Abstract
Multidimensional mass spectrometry techniques, combining matrix-assisted laser desorption/ionization (MALDI) or electrospray ionization (ESI) with tandem mass spectrometry (MS2), multistage mass spectrometry (MSn) or ion mobility mass spectrometry (IM-MS), have been employed to gain precise structural insight on the compositions, sequences and architectures of small oligomers of a hyperbranched glycopolymer, prepared by atom transfer radical copolymerization of an acrylate monomer (A) and an acrylate inimer (B), both carrying mannose ester pendants. The MS data confirmed the incorporation of multiple inimer repeat units, which ultimately lead to the hyperbranched material. The various possible structures of n-mers with the same composition were subsequently elucidated based on MS2 and MSn studies. The characteristic elimination of bromomethane molecule provided definitive information about the comonomer connectivity in the copolymeric AB2 trimer and A2B2 tetramer, identifying as present only one of the three possible trimeric isomers (viz. sequence BBA) and only two of the six possible tetrameric isomers (viz. sequences BBA2 and BABA). Complementary IM-MS studies confirmed that only one of the tetrameric structures is formed. Comparison of the experimentally determined collision cross-section of the detected isomer with those predicted by molecular simulations for the two possible sequences ascertained BBA2 as the predominant tetrameric architecture. The multidimensional MS approaches presented provide connectivity information at the atomic level without requiring high product purity (due to the dispersive nature of MS) and, hence, should be particularly useful for the microstructure characterization of novel glycopolymers and other types of complex copolymers.
Introduction
Carbohydrates participate in numerous biological functions in living systems, which are generally initiated by molecular recognition events proceeding through specific, noncovalent carbohydrate-protein interactions.1–5 Saccharides that bind weakly to protein receptors may not provide sufficient control for processes mediated in vivo by protein-carbohydrate binding. This problem has spurred the development of synthetic carbohydrates and carbohydrate-based polymers (“glycopolymers”) that interact more strongly with proteins due to their multiple sugar residues.3,4 Interest in such polymers has further increased after several studies revealed that glycopolymer-protein interactions are very similar with those between proteins and natural (poly)saccharides and suitable for a diverse array of pharmaceutical and medical applications, including drug delivery, diagnosis, immune system modulation and tissue engineering.3,6–11 Glycopolymers are also employed as superabsorbents, contact lens materials and gene delivery systems due to their biocompatibility and biodegradability.12–16 The term “glycopolymer” describes both synthetic carbohydrates as well as carbohydrate-bearing polymers in which sugar moieties are attached as pendants to a conventional synthetic polymer chain.8
The chemical properties of a glycopolymer and its suitability for a specific biomedical application depend on the spatial arrangement of its sugar units.4 Preparing and identifying specific structures is therefore essential for glycopolymers intended for medical use. Glycopolymers can exist in different architectures, including branched, dendritic and comb-shaped structures.8 As with natural carbohydrates,17 isomeric sequences of the individual repeat units are possible, posing significant challenges in deducing the correct connectivity (primary structure) and overall shape of a glycopolymer.
The progress achieved in glycotechnology over the last decades has been accompanied by a comparable development of analytical methods for the characterization of natural and synthetic carbohydrates. Among them, mass spectrometry (MS) has become an established and widely used technique for the analysis of carbohydrates, providing molecular structure information with high sensitivity and convenient sample preparation.17–24 In particular, tandem mass spectrometry (MS2) and multistage mass spectrometry (MSn) via collisionally activated dissociation (CAD) have proven to be a powerful means for the determination of the primary structure of carbohydrates.20,22–25 These methods have found similar practicality in analyses of synthetic polymer connectivity.26–39 More recently, ion mobility mass spectrometry (IM-MS)40–43 has shown promise in offering unique insight into both carbohydrate branching and stereochemistry22,44,45 as well as into synthetic polymer assembly and architecture.39,46–55 The present investigation evaluates for the first time the utility of combining MS2, MSn and IM-MS to determine the sequence (primary structure) and architecture (branching type) of a glycopolymer. The sample examined was a copolymer synthesized from an acrylate monomer (A) and an acrylate inimer (B), both containing a β-D-mannopyranoside substituent in the ester moiety (Fig. 1);56 these units can copolymerize to yield linear chains with all mannose groups as pendants or branched chains with some mannose groups as pendants and some within the chain at the branching points, as shown in Fig. 1.
Fig. 1.
Monomer and inimer used for the synthesis of the glycopolymer studied (top); the inimer includes both an initiating moiety and a polymerizable group. The monomer and inimer can copolymerize to yield linear and branched chain segments (bottom).
Experimental
Materials
The glycopolymer studied was prepared via atom-transfer radical polymerization (ATRP) of a 2:1 molar mixture of acetyl protected acryloyl-β-D-mannopyranoside (structure A in Fig. 1) and acetyl protected acryloyl-1-(2-bromo)-methyl acrylic-β-D-mannopyranoside (structure B in Fig. 1), as has been reported in the literature.56 Gel permeation chromatography (GPC) analysis, using poly(methyl methacrylate) standards for mass scale calibration, indicated an average molecular weight (Mn) of 6080 Da and a polydispersity index (Mw/Mn) of 1.86.56
Matrix-assisted laser desorption/ionization (MALDI) experiments
MALDI-MS and MS2 experiments were carried out on a Bruker UltraFlex III tandem time-of-flight (ToF/ToF) mass spectrometer (Bruker Daltonics, Billerica, MA), equipped with a Nd:YAG laser emitting at a wavelength of 355 nm; t-2-[3-(4-t-butyl-phenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) was used as matrix and sodium trifluoroacetate (NaTFA) as cationizing agent. Solutions of the matrix (20 mg/mL), polymer (20 mg/mL) and cationizing salt (10 mg/mL) were prepared in CHCl3 and mixed in the ratio 10:5:1, respectively. Approximately 0.5 µL of the final mixture was spotted on a 384-well ground-steel MALDI plate and allowed to air-dry before insertion of the plate into the vacuum system. MS2 experiments were performed using Bruker’s LIFT mode with no additional collision gas.57 Data analysis was conducted with the flex Analysis software.
Electrospray ionization (ESI) experiments
Stock solutions of the polymer and NaTFA were prepared in CHCl3 and MeOH, respectively (both at 10 mg/mL). The samples sprayed were prepared by mixing 10 µL of the polymer solution with 1 µL of the salt solution and adding 750 µL CHCl3 and 250 µL MeOH to obtain a final polymer concentration of 0.10 mg/mL in 3:1 (v/v) CHCl3/MeOH.
MSn
The final polymer solution was injected into a Bruker HCTultra II quadrupole ion trap (QIT) mass spectrometer (Bruker Daltonics, Billerica, MA)58 by direct infusion with a syringe pump at a flow rate of 180 µL/h. The temperature and flow rate of the drying gas (N2) were 300 °C and 8 L/min, respectively; the pressure of the nebulizing gas (N2) was set at 10 psi. MS2 spectra were acquired by isolating the appropriate precursor ion and accelerating it with an RF field in order to induce collisionally activated dissociation (CAD) with the He bath gas in the QIT; for MSn spectra (n = 3–4), the isolation and activation procedure were repeated with a specific fragment in the MS2 and MS3 spectra, respectively.
IM-MS
The glycopolymer architecture was examined by ion mobility mass spectrometry (IM-MS) on a Waters Synapt HDMS quadrupole/time-of-flight (Q/ToF) mass spectrometer (Waters, Milford, MA), equipped with the traveling wave version of IM-MS.59 Instrument parameters were adjusted as follows: ESI capillary voltage, 3.5 kV; sample cone voltage, 30 V; extraction cone voltage, 3.2 V; desolvation gas flow, 550 L/h (N2); trap collision energy (CE), 6.0 eV; transfer CE, 4.0 eV; trap gas flow, 1.5 mL/min (Ar); IM gas flow, 22.7 mL/min (N2); sample flow rate, 10 µL/min; source temperature, 90 °C; desolvation temperature, 180 °C; IM traveling wave velocity, 350 m/s; and IM traveling wave height, 10.5 V.
Collision cross-section determination
The collision cross-section (CCS) of oligomers with the composition A2B2 was deduced from the corresponding drift time, measured by IM-MS, after calibration of the drift time scale with ions of known CCS as reported previously.60–62 Polyalanine, cytochrome c and insulin chain A ions served as calibrants.63 The calibration curve was constructed by plotting the corrected CCSs of the calibrant ions against their corrected drift times measured by IM-MS at the same instrument settings used for the glycopolymer (see Electronic Supplementary Information).
Molecular modeling
Geometry optimization of glycopolymer structures with the composition A2B2 was performed by molecular mechanics/dynamics calculations, using the Materials Studio software (version 4.2). With each architecture, 150 candidate structures were generated and their theoretical collision cross-sections were calculated by the projection approximation method available in the MOBCAL software program.64
RESULTS AND DISCUSSION
MALDI-MS analysis
The MALDI mass spectrum of the acetyl protected glycopolymer is shown in Fig. 2. It shows four distributions with the composition [AnBm + Na]+ (m = 1–4). The mass difference between two consecutive peaks within each distribution is 402 Da, matching the mass of one A unit (monomer). Because each B unit (inimer) contributes one Br atom, oligomers with the composition AnBm should contain m Br atoms; this is confirmed by the mass-to-charge ratios and isotope patterns of the corresponding oligomer peaks, as exemplified in Fig. 2 for A3B2 (m/z 2277.44). Similarly, the other ions detected in the MALDI mass spectrum include all Br atoms provided by their inimer content.
Fig. 2.
MALDI mass spectrum of the glycopolymer studied. Four [M + Na]+ ion distributions of AnBm oligomers, m = 1–4, are clearly discerned. Each oligomer observed includes m Br atoms. The insets show the structures of A and B (with their pendants shadowed by different colors) and the measured and calculated isotope pattern of one oligomer (A3B2), which corroborates the detection of brominated acrylates upon MALDI. Monoisotopic m/z ratios are given on top of the peaks. R = CH3CO.
A higher number of inimer units in the glycopolymer lead to higher molecular weight. This is reflected by the average molecular weights (Mn) of the four AnBm distributions observed in the MALDI mass spectrum, which increase from 1878 Da for AnB1 to 2609 Da for AnB2, 3411 Da for AnB3 and 4134 Da for AnB4. Such a trend is consistent with the formation of simultaneously growing branches after inimer incorporation.
Tandem mass spectrometry analysis
AnB1 oligomers
Linear as well as branched architectures are possible for AnB>1. Conversely, the major distribution, AnB1, can only have a linear structure with the inimer and its acryloyl group at the initiating chain end and a Br atom at the terminating chain end. The MSn characteristics of oligomers A1B1, A2B1 and A3B1 were examined first, using QIT multistage mass spectrometry, in order to evaluate the fragmentation pathways of linear chain segments.
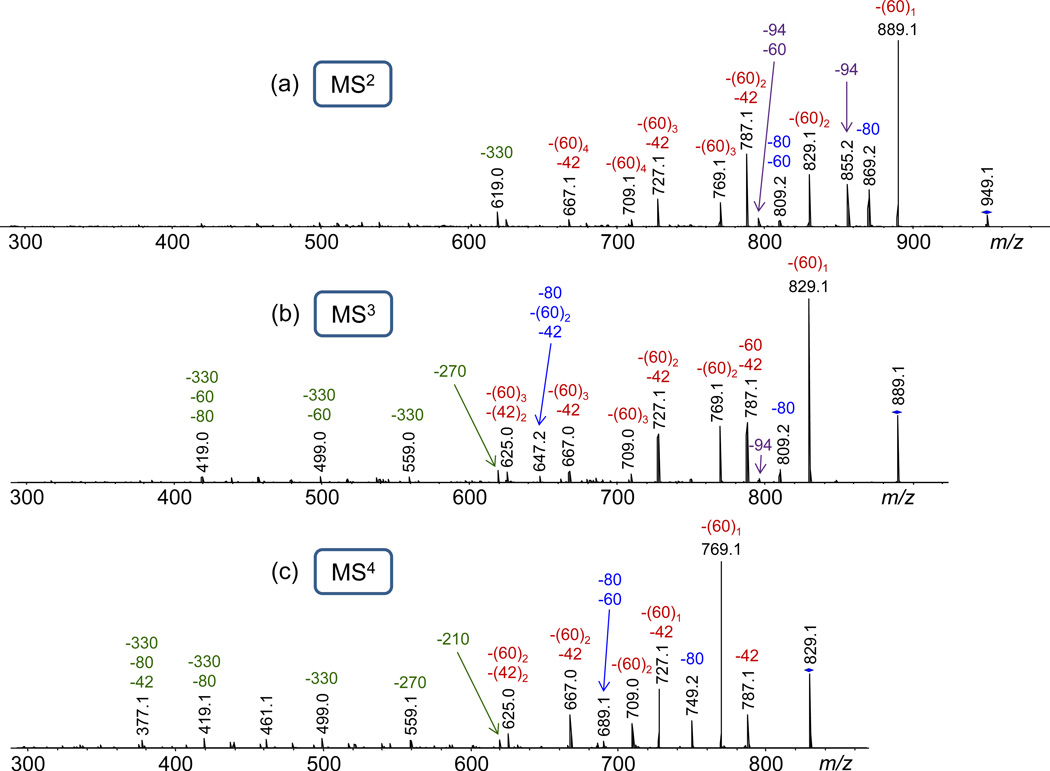
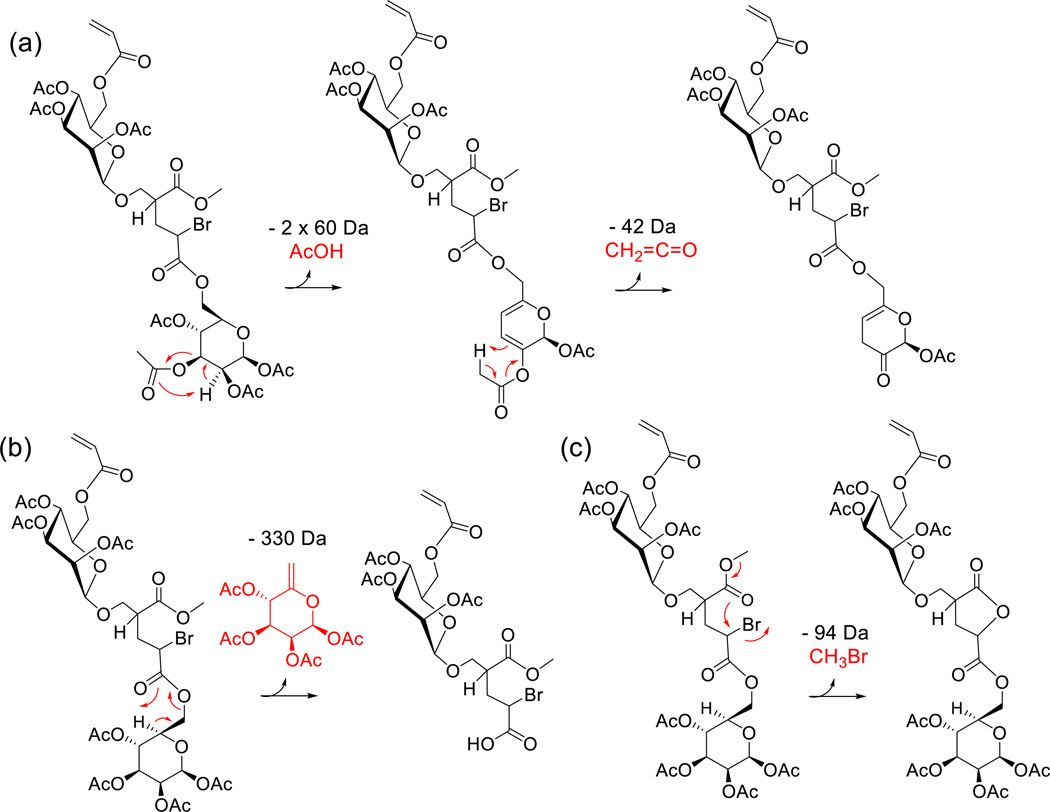
Collisionally activated A1B1 mainly undergoes consecutive and competitive decompositions via 1,5-H rearrangement at either the acetyl protecting groups or the glycopolymer backbone ester groups, giving rise to neutral losses of acetic acid (AcOH, 60 Da) and ketene (CH2CO, 42 Da), or the fully protected mannose pendant with an exocyclic double bond (330 Da), respectively (cf. Fig. 3a and Scheme 1a–b). Up to two AcOH molecules can be eliminated by this mechanism from each mannose unit of A1B1, producing a double bond within the sugar ring after each AcOH loss. After elimination of two AcOH molecules from the same mannose ring, a remaining acetyl group in the same ring can be cleaved in the form of ketene (42 Da) by 1,5-H rearrangement to one of the newly created double bonds, cf. Scheme 1. The successive elimination of two acetic acid and one ketene moieties (i.e. the reaction sequence m/z 949.1 → 889.1 → 829.1 → 787.1) is supported by the MS3 and MS4 characteristics of the CAD fragments generated after the first (m/z 889.1) and second AcOH loss (m/z 829.1), cf. Figs. 3b–3c.
Fig. 3.
(a) ESI-MS2 (CAD) mass spectrum of sodiated A1B1 (m/z 949.1); (b) MS3 (CAD) mass spectrum of m/z 889.1, formed by acetic acid (AcOH) loss from sodiated A1B1; (c) MS4 (CAD) mass spectrum of m/z 829.1, formed by AcOH loss from m/z 889.1. The numbers on top of the peaks give the monoisotopic m/z ratio (in black) and the mass of the neutral loss(es) in Da (in color); −60 and −42 indicate losses of acetic acid and ketene, respectively; −80 and −94 indicate losses of HBr and CH3Br, respectively; −330, −270 and −210 indicate losses of a mannose pendant or a mannose pendant that lost one or two AcOH molecules, respectively. The subscripts indicate the number of losses.
Scheme 1.
Charge-remote 1,5-H rearrangements in sodiated A1B1, leading to (a) AcOH and CH2CO losses from the mannose ring and (b) expulsion of the sugar pendant from monomer unit A; the Na+ has been omitted for brevity. Note that AcOH and CH2CO losses can occur at the mannose group of A (as shown) as well as the mannose group of B. (c) Intramolecular nucleophilic displacement of Br by the adjacent ester group to form a five-membered ring lactone with concomitant elimination of CH3Br. The latter reaction is possible only at a terminal unit (A or B) attached at the branching site of an inimer unit B (see text).
Two additional, major fragmentation pathways of sodiated A1B1 are the elimination of HBr (80 Da) and CH3Br (94 Da), both of which are accompanied by consecutive AcOH losses (Fig. 3a). The loss of HBr (m/z 869.2) takes place within the acrylate frame and most likely produces a conjugated double bond. On the other hand, the loss of CH3Br (m/z 855.2) is accounted for by an intramolecular nucleophilic displacement leading to the formation of a favorable, five-membered ring lactone and the expulsion of methyl bromide (cf. Scheme 1c).
The fragmentation pathway proposed for the formation of m/z 855.2 (Scheme 1c) is supported by the MALDI-MS2 mass spectrum of A1B1 acquired using MALDI-ToF/ToF instrumentation (see Experimental), with which the entire isotope cluster of the precursor ion is mass-selected for MS2 analysis.36 In this spectrum (Fig. S1), m/z 855.2 does not show the characteristic isotope pattern of Br, confirming that Br is lost with the neutral fragment. Similarly, the presence or absence of Br content in the other MS2 fragments is readily established from the corresponding isotope patterns (cf. Fig. S1).
It is noteworthy that backbone cleavages within the acrylate connectivity are not observed, in analogy to sodiated poly(butyl acrylate)s, which also dissociate mainly by 1,5-H rearrangements at the acrylate pendants and in sharp contrast to sodiated poly(methyl acrylate), which dissociates through homolytic backbone C–C bond cleavages.65 This reactivity difference must result from the lower energy requirements for dissociations via 1,5-H rearrangement (“McLafferty-type” rearrangement) as compared to dissociations proceeding through homolytic bond cleavages in the polymer chain.35,65
A2B1 and A3B1 oligomers
These larger glycopolymer n-mers were only examined by MALDI-MS2 (Fig. 4). The fragmentation pathways of sodiated A2B1 and A3B1 are fairly similar with those of A1B1. Both primarily dissociate by charge remote 1,5-H rearrangements, giving rise to consecutive and competitive losses of 60 Da, 42 Da and 330 Da from the protected sugar pendants, cf. Fig. 4. The elimination of hydrogen bromide is also observed from both A2B1 and A3B1. The peaks in the low mass range of the MS2 spectra originate largely from the sugar pendant losses; plausible structures of the most prominent fragments are provided in Fig. S2.
Fig. 4.
MALDI-MS2 mass spectra of sodiated (a) A2B1 (m/z 1351.3) and (b) A3B1 (m/z 1753.4); the numbers on top of the peaks give the monoisotopic m/z ratio (in black) and the mass of the neutral loss(es) in Da (in color). The insets show the structures of A2B1 and A3B1.
As with A1B1, neither A2B1 nor A3B1 forms any noticeable fragments from cleavages within the polyacrylate backbone. In contrast to A1B1, however, the larger n-mers do not produce a neutral CH3Br (94 Da) fragment via intramolecular nucleophilic displacement, cf. Fig. 4 vs. S1. Consequently, the latter reaction can compete with the 1,5-H rearrangements and HBr loss only if the Br atom is in γ position to the propionate carbonyl group of the inimer and CH3Br elimination leads to the formation of an entropically favored five-membered ring lactone, as is possible for A1B1 (cf. Scheme 1), but not for A>1B1 where the reacting sites are further apart from each other and hindered by rotational restrictions, due to the presence of bulky sugar pendants, in approaching each other for lactone formation.66 Observation of CH3Br loss is, thus, specific to glycopolymer in which a B (inimer) unit is connected through its initiating site to a terminal A or B unit and can be used to probe the sequence and type of branching in smaller oligomers, such as A1B2 and A2B2 (vide infra).
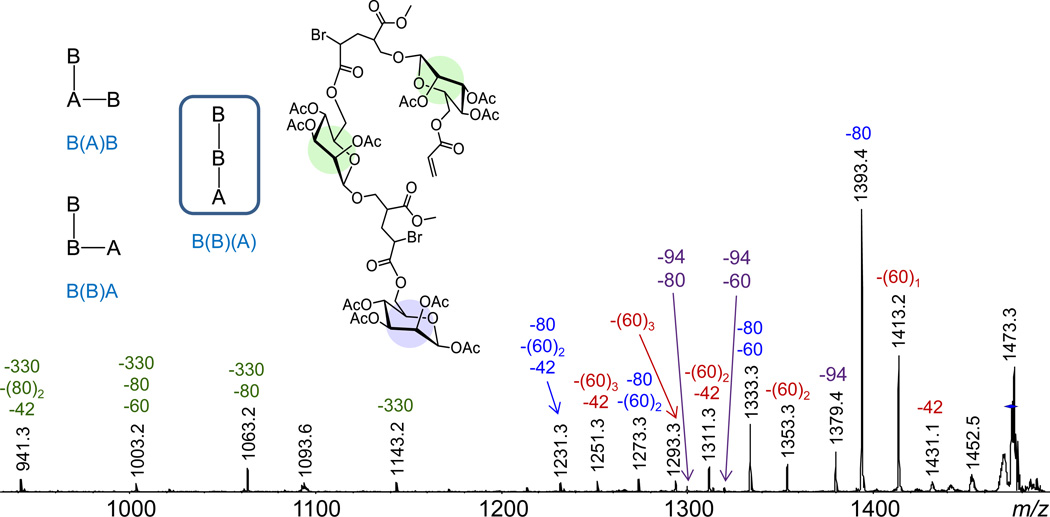
A1B2 oligomers
Three isomers with distinct sequences and/or architectures exist for glycopolymer n-mers with the composition A1B2. These are shown in Fig. 5 in schematic representation using the symbols A and B for the monomer and inimer repeat units, respectively. Horizontal bonds denote repeat unit attachment through the acrylate vinyl group, whereas vertical bonds indicate repeat unit attachment to the initiator site (branching site) of the inimer. All isomers start with a B unit and a vertical bond (because only the inimer can initiate the polymerization), but differ in the sequence and arrangement of the other two repeat units. In the text, monomer or inimer units attached to a vertical bond (i.e. repeat units connected to the initiating site of the inimer) are shown in parentheses to avoid ambiguity (cf. Fig. 5).
Fig. 5.
ESI-MS2 (CAD) mass spectra of sodiated A1B2 (m/z 1473.3); the numbers on top of the peaks give the monoisotopic m/z ratio (in black) and the mass of the neutral loss(es) in Da (in color). The inset shows schematic representations of the three isomers possible with the A1B2 stoichiometry and the actual structure of the A1B2 architecture from which CH3Br can be eliminated to form a five-membered ring lactone.
The ESI-MS2 mass spectrum of sodiated A1B2 (Fig. 5) includes abundant fragments from competitive and sequential eliminations of hydrogen bromide (80 Da), acetic acid (60 Da), ketene (42 Da) and unbranched sugar pendant (330 Da), as observed for the other n-mer stoichiometries (Fig. 4) and which affirm the glycopolymer composition but do not provide connectivity information. The loss of methyl bromide (94 Da) and consecutive losses of methyl bromide and acetic acid or hydrogen bromide are, however, also observed, indicating that the major product with A1B2 stoichiometry must contain a terminal unit attached to the branching point of the inimer B (i.e. a terminal unit connected vertically to a penultimate B). Only one of the three possible A1B2 structures, viz. B(B)(A), satisfies this requirement and is, thus, identified as the main A1B2 product.
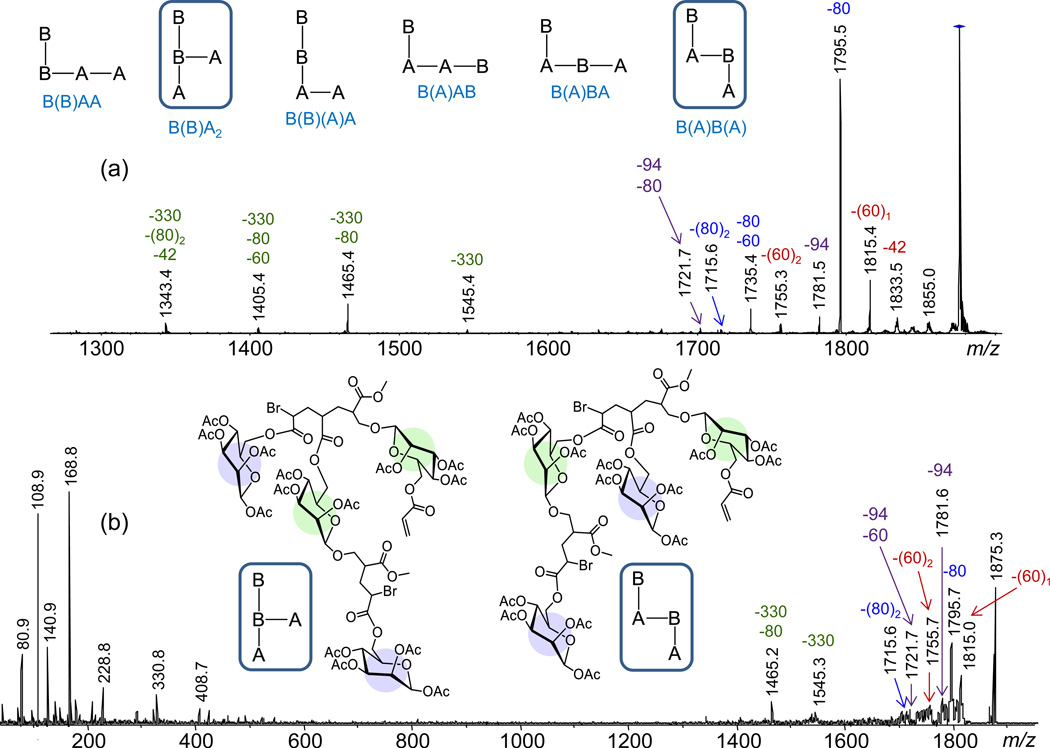
A2B2 oligomers
The number of distinct isomers for the glycopolymer composition A2B2 increases to six. These are shown in Fig. 6a in schematic representation using the symbols A and B and vertical bonds or parentheses to designate chain extension through the inimer branching point (vide supra).
Fig. 6.
(a) ESI-MS2 and (b) MALDI-MS2 mass spectra of sodiated A2B2 (m/z 1875.3); the numbers on top of the peaks give the monoisotopic m/z ratio (in black) and the mass of the neutral loss(es) in Da (in color). The insets show (a) the isomers possible with the A2B2 stoichiometry and (b) the A2B2 architectures from which CH3Br can be eliminated to form a five-membered ring lactone. The low-mass fragments are not detected in the ESI-MS2 spectrum due to the low-mass cutoff in CAD experiments with QIT instrumentation.58
Under both ESI-MS2 (Fig. 6a) as well as MALDI-MS2 (Fig. 6b) conditions, sodiated A2B2 loses CH3Br (94 Da) as well as one or more molecules of acetic acid (60 Da), ketene (42 Da) and unbranched sugar pendant (330 Da). The methyl bromide loss again reveals that this n-mer must include a terminal unit that is connected to the branching point (initiating site) of an inimer unit. The two sequences shown in Fig. 6b (their schematic acronyms are encased) possess this distinguishing structural feature, which establishes them as the prime A2B2 candidates. Confirmatory proof for this conclusion and further differentiation between the two possible architectures was sought by IM-MS (vide infra).
The oligomers found to lose CH3Br, viz. dimer BA, trimer B(B)(A) and tetramers B(B)A2 or B(A)B(A), carry one terminal B(A) unit; consequently, this elimination can only occur at one site. On the other hand, the trimer and tetramers contain two sites where HBr loss can take place, while all four n-mers have several acetyl groups from which AcOH loss(es) can proceed. These differences and the rising number of competitive and sequential dissociations that are possible with increasing oligomer size reconcile the decrease in intensity of the CH3Br loss in the ESI-MS2 spectra, relative to the total fragment ion current, when the number of comonomer repeat units increases from two (Fig. 3a) to three (Fig. 5) to four (Fig. 6a). Nonetheless, this structurally diagnostic fragment is clearly discernible and appears with an significant intensity in all cases (>6% of most abundant fragment). Interestingly, the peak intensity ratio of CH3Br loss versus the first AcOH loss is fairly constant among the oligomers undergoing both reactions: 0.23 for BA, 0.29 for B(B)(A) and 0.30 for B(B)A2/B(A)B(A); this similarity is attributed to the comparable mechanisms of these fragmentations, both of which involve charge-remote rearrangements through cyclic transition states (cf. Scheme 1).
Ion mobility mass spectrometry analysis
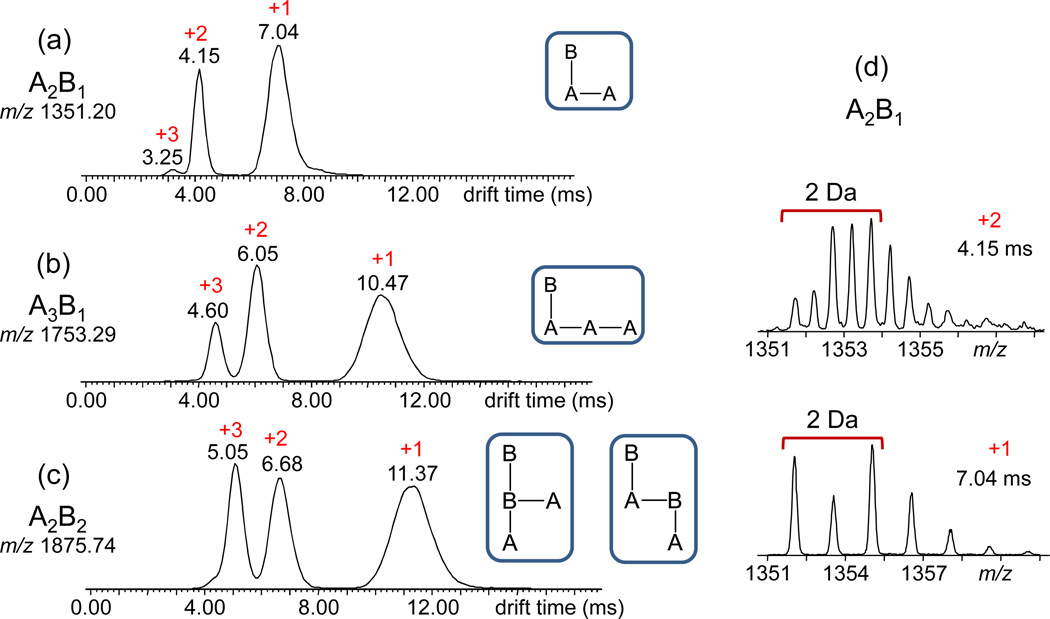
The [M + Na]+ ions of A2B1 (m/z 1351) and A3B1 (m/z 1753), which can only have one architecture (cf. Fig. 4), were investigated first. Separation of their constituents by their ion mobilities gave rise to the IM-MS drift time distributions depicted in Figs. 7a–b, which show the presence of three components in each ion beam, arising from superimposed charge states of different oligomers (cf. Fig. 7). The isotope patterns of the signals at 7.04, 4.15 and 3.25 ms in Fig. 7a reveal the compositions [A2B1 + Na]+, [A4B2 + 2Na]2+ and [A6B3 + 3Na]3+, respectively; similarly, the three signals at 10.47, 6.05 and 4.60 ms in Fig. 7b are identified by their isotope patterns as [A3B1 + Na]+, [A6B2 + 2Na]2+ and [A9B3 + 3Na]3+, respectively. Although only one sequence is possible for the singly charged components, as mentioned above, the higher charge states can exist in different types of branching depending on the sequence of A and B repeat units. The observation of single peaks for the higher charge states strongly suggests, however, that not all possible sequences are formed and/or that larger, differently branched isomers may be very similar in overall shape (architecture) to be dispersed under our IM-MS conditions. Unfortunately, the absolute ion intensities of the mobility dispersed signals were too low to study their fragmentation characteristics by IM-MS2 experiments.
Fig. 7.
ESI-IM-MS drift time distributions of sodiated (a) A2B1 (m/z 1351.3), (b) A3B1 (m/z 1753.4) and (c) A2B2 (m/z 1875.3); at each m/z value, three peaks are observed, corresponding to ions with +1 to +3 sodium charges. (d) Charge states were determined from the isotope patterns in the mass spectra extracted from each IM-separated peak, as exemplified for the +2 and +1 peaks of A2B1.
IM separation of the [M + Na]+ ion from A2B2 (m/z 1875) also leads to three signals, peaking at 11.37, 6.68 and 5.05 ms (cf. Fig. 7c). The corresponding isotope patterns reveal the compositions [A2B2 + Na]+, [A4B4 + 2Na]2+ and [A6B6 + 3Na]3+, respectively. Only a single peak is detected for the singly charged A2B2. Hence, this stoichiometry results in the formation of mainly one sequence/architecture, or both structures consistent with the MS2 data coexist but are too similar in shape/size for differentiation by IM-MS. To resolve this issue, the collision cross-section (CCS) of sodiated A2B2 was derived from the drift time of this ion in the IM cell (11.37 ms, Fig. 7c) and compared with theoretical predictions of the CCSs of the two possible candidates, B(B)A2 and B(A)B(A).
The ion mobility and, hence, drift time of an ion through the IM region depends on its charge and CCS which is a measure of the ion’s size (mass) and shape (architecture).40–42 With the traveling wave variant of IM-MS utilized in our study, drift times cannot be converted to collision cross-sections through a mathematical equation but require calibration of the drift time scale with standards of known CCS.60,61 A calibration curve was constructed using peptide and protein ions (Fig. S3),63 which renders a CCS of 327 Å2 for A2B2 (± <4%).
Molecular modeling was employed to calculate the CCS of B(B)A2 and B(A)B(A). For each isomer, 150 structures were energy-minimized by annealing simulations and their collision cross-sections were calculated by the projection approximation (PA) method. The PA method ignores scattering losses and interactions between the ion and the bath gas in the IM cell, which are included in the more rigorous trajectory method, but nevertheless has been demonstrated to generate CCSs in good agreement with experimental values for relatively small (<2000 Da), quasi spherical structures, such as the ones considered in this study (cf. Fig. 6b).67–69 The resulting CCS vs. energy plots (Fig. S4) reveal that all 150 optimized structures of each isomer are grouped closely together in one architectural family. The average calculated collision cross-section of the 150 structures is 338 Å2 for B(B)A2 and 352 Å2 for B(A)B(A). CCS differences of this magnitude can be distinguished with the IM-MS instrumentation used in this study, as was recently shown for ortho-, meta- and para-substituted polyhedral oligomeric silsesquioxane.54
It is worth noting that the drift time distributions of [A2B2 + Na]+ and [A3B + Na]+, which have comparable masses, are very similar (cf. +1 peaks in Figs. 7b and 7c). Since [A3B + Na]+ only has one sequence (vide supra), this similarity strongly suggests that [A2B2 + Na]+ too comprises mainly one sequence; the presence of a significant amount of a second architecture would have resulted in a distorted peak shape with a recognizable shoulder.51,54
The experimentally deduced collision cross-section of sodiated A2B2, 327 (±13) Å2, agrees reasonably well (within ~3%) with the calculated CCS of the branched architecture B(B)A2, 338 Å2, but is substantially different (by ~8%) from the CCS of the isomeric architecture B(A)B(A), 352 Å2. Thus, the combined MS2 and IM-MS data provide evidence that the most probable tetrameric sequence of the glycopolymer is the branched structure B(B)A2.
CONCLUSIONS
MALDI mass spectrometry confirmed the successful copolymerization of the monomer (A) and inimer (B) used to prepare a hyperbranched polyacrylate-based glycopolymer with mannose substituents in the acrylate ester pendants and at the branching points. Its molecular weight increased substantially with the number of inimer units incorporated, consistent with simultaneous growth of branches at the inimer sites.
MALDI and ESI combined with MS2 and MSn experiments showed that energetically activated [M + Na]+ ions of the glycopolymer, in which all sugar hydroxy groups were acetylated, dissociate mainly via charge remote 1,5-H rearrangement fragmentations over either the acetyl protecting groups or the mannose pendant ester groups, leading to losses of acetic acid and dehydrated mannose, respectively. Backbone C–C bond cleavages, which could have revealed information about the sequence of A and B repeat units, were not observed.
The copolymerization method utilized (ATRP), led to a glycopolymer with the composition AnBm and m Br substituents. All Br atoms remained bound to the polymer during MALDI or ESI analysis. An important finding of this study was that sodiated AnBm oligomers underwent elimination of CH3Br under MS2 conditions, if they contained an inimer unit (B) attached through its initiating site to a terminal repeat unit. Only with this specific arrangement, a rearrangement took place, causing the elimination of a terminal Br and a CH3 group from the inimer’s COOCH3 substituent. Because of its specificity, this fragmentation provided unique sequence insight on the architecture of A1B2 and A2B2 oligomers, whose MS2 characteristics could only be reconciled with the sequence B(B)(A) for the former and the sequences B(B)A2 and/or B(A)B(A) for the latter.
Supplementary information that helped to distinguish between the two mentioned alternative A2B2 structures was provided by IM-MS. Dispersion of the sodiated A2B2 beam by its ion mobility indicated the presence of only one A2B2 component with a collision cross-section of 327 Å2, which matched within experimental error (4%) the CCS predicted theoretically for the B(B)A2 architecture (338 Å2) and was significantly different from the CCS predicted for the isomeric B(A)B(A) architecture (352 Å2). A difference of 14 Å2 in CCS would be sufficient to separate the B(B)A2 and B(A)B(A) isomers or lead to recognizably distorted IM-MS signals under our conditions.51,54 Hence, the combined tandem MS and ion mobility MS data provided evidence that the preferred tetrameric A2B2 architecture has the branched sequence B(B)A2 (see more detailed structures or symbols in Figs. 6 and 7).
The two identified sequences, viz. B(B)(A) for the A1B2 trimer and B(B)A2 for the A2B2 tetramer, provide a hint about the polymerization mechanism. Such architectures would arise if dimer B(A) is formed first and continues to grow by reacting with the acrylate group of a second inimer unit to form B(B)(A) and subsequently with the acrylate group of another monomer to form B(B)A2. Further propagation through such steps creates more branches and ultimately, as more B and A units are copolymerized, a hyperbranched material.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the National Science Foundation (CHE-1308307 to C.W. and CHE-1112490 to A.M.K.) for financial support and George R. Newkome for the use of the Materials Studio software. K.L. was supported by a fellowship from the NIH Biotechnology Training Program, T32GM067555.
Footnotes
Electronic supplementary information (ESI) available.
REFERENCES
- 1.Sharon N, Lis H. Sci. Am. 1993;268:82–89. doi: 10.1038/scientificamerican0193-82. [DOI] [PubMed] [Google Scholar]
- 2.Lee YC, Lee RT. Acc. Chem. Res. 1995;28:321–327. [Google Scholar]
- 3.Mammen M, Chio SK, Whitesides GM. Angew. Chem. Int. Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee RT, Lee YC. Glycoconjugate J. 2000;17:543–551. doi: 10.1023/a:1011070425430. [DOI] [PubMed] [Google Scholar]
- 5.Capila I, Linhardt RJ. Angew. Chem., Int. Ed. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Bertozzi CR, Kiessling LL. Science. 2001;291:2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- 7.Ladmiral V, Melia E, Haddleton DM. Eur. Polym. J. 2004;40:431–449. [Google Scholar]
- 8.Muthukrishnan S, Jutz G, André X, Mori H, Müller AHE. Macromolecules. 2005;38:9–18. [Google Scholar]
- 9.Wang Y, Kiick KL. J. Am. Chem. Soc. 2005;127:16392–16393. doi: 10.1021/ja055102+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becer CR. Macromol. Rapid Commun. 2012;33:742–752. doi: 10.1002/marc.201200055. [DOI] [PubMed] [Google Scholar]
- 11.Miura Y. Polym. J. 2012;44:679–689. [Google Scholar]
- 12.Dickinson E, Bergenstahl B, editors. Food colloids: proteins, lipids, and polysaccharides. Vol. 192. Cambridge: The Royal Society of Chemistry; 1997. [Google Scholar]
- 13.Suh JKF, Matthew HWT. Biomaterials. 2000;21:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Goto M, Cho CS, Akaike T. Biotechnol. Lett. 2000;22:1049–1057. [Google Scholar]
- 15.Sashiwa H, Thompson JM, Das SK, Shigemasa Y, Tripathy S, Roy R. Biomacromolecules. 2000;1:303–305. doi: 10.1021/bm005536r. [DOI] [PubMed] [Google Scholar]
- 16.Spain SG, Cameron NR. Polym. Chem. 2011;2:60–68. [Google Scholar]
- 17.Zaia J. Mass Spectrom. Rev. 2004;23:161–227. doi: 10.1002/mas.10073. [DOI] [PubMed] [Google Scholar]
- 18.Bindila L, Peter-Katalinic J. Mass Spectrom. Rev. 2009;28:223–253. doi: 10.1002/mas.20197. [DOI] [PubMed] [Google Scholar]
- 19.Harvey DJ. Mass Spectrom. Rev. 2012;31:183–311. doi: 10.1002/mas.20333. [DOI] [PubMed] [Google Scholar]
- 20.Mischnick P. Adv. Polym. Sci. 2012;248:105–174. [Google Scholar]
- 21.El-Hawiet A, Kitova EN, Klassen JS. Biochemistry. 2012;51:4244–4253. doi: 10.1021/bi300436x. [DOI] [PubMed] [Google Scholar]
- 22.Kailemia MJ, Ruhaak LR, Lebrilla CB, Amster IJ. Anal. Chem. 2014;86:196–212. doi: 10.1021/ac403969n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinhold VN, Reinhold BB, Costello CE. Anal. Chem. 1995;67:1772–1784. doi: 10.1021/ac00107a005. [DOI] [PubMed] [Google Scholar]
- 24.An HJ, Lebrilla CB. Mass Spectrom. Rev. 2011;30:560–578. doi: 10.1002/mas.20283. [DOI] [PubMed] [Google Scholar]
- 25.Staples GO, Zaia J. Curr. Proteomics. 2011;8:325–336. doi: 10.2174/157016411798220871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamus G, Sikorska W, Kowalczuk M, Noda I, Satkowski MM. Rapid Commun. Mass Spectrom. 2003;17:2260–2266. doi: 10.1002/rcm.1190. [DOI] [PubMed] [Google Scholar]
- 27.Jackson AT, Scrivens JH, Williams JP, Shammel Baker E, Gidden J, Bowers MT. Int. J. Mass Spectrom. 2004;238:287–297. [Google Scholar]
- 28.Rizzarelli P, Puglisi C, Montaudo G. Rapid Commun. Mass Spectrom. 2005;19:2407–2418. doi: 10.1002/rcm.2075. [DOI] [PubMed] [Google Scholar]
- 29.Wesdemiotis C, Pingitore F, Polce MJ, Russell VM, Kim Y, Kausch CM, Connors TH, Medsker RE, Thomas RR. Macromolecules. 2006;39:8369–8378. [Google Scholar]
- 30.Terrier P, Buchmann W, Desmazières B, Tortajada J. Anal. Chem. 2006;78:1801–1806. doi: 10.1021/ac051308h. [DOI] [PubMed] [Google Scholar]
- 31.Adamus G. Rapid Commun. Mass Spectrom. 2007;21:2477–2490. doi: 10.1002/rcm.3111. [DOI] [PubMed] [Google Scholar]
- 32.Gies AP, Vergne MJ, Orndorff RL, Hercules DM. Macromolecules. 2007;40:7493–7504. [Google Scholar]
- 33.Polce MJ, Ocampo M, Quirk RP, Wesdemiotis C. Anal. Chem. 2008;80:347–354. doi: 10.1021/ac071071k. [DOI] [PubMed] [Google Scholar]
- 34.Polce MJ, Ocampo M, Quirk RP, Leigh AM, Wesdemiotis C. Anal. Chem. 2008;80:355–362. doi: 10.1021/ac701917x. [DOI] [PubMed] [Google Scholar]
- 35.Wesdemiotis C, Solak N, Polce MJ, Dabney DE, Chaicharoen K, Katzenmeyer BC. Mass Spectrom. Rev. 2011;30:523–559. doi: 10.1002/mas.20282. [DOI] [PubMed] [Google Scholar]
- 36.Scionti V, Wesdemiotis C. In: Mass Spectrometry in Polymer Chemistry. Barner-Kowollik C, Gründling T, Falkenhagen J, Weidner S, editors. Weinheim: Wiley-VCH; 2012. pp. 57–84. [Google Scholar]
- 37.Fouquet T, Chendo C, Toniazzo V, Ruch D, Charles L. Rapid Commun. Mass Spectrom. 2013;27:88–96. doi: 10.1002/rcm.6432. [DOI] [PubMed] [Google Scholar]
- 38.Yol AM, Dabney DE, Wang S-F, Laurent BA, Foster MD, Quirk RP, Grayson SM, Wesdemiotis C. J. Am. Soc. Mass Spectrom. 2013;24:74–82. doi: 10.1007/s13361-012-0497-5. [DOI] [PubMed] [Google Scholar]
- 39.Yol AM, Wesdemiotis C. React. Funct. Polym. 2014;80:95–108. [Google Scholar]
- 40.Bowers MT, Kemper PR, von Helden G, van Koppen PAM. Science. 1993;260:1446–1451. doi: 10.1126/science.260.5113.1446. [DOI] [PubMed] [Google Scholar]
- 41.Clemmer DE, Jarrold MF. J. Mass Spectrom. 1997;32:577–592. [Google Scholar]
- 42.Kanu AB, Dwivedi P, Tam M, Matz L, Hill H., Jr J. Mass Spectrom. 2008;43:1–22. doi: 10.1002/jms.1383. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins CL, Trimpin S, editors. Ion Mobility Spectrometry-Mass Spectrometry. Boca Raton: CRC Press; 2011. [Google Scholar]
- 44.Lapthorn C, Pullen F, Chowdhry BZ. Mass Spectrom. Rev. 2013;32:43–71. doi: 10.1002/mas.21349. [DOI] [PubMed] [Google Scholar]
- 45.Fenn LS, McLean JA. Meth. Mol. Biol. 2013;951:171–194. doi: 10.1007/978-1-62703-146-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan Y-T, Li X, Soler M, Wang JL, Wesdemiotis C, Newkome GR. J. Am. Chem. Soc. 2009;131:16395–16397. doi: 10.1021/ja907262c. [DOI] [PubMed] [Google Scholar]
- 47.Broker ER, Anderson SE, Northrop BH, Stang PJ, Bowers MT. J. Am. Chem. Soc. 2010;132:13486–13494. doi: 10.1021/ja105702y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Guo L, Casiano-Maldonado M, Zhang D, Wesdemiotis C. Macromolecules. 2011;44:4555–4564. [Google Scholar]
- 49.Hoskins JN, Trimpin S, Grayson SM. Macromolecules. 2011;44:6915–6918. [Google Scholar]
- 50.Chan Y-T, Li X, Carri GA, Moorefield CN, Newkome GR, Wesdemiotis C. J. Am. Chem. Soc. 2011;133:11967–11976. doi: 10.1021/ja107307u. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Chan Y-T, Casiano-Maldonado M, Yu J, Carri GA, Newkome GR, Wesdemiotis C. Anal. Chem. 2011;83:6667–6674. doi: 10.1021/ac201161u. [DOI] [PubMed] [Google Scholar]
- 52.Scionti V, Katzenmeyer BC, Solak N, Li X, Wesdemiotis C. Eur. J. Mass Spectrom. 2012;18:113–137. doi: 10.1255/ejms.1175. [DOI] [PubMed] [Google Scholar]
- 53.Snelling JR, Scarff CA, Scrivens JH. Anal. Chem. 2012;84:6521–6529. doi: 10.1021/ac300779p. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Guo K, Su H, Li X, Feng X, Wang Z, Zhang W, Zhu S, Wesdemiotis C, Cheng SZD, Zhang W-B. Chem. Sci. 2014;5:1046–1053. [Google Scholar]
- 55.Solak Erdem N, Alawani N, Wesdemiotis C. Anal. Chim. Acta. 2014;808:83–93. doi: 10.1016/j.aca.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 56.Lin K, Kasko AM. Biomacromolecules. 2013;14:350–357. doi: 10.1021/bm3015285. [DOI] [PubMed] [Google Scholar]
- 57.Suckau D, Reseman A, Schuerenberg M, Hufnagel P, Franzen J, Holle A. Anal. Bioanal. Chem. 2003;376:952–965. doi: 10.1007/s00216-003-2057-0. [DOI] [PubMed] [Google Scholar]
- 58.Bruker Esquire/HCT Series User Manual, version 1.3. 1, chapter 3. Bremen, Germany: Bruker Daltonik GmbH; 2008. [Google Scholar]
- 59.Pringle SD, Giles K, Wildgoose JL, Williams JP, Slade SE, Thalassinos K, Bateman RH, Bowers MT, Scrivens JH. Int. J. Mass Spectrom. 2007;261:1–12. [Google Scholar]
- 60.Ruotolo BT, Benesch J, Sandercock A, Hyung S-J, Robinson CV. Nat. Protoc. 2008;3:1139–1152. doi: 10.1038/nprot.2008.78. [DOI] [PubMed] [Google Scholar]
- 61.Thalassinos K, Grabenauer M, Slade SE, Hilton GR, Bowers MT, Scrivens JH. Anal. Chem. 2009;81:248–254. doi: 10.1021/ac801916h. [DOI] [PubMed] [Google Scholar]
- 62.Katzenmeyer BC, Cool LR, Williams JP, Craven K, Brown JM, Wesdemiotis C. Int. J. Mass Spectrom. 2014 in press. [Google Scholar]
- 63. [accessed on 26 November 2014]; http://www.indiana.edu/~clemmer/Research/Cross%20Section%20Database/Peptides/polyaminoacid_cs.htm and http://www.indiana.edu/~clemmer/Research/Cross%20Section%20Database/Proteins/protein_cs.htm. [Google Scholar]
- 64.Mesleh MF, Hunter JM, Shvartsburg AA, Schatz GC, Jarrold MF. J. Phys. Chem. 1996;100:16082–16086. [Google Scholar]
- 65.Chaicharoen K, Polce MJ, Singh A, Pugh C, Wesdemiotis C. Anal. Bioanal. Chem. 2008;392:595–607. doi: 10.1007/s00216-008-1969-0. [DOI] [PubMed] [Google Scholar]
- 66.Mischnick P, Momcilovic D. Adv. Carbohydr. Chem. Biochem. 2010;64:117–210. doi: 10.1016/S0065-2318(10)64004-8. [DOI] [PubMed] [Google Scholar]
- 67.Henderson SE, Li J, Counterman AE, Clemmer DE. J. Phys. Chem. B. 1999;103:8780–9785. [Google Scholar]
- 68.Knapman TW, Berryman JT, Campuzano J, Harris SA, Ashcroft AE. Int. J. Mass Spectrom. 2010;298:17–23. [Google Scholar]
- 69.Bleiholder C, Wyttenbach T, Bowers MT. Int. J. Mass Spectrom. 2011;308:1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.