Abstract
Atypical pupillary light reflexes (PLR) has been observed in children with autism spectrum disorders (ASD), which suggests potential autonomic nervous system (ANS) dysfunction in ASD. ANS is also involved in modulating sensory processing and sensory dysfunction has been widely reported in children with ASD. However, the potential association between physiological measurements of PLR and behavioral observations (e.g. sensory behaviors) has not been examined extensively in literature. In this study, we investigated the potential correlation between PLR and frequently observed sensory behaviors in children with ASD. We found a significant association between PLR constriction amplitude and a set of sensory behaviors in the ASD group but not in typically developing children. Children with ASD who showed more atypical sensory behaviors also had smaller PLR constriction amplitudes. A smaller PLR constriction amplitude suggests lower parasympathetic modulation. This observation implies that some atypical sensory behaviors in children with ASD could be associated with decreased parasympathetic modulation.
Keywords: pupillary light reflex, autism, sensory, autonomic nervous system
1. Introduction
Autism spectrum disorders (ASDs) are complex developmental disorders with symptoms in social functioning, communication, and restricted or repetitive behaviors. Multiple atypical neurological and behavioral measures have been found in ASD. We recently discovered that children with ASD showed significantly different pupillary light reflex (PLR) than typically developing children (Fan, Miles, Takahashi, & Yao, 2009; Daluwatte et al., 2013). PLR measures the dynamic changes in pupil size induced by optical luminance changes. Children with ASD showed multiple atypical PLR parameters including longer latency, less constriction amplitude, and shorter constriction/redilation times. We also found a significant age effect in PLR latency in children with typical development that was not observed in children with ASD (Daluwatte et al., 2013).
The pupil size is controlled by two antagonist iris muscles, the sphincter and dilator, which produce pupil constriction and dilation, respectively. The sphincter is mainly innervated by the parasympathetic nervous system and the dilator innervated by the sympathetic system (Barbur, 2004). The sympathetic tract passes through the ciliary ganglion without synapsing and emerges as the long ciliary nerves entering the eye along the optic nerve. These postganglionic sympathetic nerves travel within the suprachorodial space to innervate the iris dilator muscle (Appenzeller, 1999). The preganglionic parasympathetic nerve branches off to the ciliary ganglion and emerges as several short ciliary nerves which innervate the iris sphincter muscle (Appenzeller, 1999). Due to such underlying control mechanisms from the autonomic nervous system (ANS), PLR provides a simple yet reliable clinical assessment of ANS dysfunction (Barbur, 2004; Bremner, 2009).
In addition to pupillary pathway, ANS dysfunction in cardiovascular system has also been reported in children with ASD (Ming et al., 2011; Ming, Julu, Brimacombe, Connor, & Daniels, 2005; Bal et al., 2010). In fact, ANS in general is involved in a multitude of physiological and behavioral activities beyond the pupillary and cardiovascular controls. For example, ANS is known to play a role in modulating the sensory processing (Saper, 2002; Vallbo, Hagbarth, Torebjork, & Wallin, 1979; Kootz & Cohen, 1981). Human senses can be divided into five traditional groups (vision, auditory, taste, smell, and touch) and various non-traditional senses such as temperature and pain. Sensory system is an essential part of the neurological system that transduces the physical world to our perception. There are four basic patterns in sensory processing: low registration, sensory seeking, sensory sensitivity, and sensation avoiding (Dunn, 1997). Abnormality in sensory processing has been frequently reported in children with ASD (Klintwall et al., 2011; Kientz & Dunn, 1997; Tomchek & Dunn, 2007). For example, children with ASD avoid auditory stimulation by withdrawal while seeking for proprioceptive and vestibular stimulation by repetitive behaviors such as rocking, spinning, or flapping their hands (Case-Smith & Bryan, 1999). While identifying atypical sensory behaviors in ASD, it is also important to understand its association with physiological measures to better understand the causes and effects of such atypical sensory behaviors in ASD.
Due to the widespread implication of ANS dysfunction in ASD, it is valuable to understand whether different ANS measures may be correlated with atypical sensory behaviors observed in ASD. Indeed, Woodard et al. (2012) recently reported an association between sensory processing and heart rate responses to a variety of sensory stimuli in children with ASD. We investigated in this study the potential association between PLR and atypical sensory behaviors observed in children with ASD. We hypothesize that some atypical sensory behaviors observed in children with ASD are associated with PLR parameters because both are regulated by the ANS.
2. Methods
2.1 Participants
Data on frequently observed atypical sensory behaviors in children with ASD were collected from a group of children who participated in a PLR study (Daluwatte et al., 2013). Children with ASD (referred to as the “ASD” group) were recruited from regional autism clinics and their diagnoses were evaluated and confirmed by the pediatrician and/or neuropsychologist. Children with typical development (referred to as the “TD” group) were recruited from the local community. All children in the TD group were screened for autism using the Social Communication Questionnaire Lifetime (Eaves, Wingert, Ho, & Mickelson, 2006) and scored below the clinical cutoff of 15.
The ASD group included 152 children from 5 to 19 years with an average age of 10.7 ± 3.4 years. Among the children with ASD, 86 were diagnosed with autism, 32 with Asperger syndrome and 34 with PDD-NOS. The TD group included 107 children from 6 to 17 years (10.9 ± 2.9 years). There were 135 boys (10.9 ± 3.5 years), 17 girls (9.8 ± 2.6 years) in the ASD group, and 79 boys (11.1 ± 3.1 years), 28 girls (10.6 ± 2.4 years) in the TD group. The ASD group was further divided between those who took some medication (stimulants, atypical antipsychotics, serotonin reuptake inhibitors, antihistamines, antiepileptics etc.) within 48 hours before the PLR test and those with no medication use. In the “w/ med” group, seventy children were exposed to one or more medications; whereas 82 were medication free (the “w/o med” group). No one in the TD group had taken medication. Sensory behavioral data were not available for 4 participants in the ASD group and one participant in the TD group.
This study was approved by the Institutional Review Board of the University of Missouri. All participants and their legal guardians provided written informed assent and consent prior to participating.
2.2 Instrument and procedure
2.2.1 PLR measurement
The binocular pupillography recording system used to measure PLR was described previously in detail (Daluwatte et al., 2013). The system used near-infrared cameras (GC660, Allied Vision Technologies, Stadtroda, Germany) to record pupil images at a speed of 115 frames-per-second (fps). PLR was stimulated using a 100 ms green light flash which was produced using 530 nm green LEDs. PLR was measured at four stimulation intensities in both light-adapted (LA) and dark-adapted (DA) conditions (LA 69.3 cd/m2, LA 872.1 cd/m2, LA 8721.1 cd/m2 and DA 63.1 cd/m2).
As explained in Daluwatte et al., (2013), a total of five PLR parameters were obtained: (1) resting pupil diameter; (2) PLR latency (time from stimulus to onset of constriction); (3) constriction time (time from onset of constriction to reach minimal diameter); (4) redilation time (time for the pupil to recover half of the constriction since minimal pupil diameter); (5) constriction amplitude (maximal relative change in pupil area during constriction). PLR parameters from both eyes during 8 repeated measurements were averaged to obtain the PLR parameters at each stimulus.
2.2.2 Sensory Behaviors
A set of sensory behaviors (29 items) was selected from the Sensory Profile (the “Caregiver Questionnaire” from Pearson Education, Inc., San Antonio, TX) (Dunn, 1994; Dunn, 1999; Kientz & Dunn, 1997). These items were chosen on the basis of being seldom embraced by typical children and because they queried sensory behaviors often reported by parents in their children with ASD. The items we used included auditory, visual, taste/smell, movement, touch, activity level and body position subsets. The details of the sensory items used in this study are available from the National Database for Autism Research (NDAR) website at: https://ndar.nih.gov/ndar_data_dictionary.html?short_name=sensory_profile_199401. This questionnaire was completed by a parent or guardian for each participant. All items were rated on a Likert scale (1=Always, 2=Often, 3=Sometimes, 4=Rarely, and 5=Never). The total sensory score was calculated by summing scores for each item and was used to represent overall sensory abnormality. A lower sensory total score indicates greater atypical sensory behavior.
2.3 Statistical analysis
Reliability of the sensory total score was evaluated by calculating the “Cronbach’s Coefficient of Alpha” and an alpha value greater than 0.7 was considered reliable (Kline, 2000). The Kolmogorov-Smirnov test was used to verify normal distribution for all measured PLR parameters. The Wilcoxon Rank Sum test was used to compare the group differences in sensory total score between the two groups (ASD and TD). A p value <0.05 was considered significant.
To study the association between PLR parameters and sensory scores, linear correlations were first analyzed with Spearman rank correlation (PROC CORR procedure in SAS). The partial least squares (PLS) regression (the “PROC PLS” procedure in SAS) was then performed to select a subset of sensory behaviors (predictor variables) which explains the maximum variance in PLR parameters. Since the dataset consists of continuous response variables (PLR parameters) and ordinal predictor variables (sensory behaviors), the data processing method described by Russolillo and Lauro (2011) was used. A relatively small coefficient (absolute value <0.1) and a small variable importance for projection (VIP) statistic of Wold (<0.8) were selected as the exclusion criteria for predictors. After selecting a subset of sensory items that best predict each PLR parameter, we calculated the “sensory score A” using the total score from these items and the “sensory score B” using the items which were not selected and reevaluated the Spearman rank correlation with the respective PLR parameters. Post-hoc one-way analysis of variance (ANOVA) was performed on PLR parameters while treating each selected subset of items as independent variable. Effect size (f) was calculated as explained by (Cohen, 1992) and 0.1, 0.25 and 0.4 were considered small, medium and large, respectively.
3. Results
The obtained Cronbach’s Coefficient of Alpha was 0.9 for the ASD group and 0.8 for the TD group, suggesting the total sensory score was a reliable measure of sensory behaviors in both groups. The mean and standard deviations of the total sensory score in the ASD and TD groups were 98.5±16.5 and 131.1±8.8, respectively. The ASD group had a significantly lower total sensory score than that of the TD group (p<0.05 in Wilcoxon Rank Sum test), indicating greater atypical sensory behaviors in children with ASD. In the ASD group the sensory score slightly increased with age (Spearman rank correlation coefficient = 0.2 p = 0.004). However the gender, ASD subtype and medication effects did not have a significant effect on the sensory scores in the ASD group (p > 0.05 in Wilcoxon Rank Sum test). In the TD group neither gender nor age showed a significant effect.
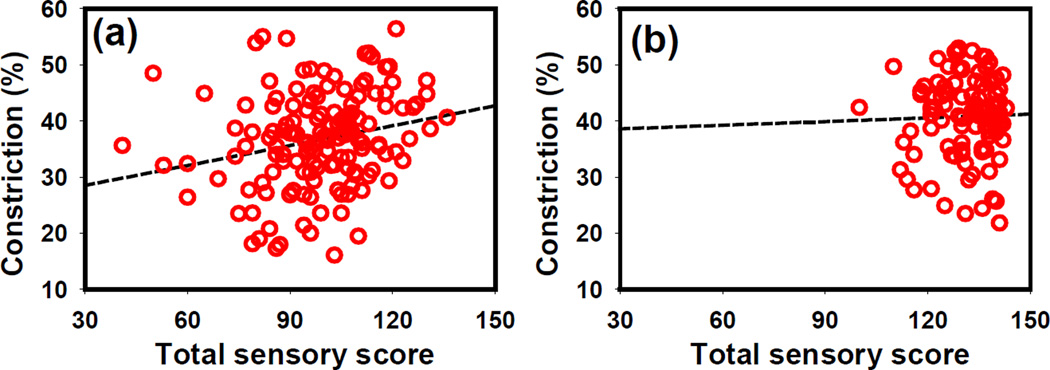
The Spearman rank correlation coefficients between the total sensory score with each PLR parameter at each stimulus are summarized in Table 1. Fig. 1 provides a graphic rendition of the association between constriction amplitude and total sensory scores. PLR constriction amplitude correlated with total sensory score in all light-adapted (LA) tests in ASD group (p < 0.05) (Fig. 1a). This correlation was not observed in typically developing children (p > 0.05) (Fig. 1b). In the TD group, the light-adapted resting pupil size showed a slight negative correlation with total sensory score (p < 0.05) and the redilation time measured in dark adaptation (DA 63.1 cd/m2) was positively correlated with total sensory score (p < 0.05). No other PLR parameters showed a statistically significant correlation with sensory total score in either groups (ASD and TD).
Table 1.
Correlation coefficients (Spearman rank) between total sensory score and PLR parameters at each stimulus in TD and ASD groups. LA: light-adaptation; DA: dark-adaptation.
| Stimulus (cd/m2) | |||||
|---|---|---|---|---|---|
| LA 69.3 | LA 872.1 | LA 8721.1 | DA 63.1 | ||
| ASD | Resting pupil diameter | −0.09 | 0.008 | ||
| Latency | −0.12 | −0.16 | −0.08 | −0.02 | |
| Constriction time | −0.04 | 0.10 | 0.003 | 0.05 | |
| Redilation time | 0.07 | 0.12 | −0.005 | 0.06 | |
| Constriction amplitude | 0.20* | 0.25** | 0.26** | 0.07 | |
| TD | Resting pupil diameter | −0.22* | −0.13 | ||
| Latency | 0.05 | −0.08 | −0.03 | −0.14 | |
| Constriction time | −0.004 | −0.02 | −0.05 | 0.11 | |
| Redilation time | −0.03 | −0.04 | 0.14 | 0.22* | |
| Constriction amplitude | 0.02 | −0.06 | 0.003 | 0.05 | |
p <0.05.
p <0.01.
Fig. 1.
The correlation between PLR constriction amplitude (at LA 8721.1 cd/m2 stimulus intensity) and total sensory score in the (a) ASD and (b) TD groups. The Spearman rank correlation r = 0.26, p < 0.01 in the ASD group; r = 0.003, p > 0.05 in the TD group at LA 8721.1 cd/m2. Lower sensory scores indicate greater atypical sensory behavior.
Since the most consistent correlation was found between constriction amplitude and total sensory score in children with ASD, we further studied the correlation in the subgroups of this population (Table 2). This correlation was statistically significant for children with ASD who were not taking any medications, but not for the group who took medications before the test. Within the “w/o med” ASD group, this correlation was consistently significant for the Autism group, but not in Asperger’s or PDD-NOS groups (Table 2). None of the other PLR parameters demonstrated significant correlation with sensory scores, when divided by medication use or ASD diagnosis.
Table 2.
Correlation coefficients (Spearman rank) between total sensory score and PLR constriction amplitude at each stimulus in ASD sub-groups. LA: light-adaptation; DA: dark-adaptation.
| Number of participants |
Stimulus (cd/m2) | ||||
|---|---|---|---|---|---|
| LA 69.3 | LA 872.1 | LA 8721.1 | DA 63.1 | ||
| Medication | |||||
| Without medication | 82 | 0.22 | 0.30** | 0.33** | 0.14 |
| With medication | 70 | 0.13 | 0.19 | 0.18 | 0.01 |
| Diagnosis (w/o med) | |||||
| Autism | 49 | 0.33* | 0.36* | 0.50** | 0.19 |
| Asperger | 16 | 0.12 | 0.04 | 0.06 | 0.25 |
| PDD-NOS | 17 | 0.16 | 0.27 | 0.17 | 0.03 |
p <0.05.
p <0.01.
As shown in Table 2, PLR constriction amplitude measured at stimulus LA 8721.1 cd/m2 had the most significant correlation (highest r value and smallest p value) with total sensory score. PLS regression was performed in the “w/o med” ASD group to select a subset of sensory behaviors which explains the maximum variance in constriction amplitude at stimulus LA 8721.1 cd/m2. The following sensory behaviors were selected from the PLS regression model: “Avoids getting ‘messy’”, “Avoids going barefoot”, “Hangs on people, furniture or objects”, “Avoids playground equipment or moving toys” and “Expresses distress during grooming”. This subset of sensory items explained 18.6% of the total variance in PLR constriction amplitude. The PLS regression coefficients are reported in Table 3. The importance for projection (VIP) statistic of Wold is higher than 0.8 for each listed sensory item (predictor variable).
Table 3.
Parameter estimates obtained from the PLS regression model and Post-hoc ANOVA analysis for constriction amplitude in the “w/o med” ASD group.
| Sensory Behavior | Coefficient | F | p | f |
|---|---|---|---|---|
| Avoids getting ‘messy’ | 0.13 | 11.15 | 0.001 | 0.13 |
| Avoids going barefoot | 0.11 | 8.09 | 0.006 | 0.10 |
| Hangs on people, furniture or objects | 0.10 | 5.50 | 0.022 | 0.07 |
| Avoids playground equipment or moving toys | 0.11 | 4.35 | 0.040 | 0.06 |
| Expresses distress during grooming | 0.08 | 4.86 | 0.031 | 0.06 |
Post-hoc one-way ANOVA confirmed significant effects for all 5 sensory items on PLR constriction amplitude at stimulus LA 8721.1 cd/m2 in “w/o med” ASD group with the corresponding F and p-values shown in Table 3. The two items “Avoids getting ‘messy’” and “Avoids going barefoot” had a small effect size (Cohen, 1992). The remaining three, although significant, didn’t reach a meaningful effect size.
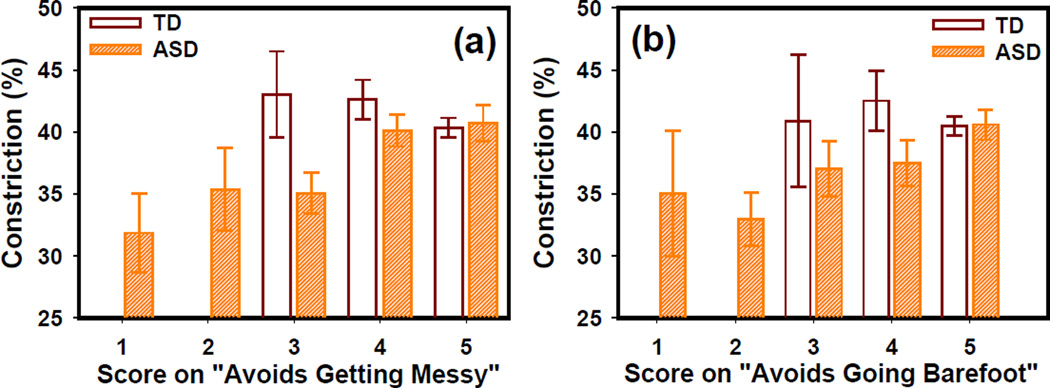
As shown in Fig. 2a and 2b, the constriction amplitude increases with the sensory scores on “Avoids getting ‘messy’” and “Avoids going barefoot” in the “w/o med” ASD group. However, the TD group showed similar PLR constriction at different sensory scores. At a score 5 (i.e. never had the symptom), the constriction amplitude obtained in the “w/o med” ASD group was closer to that obtained in the TD group.
Fig. 2.
PLR constriction amplitude at different scores for the two behavior items: (a) Avoids getting messy and (b) Avoids going barefoot. The error bars indicate the standard error. Score key: 1=Always, 2=Often, 3=Sometimes, 4=Rarely and 5=Never.
The correlation between constriction amplitude and sensory behaviors was re-evaluated by comparing two different sensory scores. The score A was calculated using scores from the five sensory items selected by the PLS regression model (Table 3); whereas the score B was calculated using scores from sensory items not selected by the PLS regression model. The PLR constriction amplitude was correlated with both score A and score B in all LA tests (Table 4) in ASD group. As expected, the correlation with Sensory score A showed greater significance than Sensory score B. No correlation was observed in typically developing children with either scores A or B (Table 4).
Table 4.
Spearman rank correlation “w/o med” for the correlation of PLR constriction amplitude with sensory score A and sensory score B in ASD group. LA: light-adaptation; DA: dark-adaptation.
| Sensory Score Groups |
Stimulus (cd/m2) | ||||
|---|---|---|---|---|---|
| LA 69.3 | LA 872.1 | LA 8721.1 | DA 63.1 | ||
| ASD | Sensory score A | 0.23 | 0.32** | 0.39** | 0.23* |
| Sensory score B | 0.21 | 0.24* | 0.25* | 0.03 | |
| TD | Sensory score A | −0.02 | −0.11 | −0.06 | 0.01 |
| Sensory score B | 0.06 | −0.02 | 0.04 | 0.04 | |
p <0.05.
p <0.01.
Since a significant age effect was observed on sensory score in ASD group, we analyzed the age effect on the correlation between constriction amplitude and sensory score A using a generalized linear model. The age group, score A and interaction between score A and age group were used as factors. The PLR constriction amplitude was used as response variable. The “w/o med” ASD group was divided into three age groups: 5–9 years, 10–13 years and 14–19 years. The interaction between score A and age group was not significant (F2,69 = 1.15 p = 0.3). The age group had no significant effect on PLR constriction amplitude (F2,69 = 0.96 p = 0.4). The score A was significant as a fixed effect (F1,69 = 10.52 p = 0.0018). This suggests that the significant association between sensory score A and PLR constriction amplitude was independent of the age group.
4. Discussion
Our results substantiate that children with ASD commonly have significant sensory dysfunction compared with children of typical development. Such sensory dysfunction may be caused or exacerbated by autonomic nervous system dysfunction. We found that ASD children with more atypical sensory behaviors (i.e. lower sensory scores) also had smaller PLR constriction amplitudes (Fig. 1). The fact that atypical sensory behavior was only associated with the PLR constriction underscores the notation that different PLR parameters are affected by different aspects of the neurological system (Barbur, 2004; Clarke, 2007; Davis, Daluwatte, Colona, & Yao, 2013). Previously, we reported that children with ASD generally have smaller constriction amplitudes (Fan et al., 2009; Daluwatte et al., 2013) suggesting a lower parasympathetic modulation since PLR constriction is predominantly under parasympathetic control (Barbur, 2004; Clarke, 2007; Davis et al., 2013). In post-hoc analysis we saw that children with ASD who reported “never” on the five sensory items (Table 3) had greater PLR constriction amplitude than those who reported “always”. This suggests an association between some atypical sensory behaviors and impaired parasympathetic function.
ANS dysfunction in ASD was initially observed on cardiovascular data (Ming et al., 2005). Several studies reported a higher average heart rate in children with ASD than in the typically developing controls (Daluwatte et al., 2013; Ming et al., 2005; Palkovitz & Wiesenfeld 1980; Kootz & Cohen 1981; Bal et al., 2010). A higher heart rate suggests an increased sympathetic tone or/and a diminished parasympathetic control. Woodard et al., (2012) also reported a strong negative correlation between the Infant/Toddler Sensory Profile (Dunn., 2002), sensory processing domain and heart rate in children with ASD but no correlation in typically developing children.
The lack of correlation in children of typical development suggests that the various components of autonomic nervous system (PLR and cardiovascular) function relatively independently in healthy children. However, in ASD some generalized impairment of parasympathetic modulation may override the normal control thus contributing to certain atypical sensory behaviors. It is also possible the current testing techniques are not sensitive enough to identify similar associations in our control group of typical children. We have reported that heart rate and PLR constriction amplitude are negatively correlated in ASD (Daluwatte et al., 2013), which is expected and important since both are affected by the balance of the parasympathetic and sympathetic controls. All these evidence implied that certain atypical sensory behaviors in children with ASD are linked to lowered parasympathetic modulation. This conclusion was corroborated by a recent report (Schaaf et al., 2010) that children with most severe sensory modulation dysfunction showed significantly lower baseline vagal tone and lower vagal tone during auditory stimuli.
We questioned why constriction amplitude was the only PLR parameter that showed consistent association with sensory behaviors. Both constriction time and constriction amplitude are indicators of parasympathetic modulation (Barbur, 2004) and are correlated (Pearson product moment correlation: r≈−0.5, p < 0.0001 for both ASD and TD groups). However, no association between constriction time and the sensory score was observed. This may suggest that constriction time and amplitude assess different information (Davis et al., 2013). Constriction amplitude measures the amount of response, while constriction time mainly indicates the speed of the response. Since the sensory behavior assessment we used also is an indicator of the degree of sensory responses rather than the speed of response, these results may be functionally consistent. In addition, considering the statistical variance of each PLR parameter, we noted the constriction amplitude has the highest coefficient of variance (0.23–0.64 in ASD group). This may suggest that the constriction amplitude reflects the ANS fluctuations better than other PLR parameters. It is interesting to note that no significant correlation with the constriction amplitude was observed in the dark adaptation condition. This is most likely due to the saturation effect in the PLR response obtained in the dark. Consistent with this supposition, the coefficient of variance of constriction with dark adaptation is smaller than that in light adaptation (0.18 vs 0.23–0.64).
The correlation between PLR constriction and sensory score only occurred for children with ASD who were not on medication prior to testing, and not in ASD children who took medications or in typically developing children. Interestingly, medication did not show a significant effect on either sensory scores or PLR parameters in our study population (Daluwatte et al., 2013). However, it is likely that medication does affect the ANS function in a subtle way that breaks the correlation. It is also interesting to note that the correlation between sensory behavior and PLR constriction was significant in the autism group, but not in Asperger’s group or PDD-NOS group. This observation requires further investigation because of the relatively small number of subjects in the Asperger group and PDD-NOS group.
5. Conclusion
In summary, we studied associations between PLR with atypical sensory behaviors in children with ASD and typical development. For children with ASD the degree of sensory dysfunction significantly correlated with decreased PLR constriction amplitude. Such correlations were not observed in children with typical development. These results suggest that abnormal sensory behavior is associated with ANS dysfunction in ASD. One limitation of this study was the limited number of sensory questions used. Performing the PLS regression with a complete Sensory Profile (Dunn, 2002; Robertson & Simmons, 2013) is expected to better elucidate the association of different sensory behaviors with measures of ANS dysfunction.
Highlights.
Degree of sensory dysfunction is significantly correlated with pupillary light reflex (PLR) constriction amplitude in children with autism spectrum disorders (ASDs) but not in children with typical development.
Children with ASDs who had a smaller PLR constriction amplitude also showed more atypical sensory behaviors.
Acknowledgements
This study was partially supported by National Institute of Neurological Disorders and Stroke (1R21NS070299-01) and Department of Defense Autism Research Program (DoD W81XWH-10-1-0474). Views and opinions of, and endorsements by the authors do not reflect those of the NIH-NINDS or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appenzeller O. The Autonomic Nervous System Part I. Normal Functions. 1st ed. New York: Elsevier; 1999. [Google Scholar]
- Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders. 2010;40:358–370. doi: 10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- Barbur JL. Learning from the pupil : Studies of basic mechanisms and clinical applications. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge: MIT Press; 2004. pp. 641–656. [Google Scholar]
- Bremner F. Pupil evaluation as a test for autonomic disorders. Clinical Autonomic Research. 2009;19:88–101. doi: 10.1007/s10286-009-0515-2. [DOI] [PubMed] [Google Scholar]
- Case-Smith J, Bryan T. The effects of occupational therapy with sensory integration emphasis on preschool-age children with autism. American Journal of Occupational Therapy. 1999;53:489–497. doi: 10.5014/ajot.53.5.489. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin; 1992. [DOI] [PubMed] [Google Scholar]
- Clarke RJ. Shaping the pupil's response to light in the hooded rat. Experimental Brain Research. 2007;176:641–651. doi: 10.1007/s00221-006-0649-6. [DOI] [PubMed] [Google Scholar]
- Daluwatte C, Miles JH, Christ SE, Beversdorf DQ, Takahashi TN, Yao G. A typical pupillary light reflex and heart rate variability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2013;43:1910–1925. doi: 10.1007/s10803-012-1741-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BC, Daluwatte C, Colona NC, Yao G. Effects of cold-pressor and mental arithmetic on pupillary light reflex. Physiological Measurement. 2013;34:873–882. doi: 10.1088/0967-3334/34/8/873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. Performance of Typical Children on the Sensory Profile: An Item Analysis. American Journal of Occupational Therapy. 1994;48:967–974. doi: 10.5014/ajot.48.11.967. [DOI] [PubMed] [Google Scholar]
- Dunn W. The impact of sensory processing abilities on the daily lives of young children and families: A conceptual model. Infants & Young Children. 1997;9(4):23–35. [Google Scholar]
- Dunn W. The Sensory Profile Manual. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- Dunn W. The Infant/Toddler Sensory Profile Manual. San Antonio: The Psychological Corporation; 2002. [Google Scholar]
- Eaves LC, Wingert HD, Ho HH, Mickelson ECR. Screening for autism spectrum disorders with the social communication questionnaire. Journal of Developmental and Behavioral Pediatrics. 2006;27:S95–S103. doi: 10.1097/00004703-200604002-00007. [DOI] [PubMed] [Google Scholar]
- Fan X, Miles JH, Takahashi N, Yao G. Abnormal transient pupillary light reflex in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:1499–1508. doi: 10.1007/s10803-009-0767-7. [DOI] [PubMed] [Google Scholar]
- Kientz MA, Dunn W. A comparison of the performance of children with and without autism on the sensory profile. American Journal of Occupational Therapy. 1997;51:530–537. doi: 10.5014/ajot.51.7.530. [DOI] [PubMed] [Google Scholar]
- Kline P. Handbook of Psychological Testing. 2nd ed. New York: Routledge; 2000. [Google Scholar]
- Klintwall L, Holm A, Eriksson M, Carlsson LH, Olsson MB, Hedvall A, Gillberg C, Fernell E. Sensory abnormalities in autism A brief report. Research in Developmental Disabilities. 2011;32:795–800. doi: 10.1016/j.ridd.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Kootz JP, Cohen DJ. Modulation of sensory intake in autistic children. Cardiovascular and behavioral indices. Journal of the American Academy of Child Psychiatry. 1981;20:692–701. doi: 10.1097/00004583-198102000-00002. [DOI] [PubMed] [Google Scholar]
- Ming X, Bain JM, Smith D, Brimacombe M, Gold Von-Simson G, Axelrod FB. Assessing autonomic dysfunction symptoms in children: A pilot study. Journal of Child Neurology. 2011;26:420–427. doi: 10.1177/0883073810381921. [DOI] [PubMed] [Google Scholar]
- Ming X, Julu POO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain and Development. 2005;27:509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Palkovitz RJ, Wiesenfeld AR. Differential autonomic responses of autistic and normal children. Journal of Autism and Developmental Disorders. 1980;10:347–360. doi: 10.1007/BF02408294. [DOI] [PubMed] [Google Scholar]
- Robertson AE, Simmons DR. The relationship between sensory sensitivity and autistic traits in the general population. Journal of Autism and Developmental Disorders. 2013;43:775–784. doi: 10.1007/s10803-012-1608-7. [DOI] [PubMed] [Google Scholar]
- Russolillo G, Lauro CNA. Proposal for Handling Categorical Predictors in PLS Regression Framework. In: Fichet B, Piccolo D, Verde R, Vichi M, editors. Classification and Multivariate Analysis for Complex Data Structures. New York: Springer; 2011. pp. 343–350. [Google Scholar]
- Saper CB. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Schaaf RC, Benevides T, Blanche EI, Brett-Green BA, Burke JP, Cohn ES, Koomar J, Lane SJ, Miller LJ, May-Benson TA, Parham D, Reynolds S, Schoen SA. Parasympathetic functions in children with sensory processing disorder. Frontiers in Integrative Neuroscience. 2010;4:4–11. doi: 10.3389/fnint.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. American Journal of Occupational Therapy. 2007;61:190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiological Reviews. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Woodard CR, Goodwin MS, Zelazo PR, Aube D, Scrimgeour M, Ostholthoff T, Brickley M. A comparison of autonomic, behavioral, and parent-report measures of sensory sensitivity in young children with autism. Research in Autism Spectrum Disorders. 2012;6:1234–1246. [Google Scholar]