentrations to make a significant contribution. For example, ATP binds Ca2+ but despite its high concentration contributes very little to Ca2+ buffering due to its low affinity. Our simulations suggested that for the worst case of noise near the maximum we encountered, a nerve terminal with two buffers (Kd,E1~0.1 μM, Kd,E2~1 μM, BT,E1=BT,E2~100 μM) could be adequately fitted by a model with a single buffer, and a second species did not improve the fit. Significantly, in these simulations, estimations of Kd,D remained robust. In view of the fact that two endogenous buffers were required to fit many of our data sets, the Kd and concentration obtained from the single endogenous buffer fits likely reflect aggregate behavior of multiple buffers. Our primary goal here was to illustrate the method and a more thorough analysis of endogenous buffers will be reported separately.
We used our method to estimate the Kd of the fluorescent dye fluo-8 in situ. As a dye calibration method this approach is a significant improvement over previous methods as it avoids relying on in vitro properties of other Ca2+ chelators. While the assumptions underlying the previous calibration methods are difficult to assess and violations difficult to correct, our method was sufficiently robust that we were able to test many of our assumptions. Our estimate for the Kd of fluo-8 was remarkably close to the in vitro estimate. This example suggests that the environment of these nerve terminals does not perturb the binding of this particular dye. However, the large variations reported in the literature [31,32,36] suggest that more dyes should be subjected to this form of analysis.
Titration of Ca2+ binding in situ provided estimates of the Kd and concentration of endogenous Ca2+ buffers. Such molecules shape the spatial and temporal dynamics of Ca2+ signals and play a critical role in determining the physiological consequences of Ca2+ entry [4,46–53]. The saturation of endogenous buffers has been shown to contribute to synaptic facilitation [49,54,55], and dysregulation of cellular Ca2+ buffering has serious pathological consequences [56–58]. More detailed studies of Ca2+ signaling in situ will be necessary to understand the molecular basis of these diseases. Thus, the quantitative characterization of Ca2+ binding processes in situ should have a number of useful applications.
Supplementary Material
Highlights.
Endogenous Ca2+ buffers can be probed in situ by titrating Ca2+ binding sites.
Our extension exploits information available in Ca2+ fluorometry more effectively.
We analyzed fluo-8 Ca2+ 2-photon imaging data from pituitary nerve terminals.
We estimated the excluded volume and endogenous Ca2+ buffer Kd and concentration.
An estimate of the in situ Kd of fluo-8 was very close to the in vitro value.
Acknowledgments
This research was supported by NIH grants R01NS44057 and R01NS072905.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russell JT. Imaging calcium signals in vivo: a powerful tool in physiology and pharmacology. Br J Pharmacol. 2011;163:1605–25. doi: 10.1111/j.1476-5381.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel F, Lohmann C. Probing synaptic function in dendrites with calcium imaging. Exp Neurol. 2013;242:27–32. doi: 10.1016/j.expneurol.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Grewe BF, Langer D, Kasper H, Kampa BM, Helmchen F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nat Methods. 2010;7:399–405. doi: 10.1038/nmeth.1453. [DOI] [PubMed] [Google Scholar]
- 4.Neher E, Augustine GJ. Calcium gradients and buffers in bovine chromaffin cells. [accessed June 20, 2013];J Physiol. 1992 450:273–301. doi: 10.1113/jphysiol.1992.sp019127. http://jp.physoc.org/content/450/1/273.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augustine G, Neher E. Calcium requirements for secretion in bovine chromaffin cells. [accessed June 21, 2013];J Physiol. 1992 :247–271. doi: 10.1113/jphysiol.1992.sp019126. http://jp.physoc.org/content/450/1/247.short. [DOI] [PMC free article] [PubMed]
- 6.Neher E. The use of fura-2 for estimating Ca buffers and Ca fluxes. [accessed January 09, 2014];Neuropharmacology. 1995 34:1423–42. doi: 10.1016/0028-3908(95)00144-u. http://www.ncbi.nlm.nih.gov/pubmed/8606791. [DOI] [PubMed] [Google Scholar]
- 7.Stuenkel EL. Regulation of intracellular calcium and calcium buffering properties of rat isolated neurohypophysial nerve endings. [accessed January 09, 2014];J Physiol. 1994 481(Pt 2):251–71. doi: 10.1113/jphysiol.1994.sp020436. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1155926&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tank DWD, Regehr WWG, Delaney KRK. a quantitative analysis of presynaptic calcium dynamics that contribute to short-term enhancement. [accessed January 09, 2014];J Neurosci. 1995 15:7940–7952. doi: 10.1523/JNEUROSCI.15-12-07940.1995. http://www.jneurosci.org/content/15/12/7940.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca(2+) ions in dendritic spines. [accessed January 09, 2014];Neuron. 2002 33:439–52. doi: 10.1016/s0896-6273(02)00573-1. http://www.ncbi.nlm.nih.gov/pubmed/11832230. [DOI] [PubMed] [Google Scholar]
- 10.Fontana G, Blaustein MP. Calcium buffering and free Ca2+ in rat brain synaptosomes. J Neurochem. 1993;60:843–850. doi: 10.1111/j.1471-4159.1993.tb03228.x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Baldawi NF, Abercrombie RF. Cytoplasmic calcium buffer capacity determined with Nitr-5 and DM-nitrophen. Cell Calcium. 1995;17:409–421. doi: 10.1016/0143-4160(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 12.Fleet A, Ellis-Davies G, Bolsover S. Calcium buffering capacity of neuronal cell cytosol measured by flash photolysis of calcium buffer NP-EGTA. Biochem Biophys Res Commun. 1998;250:786–790. doi: 10.1006/bbrc.1998.9377. [DOI] [PubMed] [Google Scholar]
- 13.Daub B, Ganitkevich VY. An estimate of rapid cytoplasmic calcium buffering in a single smooth muscle cell. Cell Calcium. 2000;27:3–13. doi: 10.1054/ceca.1999.0084. [DOI] [PubMed] [Google Scholar]
- 14.Trafford AW, Diaz ME, Eisner DA. A novel, rapid and reversible method to measure Ca buffering and time-course of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflügers Arch. 1999;437:501–503. doi: 10.1007/s004240050808. [DOI] [PubMed] [Google Scholar]
- 15.Mogami H, Gardner J, Gerasimenko OV, Camello P, Petersen OH, Tepikin AV. Calcium binding capacity of the cytosol and endoplasmic reticulum of mouse pancreatic acinar cells. J Physiol. 1999;518:463–467. doi: 10.1111/j.1469-7793.1999.0463p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berlin JR, Bassani JW, Bers DM. Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys J. 1994;67:1775–1787. doi: 10.1016/S0006-3495(94)80652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassani RA, Shannon TR, Bers DM. Passive Ca 2+ binding in ventricular myocardium of neonatal and adult rats. Cell Calcium. 1998;23:433–442. doi: 10.1016/s0143-4160(98)90100-2. [DOI] [PubMed] [Google Scholar]
- 18.Jackson MB, Redman SJ. Calcium Dynamics, Buffering, and Buffer Saturation in the Boutons of Dentate Granule-Cell Axons in the Hilus. [accessed June 20, 2013];J Neurosci. 2003 23:1612–1621. doi: 10.1523/JNEUROSCI.23-05-01612.2003. http://www.jneurosci.org/content/23/5/1612.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci. 1991;88:380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordmann JJ. Ultrastructural morphometry of the rat neurohypophysis. J Anat. 1977;123:213. [PMC free article] [PubMed] [Google Scholar]
- 21.Bielefeldt K, Rotter JL, Jackson MB. Three potassium channels in rat posterior pituitary nerve terminals. J Physiol. 1992;458:41–67. doi: 10.1113/jphysiol.1992.sp019405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu SF, Augustine GJ, Jackson MB. Adaptation of Ca2+-Triggered Exocytosis in Presynaptic Terminals. Neuron. 1996;17:501–512. doi: 10.1016/s0896-6273(00)80182-8. [DOI] [PubMed] [Google Scholar]
- 23.Jackson MB. Passive current flow and morphology in the terminal arborizations of the posterior pituitary. J Neurophysiol. 1993;69:692–702. doi: 10.1152/jn.1993.69.3.692. [DOI] [PubMed] [Google Scholar]
- 24.Mainen ZF, Maletic-Savatic M, Shi SH, Hayashi Y, Malinow R, Svoboda K. Two-Photon Imaging in Living Brain Slices. Methods. 1999;18:231–239. doi: 10.1006/meth.1999.0776. http://dx.doi.org/10.1006/meth.1999.0776. [DOI] [PubMed] [Google Scholar]
- 25.Dodt HU, Zieglgänsberger W. Infrared videomicroscopy: a new look at neuronal structure and function. Trends Neurosci. 1994;17:453–458. doi: 10.1016/0166-2236(94)90130-9. http://dx.doi.org/10.1016/0166-2236(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 26.Branchaw JL, Banks MI, Jackson MB. Ca2+- and Voltage-Dependent Inactivation of Ca2+ Channels in Nerve Terminals of the Neurohypophysis. [accessed July 02, 2013];J Neurosci. 1997 17:5772–5781. doi: 10.1523/JNEUROSCI.17-15-05772.1997. http://www.jneurosci.org/content/17/15/5772.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maravall M, Mainen ZFF, Sabatini BLL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J. 2000;78:2655–67. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R. R Development Core Team. R: A Language and Environment for Statistical Computing. R Found Stat Comput. 2011;1:409. doi: 10.1007/978-3-540-74686-7. [DOI] [Google Scholar]
- 29.Efron B, Efron B. SIAM. 1982. The jackknife, the bootstrap and other resampling plans. [Google Scholar]
- 30.Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, Lipp P. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 2000;28:213–23. doi: 10.1054/ceca.2000.0152. [DOI] [PubMed] [Google Scholar]
- 31.Thomas P, Surprenant a, Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990;5:723–33. doi: 10.1016/0896-6273(90)90226-6. http://www.ncbi.nlm.nih.gov/pubmed/2223095. [DOI] [PubMed] [Google Scholar]
- 32.Kurebayashi N, Harkins aB, Baylor SM. Use of fura red as an intracellular calcium indicator in frog skeletal muscle fibers. Biophys J. 1993;64:1934–1960. doi: 10.1016/S0006-3495(93)81564-9. http://dx.doi.org/10.1016/S0006-3495(93)81564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petr MJ, Wurster RD. Determination of in situ dissociation constant for Fura-2 and quantitation of background fluorescence in astrocyte cell line U373-MG. [accessed January 09, 2014];Cell Calcium. 1997 21:233–40. doi: 10.1016/s0143-4160(97)90047-6. http://www.sciencedirect.com/science/article/pii/S0143416097900476. [DOI] [PubMed] [Google Scholar]
- 34.Ljubojević S, Walther S, Asgarzoei M, Sedej S, Pieske B, Kockskämper J. In Situ Calibration of Nucleoplasmic versus Cytoplasmic Ca2+ Concentration in Adult Cardiomyocytes. Biophys J. 2011;100:2356–2366. doi: 10.1016/j.bpj.2011.03.060. http://dx.doi.org/10.1016/j.bpj.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagen BM, Boyman L, Kao JPY, Lederer WJ. A comparative assessment of fluo Ca2+ indicators in rat ventricular myocytes. Cell Calcium. 2012;52:170–81. doi: 10.1016/j.ceca.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harkins AB, Kurebayashi N, Baylor SM. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys J. 1993;65:865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du C, MacGowan Ga, Farkas DL, Koretsky aP. Calibration of the calcium dissociation constant of Rhod(2) in the perfused mouse heart using manganese quenching. Cell Calcium. 2001;29:217–27. doi: 10.1054/ceca.2000.0186. [DOI] [PubMed] [Google Scholar]
- 38.Loughrey CM, MacEachern KE, Cooper J, Smith GL. Measurement of the dissociation constant of Fluo-3 for Ca2+ in isolated rabbit cardiomyocytes using Ca2+ wave characteristics. Cell Calcium. 2003;34:1–9. doi: 10.1016/S0143-4160(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Z, Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. [accessed June 20, 2013];J Physiol. 1993 469:245–273. doi: 10.1113/jphysiol.1993.sp019813. http://jp.physoc.org/content/469/1/245.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neher E. Neuromuscul Junction. Elsevier; Amsterdam: 1989. Combined fura-2 and patch clamp measurements in rat peritoneal mast cells; pp. 65–76. [Google Scholar]
- 41.Bers DM. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. [accessed January 12, 2014];Am J Physiol Cell Physiol. 1982 242:C404–408. doi: 10.1152/ajpcell.1982.242.5.C404. http://ajpcell.physiology.org/content/242/5/C404.full-text.pdf+html. [DOI] [PubMed] [Google Scholar]
- 42.Müller A, Kukley M, Stausberg P, Beck H, Müller W, Dietrich D. Endogenous Ca2+ buffer concentration and Ca2+ microdomains in hippocampal neurons. J Neurosci. 2005;25:558–65. doi: 10.1523/JNEUROSCI.3799-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minta A, Kao JJPY, Tsien R, Chromophores F, Mintaz A, Tsieng RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. [accessed January 09, 2014];J Biol Chem. 1989 264:8171–8178. http://www.jbc.org/content/264/14/8171.short. [PubMed] [Google Scholar]
- 44.Bootman MD, Rietdorf K, Collins T, Walker S, Sanderson M. Ca2+-sensitive fluorescent dyes and intracellular Ca2+ imaging. Cold Spring Harb Protoc. 2013;2013:83–99. doi: 10.1101/pdb.top066050. [DOI] [PubMed] [Google Scholar]
- 45.Velázquez-Marrero C, Ortiz-Miranda S, Marrero HG, Custer EE, Treistman SN, Lemos JR. μ-Opioid Inhibition of Ca2+ Currents and Secretion in Isolated Terminals of the Neurohypophysis Occurs via Ryanodine-Sensitive Ca2+ Stores. J Neurosci. 2014;34:3733–42. doi: 10.1523/JNEUROSCI.2505-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mironova LA, Mironov SL. Approximate analytical time-dependent solutions to describe large-amplitude local calcium transients in the presence of buffers. Biophys J. 2008;94:349–58. doi: 10.1529/biophysj.107.113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burnashev N, Rozov A. Presynaptic Ca2+ dynamics, Ca2+ buffers and synaptic efficacy. Cell Calcium. 2005;37:489–95. doi: 10.1016/j.ceca.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Müller M, Felmy F, Schwaller B, Schneggenburger R. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of held that accelerates the decay of Ca2+ and short-term facilitation. J Neurosci. 2007;27:2261–71. doi: 10.1523/JNEUROSCI.5582-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. [accessed January 09, 2014];Neuron. 2003 38:79–88. doi: 10.1016/s0896-6273(03)00196-x. http://www.sciencedirect.com/science/article/pii/S089662730300196X. [DOI] [PubMed] [Google Scholar]
- 50.Eggermann E, Jonas P. How the “slow” Ca(2+) buffer parvalbumin affects transmitter release in nanodomain-coupling regimes. Nat Neurosci. 2012;15:20–2. doi: 10.1038/nn.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matveev V, Zucker RS, Sherman A. Facilitation through buffer saturation: constraints on endogenous buffering properties. Biophys J. 2004;86:2691–709. doi: 10.1016/S0006-3495(04)74324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohana O, Sakmann B. Transmitter release modulation in nerve terminals of rat neocortical pyramidal cells by intracellular calcium buffers. J Physiol. 1998;513(Pt 1):135–48. doi: 10.1111/j.1469-7793.1998.135by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwaller B. Cytosolic Ca2+ buffers. Cold Spring Harb Perspect Biol. 2010;2:a004051. doi: 10.1101/cshperspect.a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rozov a, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol. 2001;531:807–26. doi: 10.1111/j.1469-7793.2001.0807h.x. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2278500&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vyleta NP, Jonas P. Loose coupling between Ca2+ channels and release sensors at a plastic hippocampal synapse. Science. 2014;343:665–70. doi: 10.1126/science.1244811. [DOI] [PubMed] [Google Scholar]
- 56.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol Med. 2009;15:89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawamoto EM, Vivar C, Camandola S. Physiology and pathology of calcium signaling in the brain. Front Pharmacol. 2012;3:61. doi: 10.3389/fphar.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scharfman HE. Alzheimer’s disease and epilepsy: insight from animal models. Future Neurol. 2012;7:177–192. doi: 10.2217/fnl.12.8.Alzheimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.