Abstract
Objective
We sought to retrospectively review a single-center experience using intravenous immunoglobulin (IVIG) for the treatment of refractory, active diffuse cutaneous scleroderma.
Methods
The mean modified Rodnan skin score (mRSS) at baseline was compared to the mRSS at 6, 12, 18 and 24 months post-IVIG initiation by the paired t-test. Changes in mRSS at 6 and 12 months were also compared to data from historical controls of 3 large, negative, multicenter, randomized clinical trials of other medications (D-penicillamine (D-pen), Recombinant Human Relaxin (Relaxin), and Oral Bovine Type I Collagen (Collagen)) and to patients treated with mycophenolate mofetil (MMF) alone using the Student’s t-test.
Results
Thirty patients were treated with adjunctive IVIG (2 gm/kg/month) for refractory, active diffuse cutaneous scleroderma. The mean baseline mRSS of our cohort was 29.6±7.2, and this significantly decreased to 24.1±9.6 (N=29; p=0.0011) at 6 months, 22.5±10.0 (N=25; p=0.0001) at 12 months, 20.6±11.8 (N=23; p=0.0001) at 18 months and 15.3±6.4 (N=15; p<0.0001) at 24 months. The mean change in mRSS at 6 months was not significantly different in the IVIG group (−5.3±7.9) compared to the Relaxin trial (−4.8±6.99, p=0.74) or MMF group (−3.4±7.4, p=0.26); however, at 12 months the mean change in mRSS was significantly better in the IVIG group (−8±8.3) than in the D-pen (−2.47±8.6, p=0.005) and Collagen (−3.4±7.12, p=0.005) groups and was comparable to the group of primary MMF responders (−7.1±9, p=0.67).
Conclusion
This observational study suggests that IVIG may be an effective adjunctive therapy for active diffuse cutaneous scleroderma in patients failing other therapies.
KEY INDEXING TERMS: diffuse scleroderma, intravenous immunoglobulins
INTRODUCTION
Systemic sclerosis (SSc, scleroderma) is a rare autoimmune disease characterized pathologically by fibrosis of the skin and internal organs, and a widespread arterial vasculopathy. Patients with active diffuse cutaneous disease often develop significant disability due to progressive skin thickening, intense discomfort from pruritus and burning in the skin, and limitation in joint range of motion due to deep tissue fibrosis causing tendon friction rubs and the development of contractures. To date, there is no proven therapy that consistently and effectively treats active cutaneous disease, although several immunosuppressive agents are thought to have some positive effects (1).
While intravenous immunoglobulin therapy (IVIG) is commonly used to treat other autoimmune diseases (2), its effects on patients with scleroderma remain unclear. In our scleroderma center, we have treated patients with IVIG if they have persistently active diffuse cutaneous disease that is refractory to other immunosuppressive agents. In this study, our primary objective was to retrospectively review our experience using IVIG for the treatment of active diffuse cutaneous scleroderma. We also examined changes in pulmonary function, organ involvement and disability in patients treated with IVIG.
PATIENTS AND METHODS
Subjects were identified from the Johns Hopkins Scleroderma Center database, which has prospectively collected clinical information on consenting scleroderma patients at 6 month intervals since 2000. All subjects included in this study had consented to participate in our IRB-approved database. Clinical and laboratory data from patients previously treated with IVIG were reviewed. Patients who were treated with IVIG for active diffuse cutaneous scleroderma, with or without another treatment indication such as myositis, were included in this study if longitudinal skin score data at pre-specified intervals and IVIG initiation dates were available. Presence of active cutaneous disease was determined by the treating physician’s clinical judgment. Our physician assessment of activity is based on a composite of the following clinical features: skin thickening as assessed by the modified Rodnan skin score (mRSS), edematous skin changes, patient complaints of continued pruritus or discomfort of the skin, worsening flexibility or active tendon friction rubs (TFR). Patients without active cutaneous disease who received IVIG for the sole purpose of treating myositis or other indications were excluded. Our standard regimen for IVIG was 6 monthly cycles dosed at 2 grams/kg/month. Each cycle was administered over a course of three to five days, depending on the patient’s tolerability. To be included, patients must have completed at least three cycles of IVIG infusions within the first six months of planned treatment. If a patient had multiple rounds of treatment, with at least a three-month cessation of treatment between rounds, the first set of treatments was used for analysis.
Detailed clinical information including data on age, gender, race, disease duration, concomitant and prior immunosuppressive medication use, pulmonary function test (PFT) and echocardiography parameters, disease severity scores, autoantibody status, and functional status (e.g. HAQ) were obtained from the clinical research database and medical records review. Patients were classified as having diffuse cutaneous disease if skin thickening was present on the trunk, abdomen, or proximal to the elbows and knees (3). Treatment adverse effects were obtained from careful chart review. Reasons for discontinuing IVIG, either based on the physician’s judgment of disease activity or adverse effects, were recorded.
Our primary outcomes of interest were change in the modified Rodnan skin score (mRSS) at 6 and 12 months compared to baseline, patient perception of disease activity as assessed by the Health Assessment Questionnaire (HAQ), physician assessment, and improvement in tendon friction rubs (TFR). Skin scores were obtained from the clinical research database, and all interval data points not captured in the database were abstracted from medical records where complete skin score data were recorded. Baseline mRSS were defined as the most recent score obtained during or up to three months prior to the month of initial IVIG treatment. Scores at the 6-, 12-, 18-, and 24-month windows were determined by time elapsed from the month of initial IVIG treatment and were restricted to +/− 2 months of the target month. If mRSS data were not available within this window, this time point was considered to have missing data. The patient’s self assessment of disease activity was assessed by the HAQ disability index (DI) and a visual analog scale on the HAQ in response to the following question: “Overall, considering pain, discomfort, limitations in your daily life and other changes in your body and life, how severe would your rate your disease today?” The physician’s assessment of improvement or stabilization was based on the trajectory of the modified Rodnan skin score, tendon friction rubs, flexibility, and cutaneous pruritus or discomfort as recorded in our database and patient visit notes. We also examined whether tendon friction rubs resolved among patients who had TFR at baseline.
Data from historical controls were obtained from the pooled analysis of three large, negative randomized clinical trials of D-penicillamine (D-pen), recombinant human relaxin (Relaxin), and oral bovine type I collagen (Collagen) (4). Because of the negative outcome of these trials, we considered these data to be a close representation of the natural history of disease in scleroderma patients. In addition, the IVIG cohort was compared to patients from our center treated with mycophenolate mofetil (MMF) alone (5). As the primary data were available from the MMF study (5), we were able to compare our IVIG cohort to a group of patients treated with mycophenolate alone, who were typically deemed clinical responders. When interpreting these results, it is important to note that a large proportion of patients in the IVIG cohort were on combination therapy with MMF and IVIG if patients did not have a robust clinical response to MMF alone. Therefore, this analysis, in part, compared a group of MMF non-responders (the IVIG cohort) to a group of MMF responders (MMF alone group). The mean change in mRSS at 6 months was available for the Relaxin and MMF groups, and the mean change in mRSS at 12 months was available for the D-pen, Collagen, and MMF groups. These data were compared to that of the IVIG cohort as detailed below.
Statistical Analysis
Baseline mRSS of the IVIG cohort was compared to the mRSS at 6, 12, 18 and 24 months by the paired Student’s t-test. Based on previously published data, we defined a decrease in mRSS of at least 5 points to be a minimal clinically important difference and indicative of a positive response to IVIG treatment (6). We examined whether baseline clinical characteristics were predictive of this “cutaneous responder” status at 6 and 12 months by simple logistic regression analysis. Potential predictors of cutaneous response that were examined include age at scleroderma onset, race, gender, disease duration at IVIG initiation, number of IVIG treatments, IVIG treatment duration, reasons for discontinuing IVIG, concomitant immunosuppressive therapies, and autoantibody status (topoisomerase-1 and RNA polymerase III). We also examined whether these factors were predictive of the mean difference in mRSS at 6 and 12 months by simple linear regression analysis.
We further examined whether forced vital capacity (FVC), diffusing capacity (DLCO), disability (HAQ-DI and the HAQ patient self assessment of disease activity), and organ involvement (defined by modified Medsger severity scales (7)) differed between baseline and 12 months by the paired t-test for continuous variables and by the Wilcoxon signed rank sum test for ordinal variables. Descriptive data were also tabulated to detail improvements in creatinine kinase (CK) in patients who also had active myositis.
We compared the baseline characteristics of each negative clinical trial and of the MMF group to the IVIG cohort by the Student’s t-test. The change in mRSS at 6 months was compared between the IVIG cohort and the Relaxin and MMF groups using a Student’s t-test. Similar comparisons at the 12-month time point were performed comparing the IVIG cohort to the D-pen, Collagen and MMF groups.
Additional sensitivity analyses that restrict data point windows to tighter timeframes are detailed in the Supplement (see Additional file 1). All statistical analyses were performed using Stata version 13.0 (College Station, TX, USA), and p-values <0.05 were considered to be statistically significant.
RESULTS
Thirty patients were treated for active diffuse cutaneous disease between 2004 and 2012 and met our inclusion criteria (Table 1). Of these, 24 (80%) were female and 26 (86.7%) were white. The mean age at IVIG initiation was 46.6 years (SD 12.3), and the mean disease duration at IVIG initiation was 24.6 months (SD 19.9). The mean baseline mRSS was 29.6 (SD 7.2). Five patients (16.7%) had concomitant myositis. The majority of patients (90%) had attempted other immunomodulatory or anti-fibrotic therapies without significant benefit based on the treating physician’s clinical judgment (Table 1, Figure 1). Three patients initiated therapy with IVIG as part of first line therapy: 1) one individual was treated with IVIG monotherapy in light of a recent malignancy and concern about risk of cancer recurrence with traditional immunosuppressive strategies, 2) another patient initiated IVIG in combination with cyclophosphamide after only a short course of mycophenolate (2 weeks of 3 grams daily) due to rapidly progressive skin disease, and 3) one patient initiated concomitant mycophenolate and IVIG in the context of a myositis overlap.
Table 1.
Baseline characteristics of IVIG cohort with comparison to the Relaxin, D-Pen, Collagen and MMF studies.
| Variable | IVIG (n=30) |
Relaxin (n=231) |
D-Pen (n=134) |
Collagen (n=168) |
MMF (n=87) |
|---|---|---|---|---|---|
| Age at drug initiation, years, mean (SD) | 46.6 (12.3) | 47.3 (10.3) | 43.7 (12.4) | 50.8 (12.2) | 49.6 (11.2) |
| Duration from 1st non-Raynaud’s scleroderma symptom to drug initiation, months, mean (SD) | 24.6 (19.9) | 26.4 (16.4) | 9.5 (4.1)* | 41.8 (31.9)* | 24.4 (30.5) |
| Female gender, % | 80.0 | 85.2 | 77.6 | 79.2 | 83.9 |
| Race, % | |||||
| White | 86.7 | 74.0 | 67.9* | 76.2 | 78.0 |
| Black | 3.3 | 13.3 | 19.4* | 16.1 | 17.1 |
| Other | 10.0 | 12.8 | 12.7 | 7.7 | 4.9 |
| Modified Rodnan skin score, mean (SD) | 29.6 (7.2) | 27.3 (6.9) | 21.0* (8.0) | 26.1* (7.8) | 24.5 (9.5)* |
| Health Assessment Questionnaire DisabilityIndex, mean (SD), N=28 | 1.29 (0.70) | 1.09 (0.65) | |||
| Concomitant myositis, no. (%) | 5 (16.7) | 2 (2.3)* | |||
| Tendon friction rubs, no. (%) | 10 (33.3) | ||||
| Baseline CPK, mean (SD), N=18 | 265.8 (440.6) | ||||
| Renal crisis, no. (%) | 0 (0) | ||||
| Pulmonary function (% predicted), mean (SD) | |||||
| Forced vital capacity (FVC), N=29 | 83.1 (19.2) | ||||
| Diffusing capacity (DLCO), N=28 | 76.8 (20.2) | ||||
| Right ventricular systolic pressure (mmHg), mean (SD), N=19 | 32.1 (6.5) | ||||
| Medications used prior to IVIG initiation, no. (%) | |||||
| Prednisone | 19 (63.3) | ||||
| Methotrexate | 8 (26.7) | ||||
| Azathioprine | 3 (10.0) | ||||
| Cyclophosphamide | 8 (26.7) | ||||
| D-penicillamine | 3 (10.0) | ||||
| Hydroxychloroquine | 8 (26.7) | ||||
| Minocycline | 1 (3.3) | ||||
| Colchicine | 1 (3.3) | ||||
| TNF-inhibitor | 2 (6.7) | ||||
| Mycophenolate mofetil | 25 (83.3) | ||||
| Leflunomide | 1 (3.3) | ||||
| Imatinib | 2 (6.67) | ||||
| Rapamycin | 1 (3.3) | ||||
| Other** | 4 (13.3) | ||||
| Autoantibody status, no. (%) | |||||
| Anti-nuclear antibody (ANA) | 29 (96.7) | ||||
| Anti-centromere | 0 (0) | ||||
| Anti-topoisomerase 1 (Scl70) | 5 (16.7) | ||||
| Anti-RNA polymerase III, N=23 | 14 (60.9) | ||||
| Anti-ribonucleoprotein (RNP), N=29 | 2 (6.9) |
p<0.05 for comparison with IVIG cohort
One patient was treated with gold for RA, one patient was treated with alefacept in a clinical trial, and two patients were treated with chemotherapeutic agents (cetuximab and docetaxel, respectively).
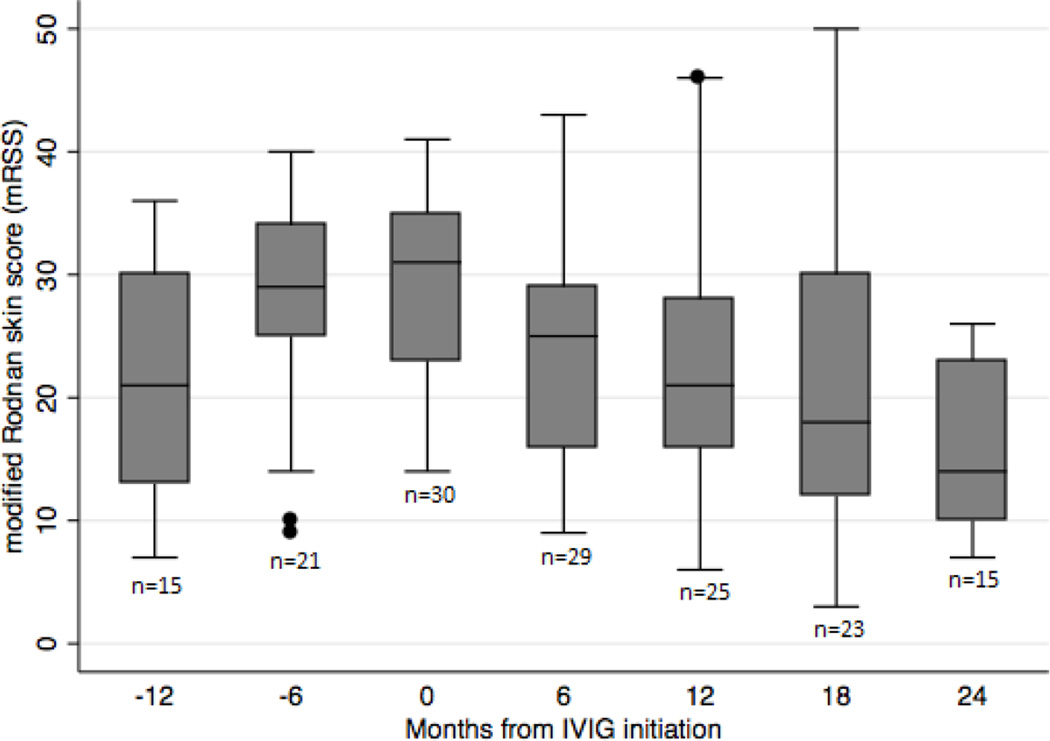
Figure 1.
Trend in mean mRSS over time before and after IVIG initiation.
Patients received IVIG as part of routine clinical care, dosed at 2 gm/kg/month (Table 2). A typical round of treatment consisted of 6 monthly cycles of therapy. On average, patients completed 8.5 cycles of IVIG therapy (SD 5.3, range 3– 24). Concomitant immunomodulatory or anti-fibrotic therapies included mycophenolate mofetil (70%), prednisone (33.3%), cyclophosphamide (20%), methotrexate (10%), imatinib (3.3%) and hydroxychloroquine (3.3%).
Table 2.
Treatment details
| Variable | N=30 |
|---|---|
| Mean treatment length (months), mean (SD) | 9.4 (6.9) |
| Total number of IVIG cycles, mean (SD) | 8.5 (5.3) |
| Concomitant Medications*, no. (%) | |
| Prednisone | 10 (33.3) |
| Methotrexate | 3 (10.0) |
| Cyclophosphamide | 6 (20.0) |
| Hydroxychloroquine | 1 (3.3) |
| Mycophenolate mofetil | 21 (70.0) |
| Imatinib | 1 (3.3) |
| Side effects, no. (%) | |
| Headache (note 1 with aseptic meningitis) | 12 (40.0) |
| Fatigue | 7 (23.3) |
| Nausea and/or emesis | 4 (13.3) |
| Fever | 3 (10.0) |
| Arthralgias and/or myalgias | 3 (10.0) |
| Rash and/or pruritis | 3 (10.0) |
| Generalized weakness | 3 (10.0) |
| Hypertension | 2 (6.7) |
| Chest discomfort | 2 (6.7) |
| Leukopenia | 2 (6.7) |
| Transient ischemic attack | 1 (3.3) |
| Dyspnea | 1 (3.3) |
| Diarrhea | 1 (3.3) |
| Decreased appetite | 1 (3.3) |
| Palpitations | 1 (3.3) |
| Fluid retention | 1 (3.3) |
| Lightheadedness | 1 (3.3) |
| Aura/flashing lights | 1 (3.3) |
| Increased Raynaud’s activity | 1 (3.3) |
| Acute kidney injury | 1 (3.3) |
| Hyperkalemia | 1 (3.3) |
| Hypokalemia | 1 (3.3) |
| Transaminitis | 1 (3.3) |
| Reason IVIG discontinued*** | |
| Improvement** | 18 (60.0) |
| Stable** | 4 (13.3) |
| Insurance denial | 2 (6.7) |
| Progressing** | 1 (3.3) |
| Aseptic Meningitis | 1 (3.3) |
| Transient ischemic attack | 1 (3.3) |
| Patient moved out of the country | 1 (3.3) |
| Death | 1 (3.3) |
No patients were on concomitant therapy with azathioprine, D-penicillamine, minocycline, colchicine, leflunomide or TNF-inhibitors.
These were defined by the treating physician’s clinical judgment.
One patient was continuing to receive IVIG at the time of this study.
Examination of changes in cutaneous disease activity and predictors of clinical response in the IVIG cohort
The mean mRSS progressively decreased from a baseline score of 29.6 (SD 7.2) to 24.1 (SD 9.6, N=29, p=0.0011) at 6 months, 22.5 (SD 10.0, N=25, p=0.0001) at 12 months, 20.6 (SD 11.8, N=23, p=0.0001) at 18 months, and 15.3 (SD 6.4, N=15, p<0.0001) at 24 months (Figure 1). Comparison of mRSS values at baseline and at 6 and 12 months showed 16 of 29 patients with available data were cutaneous responders (i.e. mRSS declined by at least 5 points) at 6 months, and 15 of 25 patients with available data were cutaneous responders at 12 months. We also examined whether age at scleroderma onset, race, gender, disease duration, number of IVIG treatments, IVIG treatment duration, reasons for stopping IVIG, concomitant immunosuppressive therapies, and autoantibody status were associated with cutaneous responder status. Concomitant prednisone use had a borderline negative association with cutaneous responder status at 12 months (OR 0.17; 95% CI 0.028, 0.997; p=0.05), but not at 6 months. Simple logistic regression analysis demonstrated that none of the other variables examined were predictors of cutaneous responder status at either time point. Changes in mRSS at 6 and 12 months were also examined as continuous variables, and there were no associations found with any of the examined covariates.
Disability, as assessed by the HAQ-DI, decreased, but the change was not statistically significantly different between baseline (mean 1.29, SD 0.70) and month 12 (mean 1.21, SD 0.75; p=0.436). However, patients’ self-assessment of disease activity improved significantly from baseline (mean 1.63, SD 0.72) to 12 months (mean 1.07, SD 0.76; p=0.001). Based on the treating physician’s assessment of disease activity, 60% of patients had clinically improved, and 13.3% had stabilized. Ten patients in our cohort had tendon friction rubs (TFR) before initiating IVIG. Six months after initiating IVIG, seven of these patients had resolution of TFR, two patients continued to have TFR, and in one patient follow up data at 6 months were not available.
Examination of changes in pulmonary function and organ involvement in the IVIG cohort
Pulmonary function remained stable from baseline to twelve months. The mean FVC at baseline and 12 months were 83.0% predicted (SD 19.6) and 83.8% predicted (SD 16.8; N=28; p=0.678), respectively. The mean DLCO at baseline and 12 months were 76.3% predicted (SD 21.0) and 79.9% predicted (SD 23.1; N=25; p=0.331), respectively. There were no statistically significant differences in Raynaud’s (p=0.527), general (p=0.711), cardiac (p=1.0), pulmonary (p=0.905), or gastrointestinal (p=0.182) severity scores from baseline to 12 months. Renal severity scores remained unchanged for all patients from baseline to 12 months. There was, however, a borderline improvement in muscle severity (p=0.054) from baseline to 12 months. Among the 5 patients with concomitant myositis, serial CK data were available in 4 patients, all of whom demonstrated normalization of CK over time (data not shown).
Treatment adverse effects and reasons for discontinuation of therapy
Headache (40%), fatigue (23.3%), nausea with or without emesis (13.3%), fever (10%), arthralgias and/or myalgias (10%), rash and/or pruritus (10%), and generalized weakness (10%) were the most common adverse effects reported (Table 2). One patient developed aseptic meningitis and ultimately discontinued IVIG due to severe side effects, one patient discontinued therapy due to a transient ischemic attack, and one patient developed an acute renal injury 15 months after discontinuing IVIG due to improvement. As detailed above, over 70% of patients discontinued IVIG based on improvement or stabilization as assessed by the treating physician. Two patients could not continue therapy due to insurance coverage issues. One patient died of sudden cardiac arrest from a pre-existing cardiomyopathy 16 months after initiating IVIG.
Comparison of changes in mRSS to data from the Relaxin study at 6 months
There were no differences in baseline characteristics between the IVIG cohort and the Relaxin group (Table 1). The mean baseline mRSS of our IVIG cohort (29.6, SD 7.2) was not statistically significantly different from that of the Relaxin group (mean 27.3, SD 6.9, p=0.09). At 6 months, the mean change in mRSS was not significantly different between the IVIG (mean change −5.3, SD 7.9) and Relaxin (mean change −4.8, SD 6.99) groups (p=0.74).
Comparison of changes in mRSS to data from the D-pen and collagen studies at 12 months
The IVIG cohort had a significantly longer disease duration at drug initiation (mean 24.6 months, SD 19.9) relative to the D-pen subjects (mean 9.5 months, SD 4.1; p<0.0001) and a shorter disease duration compared to the Collagen population (mean 41.8 months, SD 31.9; p=0.005). While the IVIG cohort had a racial profile comparable to the Collagen group, the IVIG cohort had more whites and fewer blacks than the D-pen study (Table 1). There was no difference in age or gender profile between the IVIG cohort and either study population. The mean baseline mRSS of our IVIG cohort (mean 29.6, SD 7.2) was significantly greater than that of the D-pen (mean 21, SD 8; p<0.0001) and Collagen (mean 26.1, SD 7.8; p=0.023) trial groups. At 12 months, the mean change in mRSS was statistically significantly better in the IVIG treated cohort (mean change −8, SD 8.3) than the D-pen (−2.47, SD 8.6; p=0.005) and Collagen (−3.4, SD 7.12; p=0.005) groups.
Comparison of changes in mRSS to data from the MMF study at 6 and 12 months
There were no differences in baseline age, disease duration, gender, race, or disability between the IVIG and MMF groups. There were more patients with a concomitant myositis in the IVIG cohort than in the MMF group (p=0.004), and the mean baseline mRSS was statistically significantly higher in the IVIG cohort (mean 29.6, SD 7.2) than in the MMF cohort (mean 24.5, SD 9.5; p=0.009; Table 1). It is important to note that IVIG was typically used in patients who had not responded to other therapies including a significant proportion of patients deemed MMF failures, and therefore these patients were considered to have more aggressive skin disease. At 6 months, there was a comparable improvement in the mean change in mRSS in the IVIG (mean −5.3, SD 7.9) and MMF (mean −3.4, SD 7.4; p=0.256) cohorts. Similarly at 12 months, the two groups had similar improvement in mRSS (IVIG mean change −8, SD 8.3; MMF mean change −7.1, SD 9; p=0.674).
DISCUSSION
In this study, we sought to report our experience using IVIG in patients with severe, and often refractory, active diffuse cutaneous scleroderma. We present one of the largest series describing the use of IVIG for this indication. Given the active and progressive nature of their skin disease, patients often were treated with IVIG in combination with other immunomodulatory or anti-fibrotic therapies. A significant improvement was noted in skin thickening over time, and comparison to historical controls from negative clinical trials with 12 month follow-up data suggests that this improvement may be greater at 12 months than what would be expected by the natural history of the disease. In addition, the IVIG-treated cohort had a clinical improvement in mRSS that was similar to a group of MMF-treated patients who were typically deemed clinical responders, suggesting that addition of IVIG may stabilize previously refractory disease. Over 50% of the IVIG-treated patients had a decline in mRSS of at least 5 points at the 6 and 12 month time intervals, and prior data suggest that these are clinically significant improvements (6). While statistically significant improvements in HAQ-DI (which measures both activity and damage) were not observed, patients’ assessments of disease severity significantly improved over time. In addition, 7 of the 10 patients with TFR at baseline had resolution of their TFR. Patients also remained stable with respect to pulmonary function and organ involvement while receiving IVIG treatment. We found IVIG to be well-tolerated, and the main reason (73.3% of patients) for discontinuing therapy was clinical improvement or stability as deemed by the treating physician. These data provide supportive evidence that IVIG may be beneficial to treat diffuse cutaneous disease in scleroderma, and a prospective, double blind, randomized, placebo-controlled clinical trial is currently underway to further assess the efficacy of IVIG in scleroderma (NCT01785056).
We acknowledge that a majority of the patients in our cohort were treated with MMF in conjunction with IVIG often because they had failed therapy with MMF or other immunosuppressive agents. A comparison between our refractory cohort (the IVIG group) and a group of patients treated with MMF alone demonstrated comparable improvements in mRSS. We had expected that comparing our IVIG group who had refractory disease to a group of primary MMF responders would bias our findings to the null. Therefore, the finding that the IVIG cohort patients had an equivalent improvement in mRSS as the primary MMF responders suggests that IVIG, when used as an add-on therapy, may improve the responsiveness to treatment in patients with severe disease who are otherwise unresponsive to MMF alone.
Our study is not the first to anticipate the role of IVIG in treating fibrosis of the skin. Some previously published reports suggest anecdotal benefit of IVIG therapy in individual patients (8–10), and other studies report improved mean skin scores in small populations ranging from 3–15 patients (11–15). Only two placebo-controlled studies have been conducted to date. Kudo, H. et al. (16) have reported the results of a 6-patient clinical trial of IVIG in scleroderma patients showing changes in cytokine levels with no report of clinical outcomes. Another study by Takehara, K. et al. (17) reports the use of IVIG in a randomized, double-blind, placebo-controlled trial of 63 diffuse SSc patients. In this trial, participants were administered a single cycle of IVIG or placebo infusions. At 12 weeks, there were no differences in change in mRSS between the two groups. Participants whose skin score did not improve by at least 5 points at 12 weeks were given an additional cycle of IVIG. Those who received 2 IVIG treatments had greater improvements in skin score over time than those who initially received placebo followed by a later IVIG infusion. These data suggest that repeated IVIG cycles may influence long-term cutaneous outcomes. Our study, which evaluated patients receiving several monthly cycles of IVIG as part of routine clinical care, supports this idea.
Given the retrospective nature of our study, the lack of a placebo control, and the frequent use of concomitant immunosuppressive therapies, we cannot definitively attribute the improvement in skin thickening over time to the use of IVIG alone. To address these concerns, we compared the change in mRSS in our cohort to data from three large, negative randomized clinical trials to assess whether the improvements we detected were better than those expected from the natural history of the disease. It is important to note that our cohort had a few differences in baseline characteristics compared to these negative trials, particularly with respect to more severe skin disease at baseline and an intermediate disease duration at drug initiation compared to the D-pen and Collagen studies. We recognize that patients with higher baseline skin scores in clinical trials tend to improve, whereas patients with less severe cutaneous disease tend to worsen (18). The Relaxin trial participants were the most similar to our IVIG cohort at baseline. We did not see differences between our IVIG cohort and the Relaxin study at 6 months, and unfortunately 12 month outcome data were not available for comparison.
In conclusion, these data suggest that IVIG may be a beneficial adjunctive therapy for active diffuse cutaneous scleroderma patients who are unresponsive to more traditional immunosuppressive treatments, but further studies are necessary to affirm these findings.
Supplementary Material
Acknowledgments
Grants and support: This study was supported by the Scleroderma Research Foundation, the Cathi Keilty Memorial Fund for Scleroderma Research, and NIH/NIAMS K23 AR061439 to AAS.
Footnotes
Contributing authors:
C L Poelman, BA;L K Hummers, MD ScM, Associate Professor of Medicine;F M Wigley, MD, Professor of Medicine;C Anderson, MS; F Boin, MD, Assistant Professor of Medicine; A A Shah, MD MHS, Assistant Professor of Medicine
REFERENCES
- 1.Frech TM, Shanmugam VK, Shah AA, Assassi S, Gordon JK, Hant FN, et al. Treatment of early diffuse systemic sclerosis skin disease. Clin Exp Rheumatol. 2013;31(Suppl 76):166–171. [PMC free article] [PubMed] [Google Scholar]
- 2.Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 2012;367:2015–2025. doi: 10.1056/NEJMra1009433. [DOI] [PubMed] [Google Scholar]
- 3.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 4.Amjadi S, Maranian P, Furst DE, Clements PJ, Wong WK, Postlethwaite AE, et al. Course of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: analysis of three large multicenter, double-blind, randomized controlled trials. Arthritis Rheum. 2009;60:2490–2498. doi: 10.1002/art.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le EN, Wigley FM, Shah AA, Boin F, Hummers LK. Long-term experience of mycophenolate mofetil for treatment of diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2011;70:1104–1107. doi: 10.1136/ard.2010.142000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanna D, Furst DE, Hays RD, Park GS, Wong WK, Seibold JR, et al. Minimally important difference in diffuse systemic sclerosis: results from the D-penicillamine study. Ann Rheum Dis. 2006;65:1325–1329. doi: 10.1136/ard.2005.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26:2159–2167. [PubMed] [Google Scholar]
- 8.Asano Y, Ihn H, Asashima N, Yazawa N, Mimura Y, Jinnin M, et al. A case of diffuse scleroderma successfully treated with high-dose intravenous immune globulin infusion. Rheumatology (Oxford) 2005;44:824–826. doi: 10.1093/rheumatology/keh600. [DOI] [PubMed] [Google Scholar]
- 9.Bodemer C, Teillac D, Le Bourgeois M, Wechsler B, de Prost Y. Efficacy of intravenous immunoglobulins in sclerodermatomyositis. Br J Rheumatol. 1990;123:545–546. doi: 10.1111/j.1365-2133.1990.tb01462.x. [DOI] [PubMed] [Google Scholar]
- 10.Szekanecz Z, Aleksza M, Antal-Szalmas P, Soltesz P, Veres K, Szanto S, et al. Combined plasmapheresis and high-dose intravenous immunoglobulin treatment in systemic sclerosis for 12 months: follow-up of immunopathological and clinical effects. Clin Rheumatol. 2009;28:347–350. doi: 10.1007/s10067-008-1062-2. [DOI] [PubMed] [Google Scholar]
- 11.Amital H, Rewald E, Levy Y, Bar-Dayan Y, Manthorpe R, Engervall P, et al. Fibrosis regression induced by intravenous gammaglobulin treatment. Ann Rheum Dis. 2003;62:175–177. doi: 10.1136/ard.62.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ihn H, Mimura Y, Yazawa N, Jinnin M, Asano Y, Yamane K, et al. High-dose intravenous immunoglobulin infusion as treatment for diffuse scleroderma. Br J Rheumatol. 2007;156:1058–1060. doi: 10.1111/j.1365-2133.2007.07777.x. [DOI] [PubMed] [Google Scholar]
- 13.Levy Y, Amital H, Langevitz P, Nacci F, Righi A, Conforti L, et al. Intravenous immunoglobulin modulates cutaneous involvement and reduces skin fibrosis in systemic sclerosis: an open-label study. Arthritis Rheum. 2004;50:1005–1007. doi: 10.1002/art.20195. [DOI] [PubMed] [Google Scholar]
- 14.Levy Y, Sherer Y, Langevitz P, Lorber M, Rotman P, Fabrizzi F, et al. Skin score decrease in systemic sclerosis patients treated with intravenous immunoglobulin--a preliminary report. Clin Rheumatol. 2000;19:207–211. doi: 10.1007/s100670050158. [DOI] [PubMed] [Google Scholar]
- 15.Nacci F, Righi A, Conforti ML, Miniati I, Fiori G, Martinovic D, et al. Intravenous immunoglobulins improve the function and ameliorate joint involvement in systemic sclerosis: a pilot study. Ann Rheum Dis. 2007;66:977–979. doi: 10.1136/ard.2006.060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo H, Jinnin M, Yamane K, Makino T, Kajihara I, Makino K, et al. Intravenous immunoglobulin treatment recovers the down-regulated levels of Th1 cytokines in the sera and skin of scleroderma patients. J Dermatol Sci. 2013;69:77–80. doi: 10.1016/j.jdermsci.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Takehara K, Ihn H, Sato S. A randomized, double-blind, placebo-controlled trial: intravenous immunoglobulin treatment in patients with diffuse cutaneous systemic sclerosis. Clin Exp Rheumatol. 2013;31(Suppl 76):151–156. [PubMed] [Google Scholar]
- 18.Merkel PA, Silliman NP, Clements PJ, Denton CP, Furst DE, Mayes MD, et al. Patterns and predictors of change in outcome measures in clinical trials in scleroderma: an individual patient meta-analysis of 629 subjects with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2012;64:3420–3429. doi: 10.1002/art.34427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.