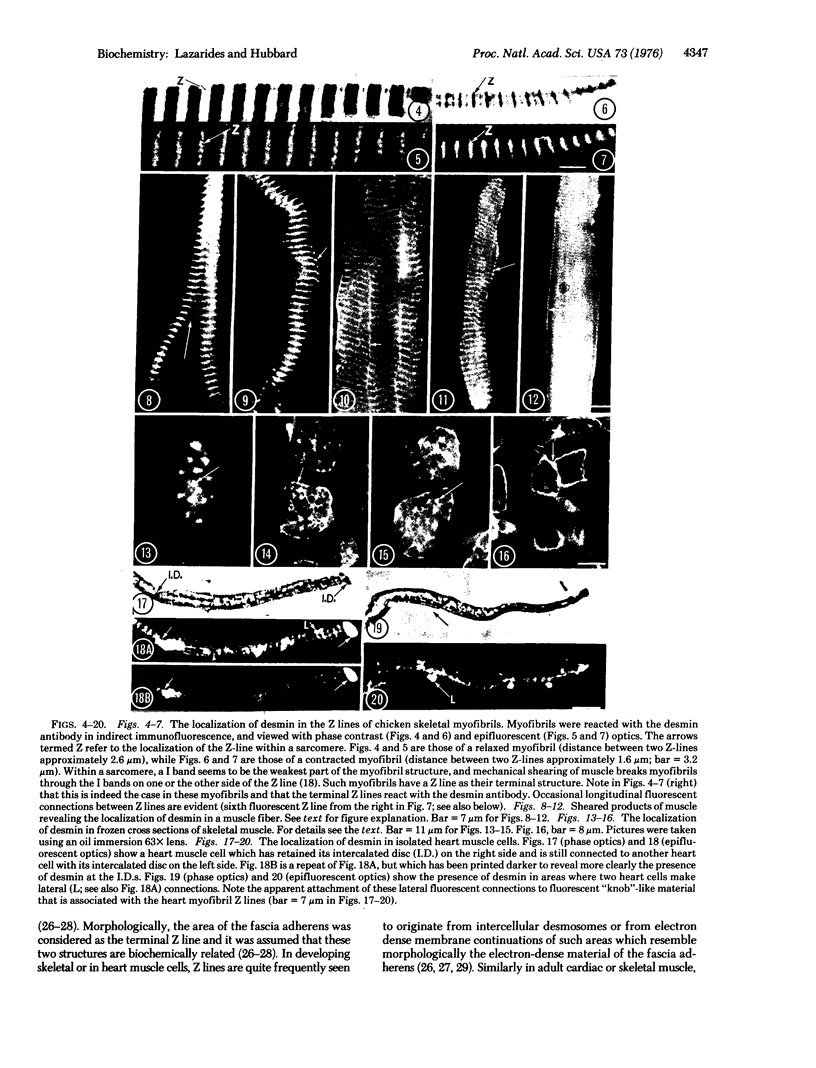
Abstract
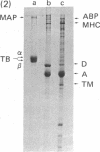
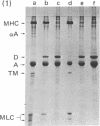
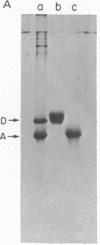
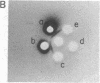
We report the immunological characterization of the subunit of the intermediate sized (100 A) filaments from muscle cells. The protein as isolated from smooth muscle (chicken gizzard) has an apparent molecular weight of 50,000. It is insoluble in buffers that solubilize myosin and the majority of actin, but becomes soluble in the presence of urea. Under a variety of experimental conditions, that include the presence of 8 M urea, this new protein comigrates with actin during purification studies. The two proteins can be separated from each other by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate, and antibodies have been elicited against the 50,000 dalton protein purified by using this technique. These antibodies crossreact with the partially purified protein in urea, but show no detectable cross reaction with actin or myosin. Indirect immunofluorescence reveals that in skeletal muscle this protein is found in close association with the Z lines of the sarcomeres and extends between the Z lines of adjacent myofibrils; it is also associated with filamentous structures that run along the length of a muscle fiber both in close association with the plasma membrane and between myofibrils at the level of their Z lines. In heart muscle, the protein shows the same distribution as in skeletal muscle. In addition, it is found intimately associated with intercalated disks and areas of membrane interaction between laterally associated heart muscle cells. The immunofluorescent localization to the subunit of the 100 A filaments suggests that in muscle cells this molecule may serve to link actin filaments at the level of the Z line (or intercalated disk) with the muscle plasma membrane. We believe that it functions in muscle primarily as a three dimensional matrix which interconnects individual myofibrils to one another and to the plasma membrane at the level of their Z lines. In this manner, this molecule may provide a framework that mechanically integrates all the contractile myofilaments during the contraction and relaxation of muscle. As a means of indicating its linking role in muscle, we have termed the protein desmin (from the Greek delta epsilon sigma mu os = link, bond).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin K. M. The fine structure and electrophysiology of heart muscle cell injury. J Cell Biol. 1970 Sep;46(3):455–476. doi: 10.1083/jcb.46.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A. Our present knowledge of the pathology of dementias. Mod Trends Neurol. 1975;6:1–16. [PubMed] [Google Scholar]
- Brecher S. The occurrence and possible role of 80-100 A filaments in PtKl cells. Exp Cell Res. 1975 Dec;96(2):303–310. doi: 10.1016/0014-4827(75)90261-x. [DOI] [PubMed] [Google Scholar]
- Cooke P. H., Chase R. H. Potassium chloride-insoluble myofilaments in vertebrate smooth muscle cells. Exp Cell Res. 1971 Jun;66(2):417–425. doi: 10.1016/0014-4827(71)90696-3. [DOI] [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol. 1976 Mar;68(3):539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Glial fibrillary acidic protein from normal and gliosed human brain. Demonstration of multiple related polypeptides. Biochim Biophys Acta. 1975 Mar 28;386(1):41–51. doi: 10.1016/0005-2795(75)90244-5. [DOI] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Heterogeneity of the glial fibrillary acidic protein in gliosed human brains. J Neurol Sci. 1974 Dec;23(4):551–563. doi: 10.1016/0022-510x(74)90027-6. [DOI] [PubMed] [Google Scholar]
- Eipper B. A. Properties of rat brain tubulin. J Biol Chem. 1974 Mar 10;249(5):1407–1416. [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W., McNutt N. S. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969 Jul;42(1):1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian M., Spiro D. Derivation of the Z line in the embryonic chick heart. J Cell Biol. 1970 Mar;44(3):683–687. doi: 10.1083/jcb.44.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneeus F. C., Davison P. F. Fibrillar proteins from squid axons. I. Neurofilament protein. J Mol Biol. 1970 Sep 28;52(3):415–428. doi: 10.1016/0022-2836(70)90410-9. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976 Feb;68(2):202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A. R. The effects of divalent cations on the ultrastructure of the perfused rat heart. J Anat. 1967 Apr;101(Pt 2):239–261. [PMC free article] [PubMed] [Google Scholar]
- Rash J. E., Biesele J. J., Gey G. O. Three classes of filaments in cardiac differentiation. J Ultrastruct Res. 1970 Dec;33(5):408–435. doi: 10.1016/s0022-5320(70)90171-1. [DOI] [PubMed] [Google Scholar]
- SZENT-GYORGYI A. G. A new method for the preparation of actin. J Biol Chem. 1951 Sep;192(1):361–369. [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V., Rice R. V. Filament organization in vertebrate smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):223–229. doi: 10.1098/rstb.1973.0027. [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Yamada K. M., Wessells N. K. Microfilaments and cell locomotion. J Cell Biol. 1971 Jun;49(3):595–613. doi: 10.1083/jcb.49.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H. Interactions between actin, myosin, and an actin-binding protein from rabbit alveolar macrophages. Alveolar macrophage myosin Mg-2+-adenosine triphosphatase requires a cofactor for activation by actin. J Biol Chem. 1975 Jul 25;250(14):5706–5712. [PubMed] [Google Scholar]
- Uehara Y., Campbell G. R., Burnstock G. Cytoplasmic filaments in developing and adult vertebrate smooth muscle. J Cell Biol. 1971 Aug;50(2):484–497. doi: 10.1083/jcb.50.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ash J. F., Singer S. J. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Dahl D., Schachner M., Shelanski M. L. Biochemistry of the filaments of brain. Proc Natl Acad Sci U S A. 1976 Feb;73(2):529–533. doi: 10.1073/pnas.73.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]