Abstract
Polyunsaturated fatty acids (PUFA) are oxidized by cytochrome P450 epoxygenases to PUFA epoxides which function as potent lipid mediators. The major metabolic pathways of PUFA epoxides are incorporation into phospholipids and hydrolysis to the corresponding PUFA diols by soluble epoxide hydrolase. Inhibitors of soluble epoxide hydrolase stabilize PUFA epoxides and potentiate their functional effects. The epoxyeicosatrienoic acids (EETs) synthesized from arachidonic acid produce vasodilation, stimulate angiogenesis, have anti-inflammatory actions, and protect the heart against ischemia-reperfusion injury. EETs produce these functional effects by activating receptor-mediated signaling pathways and ion channels. The epoxyeicosatetraenoic acids synthesized from eicosapentaenoic acid and epoxydocosapentaenoic acids synthesized from docosahexaenoic acid are potent inhibitors of cardiac arrhythmias. Epoxydocosapentaenoic acids also inhibit angiogenesis, decrease inflammatory and neuropathic pain, and reduce tumor metastasis. These findings indicate that a number of the beneficial functions of PUFA may be due to their conversion to PUFA epoxides.
Keywords: arachidonic acid (AA), epoxyeicosatrienoic acid (EET), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), epoxyeicosatetraenoic acid (EpETE), epoxydocosapentaenoic acid (EpDPE)
1. Introduction
Oxidation of PUFA by cytochrome P450 (CYP)1 epoxygenases was first detected when rat kidney or liver microsomes were incubated with arachidonic acid (AA) in the presence of NADPH and O2 [1,2]. The epoxyeicosatrienoic acids (EET) synthesized from AA were initially observed to function as lipid mediators in the cardiovascular and renal systems [3–7]. EETs are metabolized by many tissues [1,8,9], and recent findings indicate that they have effects on angiogenesis, proliferation, inflammation, pain and myocardial preconditioning [10–15]. CYP epoxygenases also oxidize linoleic acid (LA), the 18-carbon ω-6 PUFA, to epoxyoctadecenoic acids (EpOME), and the ω-3 PUFA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to epoxyeicosatetraenoic acids (EpETE) and epoxydocosapentaenoic acids (EpDPE), respectively [2]. Like EETs, the ω-3 PUFA epoxides function as lipid mediators. They have potent anti-arrhythmic actions, protect the heart against ischemia-reperfusion injury and are analgesic on inflammatory and neuropathic pain. Epoxydocosapentaenoic acids also inhibit angiogenesis and have been observed to reduce tumor metastasis. They appear to be much more potent than EETs in reducing arrhythmias, more potent in reducing blood pressure, and even somewhat more potent in reducing inflammation and pain [16–18].
2. Arachidonic acid epoxidation
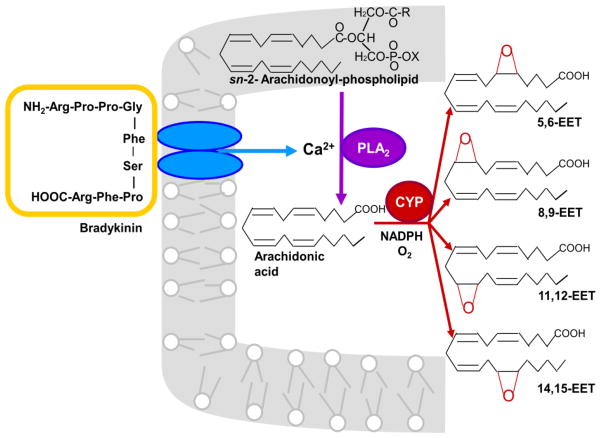
Epoxidation of AA has been studied extensively in the endothelium, the main source of EETs in the vascular system. Endothelial cells express CYP2J2 and CYP2C9, and EET production is stimulated by exposure of these cells to vasodilators like bradykinin and acetylcholine, or by shear stress [19–21]. As illustrated in Fig. 1, AA stored in phospholipids is hydrolyzed by a Ca2+-activated phospholipase A2 (PLA2) and oxidized by a CYP epoxygenase to form EETs. Although CYP epoxygenases can epoxidize each of the four double bonds of AA, most epoxygenases produce appreciable amounts of only one or two EET regioisomers. 11,12- and 14,15-EET account for 67–80% of the EETs synthesized by five purified and reconstituted CYP epoxygenases, including CYP2C8, CYP2C9 and CYP2J2, and 11,12- and 14,15-EET are the main EET regioisomers produced by most mammalian tissues [1,22].
Fig. 1.
EET synthesis in endothelial cells. In response to bradykinin or other vasodilators, AA contained in endothelial phospholipids is released by a Ca2+-activated phospholipase A2 (PLA2). CYP epoxygenases oxidize the AA to EETs in a reaction that utilizes NADPH and O2, and the EETs are released from the cell. Although CYP epoxygenases can synthesize all four EET regioisomers as illustrated in the figure, 11,12- and 14,15-EET are the most abundant EETs produced by the endothelium and many other tissues. DHA or EPA epoxygenation by CYP epoxygenases has not been investigated in intact cells, so it is uncertain whether this receptor-mediated production mechanism also applies to ω-3 PUFA epoxides.
Each EET regioisomer consists of two enantiomeric forms, R/S and S/R. The enantiomeric distributions are different for each CYP epoxygenase, and two regioisomers synthesized by the same CYP epoxygenase often have different enantiomeric distributions [1,22]. Enantiomeric dependence has been observed for some EET functions. For example, 11,12-EET-induced relaxation of rat renal microvessels and activation of renal vascular smooth muscle large-conductance Ca2+-activated K+ (BKCa) channels are mediated only by 11(R),12(S)-EET [23], whereas the activation of cardiac KATP channels is mediated only by 11(S),12(R)-EET [24].
3. Epoxidation of linoleic acid and ω-3 PUFAs
Fig. 2 illustrates the structures of the major epoxide products synthesized by CYP epoxygenases from LA, EPA and DHA. The main LA-derived epoxide produced by leukocytes, 9,10-EpOME, is called leukotoxin because it becomes cytotoxic and produces multiple organ failure and respiratory distress when it is hydrolyzed by an epoxide hydrolase to 9,10-dihydroxyoctadecaenoic acid [25]. Much higher concentrations of 9,10-dihydroxyoctadecaenoic acid than other PUFA-derived diols are present in the plasma of healthy American adults, which may reflect the high levels of linoleic acid in the Western diet [26]
Fig. 2.
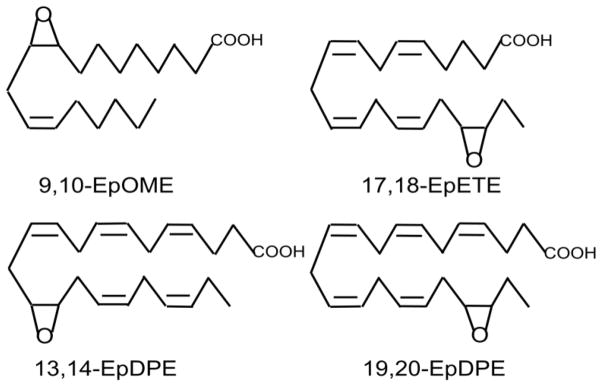
Epoxides derived from LA and ω-3 PUFAs. The most prominent regioisomers include 9,10-epoxyoctadecaenoic acid (9,10-EpOME) from LA,17,18-epoxyeicosatetraenoic acid (17,18-EpETE) from EPA and 19,20-epoxydocosapentaenoic acid (19,20-EpDPE) from DHA. Although minor, 13,14-EpDPE is a potent activator of coronary BKCa channels and reduces inflammatory and neuropathic pain.
CYP epoxygenases preferentially oxidize the ω-3 double bond of EPA and DHA. The most abundant DHA regioisomer synthesized by microsomes and by 15 human recombinant CYPs, including CYP2C8 and CYP2J2, is 19,20-EpDPE. Likewise, the most abundant EPA regioisomer synthesized by these CYPs is 17,18-EpETE [2,27,28]. An exception is CYP2C9 which oxidizes EPA mainly to 14,15-EpETE and DHA to 10,11-EpDPE [17]. EPA and DHA compete with AA for hepatic and renal microsomal epoxygenase activity and for recombinant CYP2C9, CYP1A2, CYP2C8 and CYP2J2, and thus, EET synthesis is decreased in the presence of these ω-3 PUFAs [28–30].
The CYP epoxide profiles in plasma and tissues can be modified by dietary ω-3 PUFA supplementation. When healthy human adults were supplemented with 4 g per day of ω-3 PUFA, the plasma 19,20-EpDPE and 16,17-EpDPE contents doubled, and there was a 4-fold increase in 17,18-EpETE and 14,15-EpETE [26]. Likewise, substantial increases in 19,20-EpDPE, 16,17-EpDPE and 17,18-EpETE occurred in the plasma, cerebral cortex, heart, kidney, liver, lung, pancreas and erythrocytes of rats fed a diet rich in EPA and DHA [30]. The first evidence that ω-3 PUFA epoxides have potent biological activity was the finding that EpDPEs inhibited AA-induced platelet aggregation more effectively than EETs [31].
4. Metabolism of PUFA epoxides
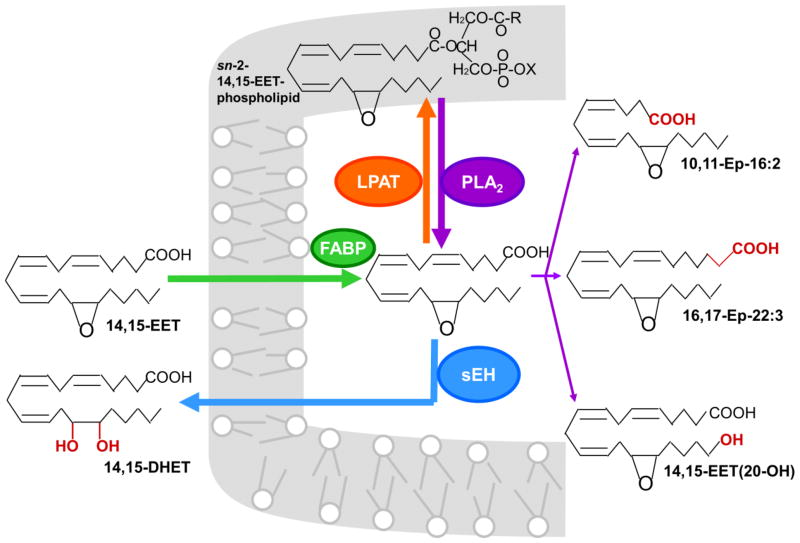
Most of the information about the metabolism of PUFA epoxides has been obtained with EETs. Fig. 3 shows the metabolic pathways using 14,15-EET, a widely studied EET, as an illustration. The major pathways are incorporation into phospholipids and hydrolysis by soluble epoxide hydrolase (sEH) which converts EETs to the corresponding dihydroxyeicosatrienoic acids (DHET) [1,8,9]. 14,15- and 11,12-EET also can be converted to the minor metabolites shown in Fig 3 under certain conditions [22],
Fig. 3.
Pathways of cellular EET metabolism. The illustration is for 14,15-EET, but the other EET regioisomers also are metabolized by the major pathways indicated by thick arrows with the exception of 5,6-EET which is a poor substrate for sEH. Cytosolic FABP binds to EETs, which may enhance EET uptake, although requirement for a transporter has not been demonstrated. Following uptake, the EET is either incorporated into phospholipids or hydrolyzed by soluble epoxide hydrolase (sEH) to the corresponding dihydroxyeicosatrienoic acids (DHET) which is excreted to the extracellular matrix. The mechanism of DHET exit from the cell has not been determined. Incorporation of the EET into phospholipids is mediated by a membrane-bound lysophospholipid acyltransferase (LPAT) that requires ATP and CoA. The membrane-incorporated EET is released by a phospholipases (PLA2) and can be either hydrolyzed by sEH or converted to minor metabolites. The minor metabolites include the partial β-oxidation product 10,11-Ep-16:2, chain elongation product 16,17-Ep-22:3 and ω-oxidation product 14,15-EET(20-OH). Appreciable amounts of these metabolites are produced only if sEH is deficient or inhibited, and some of the minor metabolites have been observed only in one cell type.
EpETEs, EpDPEs and EpOMEs also are incorporated into phospholipids and hydrolyzed by sEH [1,16,26]. However, there is no information whether the minor metabolites are produced from these PUFA epoxides.
4.1. Uptake into cells and tissues
Unesterified EETs can be taken up from the extracellular fluid by many different types of cells [9]. It is not known whether a transporter is required for EETs to cross the plasma membrane, but cytosolic fatty acid binding proteins (FABPs) bind EETs and may enhance uptake by facilitating EET desorption from the membrane [32]. The Kd for EET binding to heart-FABP ranges from 0.4 to 1.7 μM, which is 7- to 30-times higher than the Kd for AA binding. Yet, binding to FABP is sufficient to protect EETs from hydrolysis by sEH, and modeling studies suggest that binding increases the intracellular retention of unesterified EETs and prolongs their functional effects [33].
EETs, ω-3 PUFA epoxides, and the corresponding diols are present in the phospholipids and triglycerides of plasma lipoproteins [1,26]. These compounds are released when very low-density lipoproteins are hydrolyzed by lipoprotein lipase, and they represent another extracellular source of preformed PUFA epoxides for uptake by the tissues [34].
4.2. Incorporation into phospholipids
EETs are incorporated rapidly into the sn-2 position of cell phospholipids through a coenzyme A-dependent mechanism [1,9]. Studies with an insulinoma cell line suggest the acyl-CoA synthetase 4 isoform may mediate the formation of the EET-CoA intermediate required for the esterification reaction [35]. The largest amounts of EETs are present in phosphatidylcholine, but a relatively high percentage of 14,15-EET is incorporated into phosphatidylinositol [9].
The functional role of EET incorporation into cell phospholipids is still uncertain. The most likely possibility is that phospholipids provide a temporary storage site for excess EETs until they can be hydrolyzed and cleared from the cell. However, cardiac L-type Ca2+ channels are inhibited when they are reconstituted with phosphatidylcholine containing 11,12-EET at sn-2, suggesting that phospholipids containing EETs can modulate the function of membrane proteins [36]. Although EETs are estimated to comprise less than 0.01% of the total fatty acyl chains in cell phospholipids, this may be enough to affect localized membrane properties if the EETs accumulate in microdomains. Furthermore, EETs contained in phospholipids are rapidly released when endothelial cells are activated, suggesting that storage in phospholipids may provide an immediate source of EETs for the initiation of a functional response [9]. Phospholipids containing EETs also may be substrates for the synthesis of 2-epoxyeicosatrienoylglycerols, 2-arachidonoylglycerol analogues produced by kidney and spleen. These 2-epoxyeicosatrienoylglycerols bind to cannabinoid CB1 and CB2 receptors and have mitogenic activity [37,38].
4.3. Hydrolysis by soluble epoxide hydrolase
The PUFA epoxides are hydrolyzed to the corresponding diols by sEH, the member of the α/β hydrolase fold enzyme family [39]. In humans, sEH is encoded in EPXH-2, a single copy gene on chromosome 8. The enzyme is widely distributed in human tissues, with the largest amounts in liver, kidney, intestine, and the vasculature. sEH also is present in brain, and while it is expressed in some neurons, it is contained mostly in cortical and hippocampal astrocytes [40–42]. sEH functions as a 120 kDa homodimer, and dimerization is required for enzymatic activity [43]. Each subunit contains a N-terminal phosphatase domain and C-terminal hydrolase domain, and these domains function independently [44,45]. The phosphatase domain hydrolyzes isoprenoid phosphates and lysophosphatidic acid [46,47], which are phosphorylated lipids that stimulate cell growth [48].
Although sEH hydrolyzes all of the commonly occurring PUFA epoxides, human sEH has the highest catalytic efficiencies with 9,10-EpOME, 14,15-EET and 13,14-EpDPE [16]. The enzyme exhibits selectivity for the predominant enantiomeric forms present in tissues [49]. Hydrolysis begins rapidly when cells take up EETs, and some conversion of 14,15-EET to 14,15-DHET in vascular smooth muscle cells has been observed during the first 3 min of incubation [9]. Although DHETs are released into the extracellular fluid, endothelial cells can incorporate small amounts of DHETs into phosphatidylcholine and phosphatidylinositol [50]. DHETs and diols formed from EpETEs and EpDPEs also are present in rodent brain and spinal cord [16,51], as well as in the plasma of humans and piglets fed ω-3 PUFA supplements [26,52].
Other members of the α/β hydrolase fold enzyme family that hydrolyze PUFA epoxides include microsomal epoxide hydrolase (mEH) and ABHD9 (EH3) [53]. Although mEH and sEH have complementary and partially overlapping substrate selectivity, the most widely accepted role of mEH is in xenobiotic metabolism. sEH will dominate hydration of most fatty acid epoxides, except possibly in regions of the brain where mEH protein levels are relatively high. The expression of EH3 appears to be under careful regulation and is high in lung, skin and upper gastrointestinal tract [53]. This suggests a possibly important functional role of EH3, but further confirmation is required. The sEH inhibitor 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA) has been reported to inhibit EH3 [53], but the newer sEH inhibitors that contain bulky substituents on both sides of the urea group do not inhibit EH3.
4.3.1. sEH inhibition
The term sEH inhibitor as commonly used refers only to inhibition of the sEH hydrolase activity. sEH inhibition stabilize EETs, increases their incorporation into phospholipids and other metabolites, and enhances their functional responses [54], effects that have potential therapeutic benefits [55–57]. Recent findings indicate that sEH inhibitors have similar stabilizing effects on ω-3 PUFA epoxides [16,51]. Although inhibitors of the sEH phosphatase activity are available [58.59], few studies have been done with them because the physiological importance of the sEH phosphatase activity is uncertain.
4.3.1.1. Inhibitors and analogues used to study PUFA epoxide function
Selective sEH inhibitors are the most commonly used compounds to study the functional effects of PUFA epoxides [14,16,55,]. They include the potent N,N′-disubstituted urea inhibitors like AUDA, and those with higher water solubility such as trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB), and 1-(1-methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)urea (TUPS) [60]. Dual function sEH inhibitors are available now, including those that also serve as stable EET analogues such as 8-HUDE (12-(3-hexylureido) dodec-8-enoic acid) [61,62], those that modulate peroxisome proliferator-activated receptors [63], and those that inhibit 5-lipoxygenase [64]. In addition, inhibitors with new pharmacophores are being developed [65–67], including urea-containing-pyrazoles that are dual inhibitors of sEH and cyclooxygenase-2 [68]. Furthermore, in addition to inhibiting sEH, N-cyclohexyl-N′-dodecanoic acid urea (CUDA) and AUDA are weak activators of peroxisome proliferator-activated receptor (PPAR) α [69].
Other compounds used to study PUFA epoxide function include N-methylsulfonyl-6-(2-proparglyoxylphenyl)hexanamide (MS-PPOH), a selective inhibitor of CYP epoxygenase [70], and 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE), an EET antagonist [71]. Compounds containing epoxide bioisosteres that have anti-arrhythmic effects similar to 17,18-EpETE on the heart [61,72], and selective inhibitors of the vascular actions of 14,15- and 11,12-EET also are available [73,74].
4.3.2. Function of PUFA diols
DHETs and the diol metabolites of EpETE and EpDPE have little or no effect on many of the physiological functions mediated by the corresponding PUFA epoxides [16,18,30,54]. Furthermore, no phenotype was observed in EPXH2 gene deleted mice [75]. PUFA diols are much more polar than the corresponding PUFA epoxides. They accumulate primarily in the extracellular fluid and are generally thought to be inactivation products that move rapidly away from their sites of production and are excreted. However, some PUFA diols have biological activity. DHETs augment bradykinin-induced relaxation of porcine coronary artery rings [19], dilate canine and human coronary arterioles [76,77], and activate BKCa channels [78]. 14,15-DHET activates PPARα-mediated gene expression [79,80], 11,12-DHET regulates cAMP production in HEK293 cells [81], and PUFA diols including 12,13-DHOME are required for optimal progenitor cell proliferation and vascular repair [82]. Diol metabolites produced by sEH are essential for monocyte chemotaxis to monocyte chemoattractant protein-1 [83]. These findings indicate that some PUFA diols may have functional effects on physiological processes.
4.4. Additional metabolic pathways
Additional EET metabolites are produced under certain conditions. 11,12- and 14–15-EET undergo partial β-oxidation to a 16-carbon metabolites and chain-elongation to a 22-carbon metabolites that have bioactivity [22,84,85]. Likewise, 11,12-DHET undergoes partial β-oxidation to a 16-carbon metabolite in porcine aortic smooth muscle cells [9]. However, appreciable amounts of these products are formed only when sEH is deficient or inhibited [86,87], suggesting that these metabolic pathways probably have a minimal role under ordinary physiological conditions. EETs also can undergo ωoxidation, and the ω-hydroxy-EET products activate PPARα [88]. In addition, the perfused rabbit kidney converts 5,6-EET to the prostaglandin derivative 5,6-epoxy-PGE1 which produces renal vasodilation [89].
Human liver CYP3A4 and brain CYP2B6 and CYP2D6 oxidize arachidonoyl ethanolamide (anandamide) to EET ethanolamides [90,91]. 5,6-EET-ethanolamide activates the CB2 cannabinoid receptor, and 14,15-EET-ethanolamide is a weak activator of the CB1 cannabinoid receptor [91,92]. The EET-ethanolamide regioisomers can be ω-hydroxylated or hydrolyzed to the corresponding diol derivatives by sEH or microsomal epoxide hydrolase [92]. DHA-ethanolamide which has synaptogenic and neurogenic properties is synthesized in the brain [93,94], but there is no information regarding whether it can be epoxidized to EpDPE-ethanolamides.
5. Mechanism of PUFA epoxide action
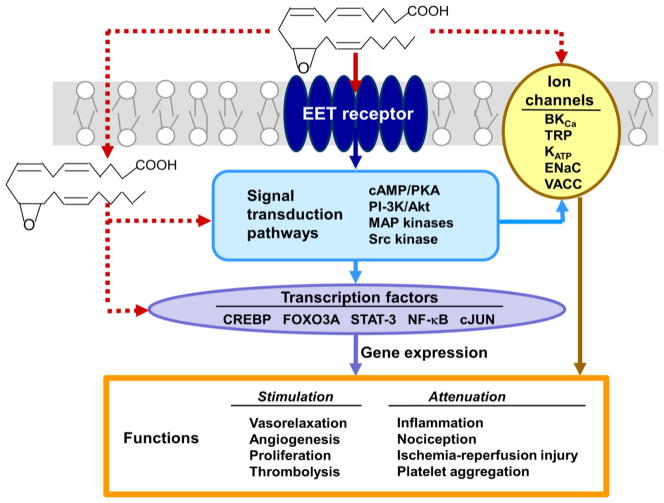
There is increasing evidence that many EETs function through a membrane receptor mechanism as illustrated schematically by the solid arrows in Fig. 4. EET-dependent vasodilation occurs through the cAMP/protein kinase A (PKA) signaling pathway activated by a receptor coupled to Gαs-protein [96,97], and other EET-dependent functions are mediated by activation of PI3K/Akt, MAP kinase or Src kinase pathways [22]. EpETEs and EpDPEs affect many of the same processes, and thus, it is likely that they act through a similar receptor-dependent mechanism. However, EETs also enter cells and can interact directly with intracellular effectors like FABPs and PPARγ [20,32], cardiac KATP channels [98], and TRPV4 Ca2+ channels [99].
Fig. 4.
Mechanisms of action of EETs. 11,12-EET is used in this illustration. Many EET functions occur through a membrane receptor-dependent mechanism which activates signal transduction pathways that modulate ion channels and transcription factors in the target cell. This mechanism is indicated by the solid arrows. Some EET responses may occur through intracellular effects or direct interactions with ion channels. Because the physiological relevance of these direct acting mechanisms is less substantial, they are illustrated by the dashed arrows. Listed are the signal transduction pathways, ion channels and transcription factors that are targeted by EETs in various cells, and the functional responses that occur in the cardiovascular, renal, and nervous system.
5.1. Putative EET-selective membrane receptor
Recent results support the probability that 14,15-EET acts by binding to a membrane receptor that activates signal transduction pathways [100]. 14,15-EET-complexs that do not enter cells produced relaxation of coronary artery rings and inhibited cAMP-induced aromatase activity with the same potency as 14,15-EET in solution [101,102]. 11,12-EET activated the vascular BKCa channel through ADP-ribosylation of the Gαs protein, providing evidence for a G-protein coupled EET receptor [96]. Furthermore, 20-iodo-14,15-epoxyeicosa-8(Z)-enoyl-3-azidophenyl-sulfonamide, a photoaffinity probe that relaxes bovine coronary arteries, labeled a 47kDa protein in bovine coronary artery and U937, endothelial and vascular smooth muscle cells. Labeling was inhibited by low concentrations of 11,12- and 14,15-EET, indicating that the 47 kDa protein may be the putative high-affinity EET receptor [103].
The results provide substantial evidence for a receptor-dependent mechanism of EET action, but some caution is indicated because the putative receptor has not been cloned, and the sulfonamide photoaffinity probe did not label transfected HEK293 cells that expressed 79 G-protein coupled receptors [103]. Moreover, it seems unlikely that a single receptor mediates all of the diverse functions of EETs [22]. In this regard, some EET responses involve receptors for other lipid mediators. For example, 14,15-EET produced vasodilation of rat mesenteric artery by activation of the prostaglandin EP2 receptor[104]. 14,15-EET also relaxed the aorta and mesenteric resistance arteries by acting as an antagonist of the thromboxane receptor [105]. Weak binding of EETs to several receptors that mediate nervous system signaling also has been observed, including the peripheral benzodiazepine, cannabinoid CB2, neurokinin NK2 and dopamine D3 receptors [106].
5.2. Modulation of ion transport
EET-dependent activation of BKCa channels produces vasodilation through hyperpolarization of the vascular smooth muscle [21]. Like EETs, EpDPEs and EpETEs are potent activators of BKCa channels. 13,14-EpDPE activates rat coronary smooth muscle BKCa channels and dilates coronary microvessels with the same efficacy as 11,12-EET [107,108], and it is 1000-times more potent than EETs in activating the BKCa channels of porcine coronary myocytes and rat small coronary arterioles [109]. 16,17-EpDPE also activates BKCa channels [108]. 17,18-EpETE produces relaxation of human pulmonary artery tissue by activating BKCa channels [110], and BKCa channels activation by 17(R),18(S)-EpETE in cerebral arteries occurs by targeting the channel α-subunit [111,112].
EETs stimulate an influx of Ca2+ by activating transient receptor potential (TRP) channels [5]. TRPV4 channels are activated by 5,6-, 8,9- and 11,12-EET [99,113,114], possibly through a direct effect of the EET on the TRP4 channel [7]. 11,12-EET activated TPRC6 channels by increasing their translocation to the plasma membrane [115,116], and 8-HUDE, the sEH inhibitor that also functions as an EET agonist, increased Ca2+ influx into pulmonary artery smooth muscle cells by increasing the expression of TRPC6 and TRPC1 channels [62]. In addition, EETs increased Ca2+ influx into vascular smooth muscle cells by activating voltage-dependent Ca2+ channels [117].
EETs inhibit Na+ channels by decreasing their open probability [118,119]. Inhibition of the epithelial Na+ (ENaC) channel is due to an ERK1/2-dependent phosphorylation of the ENaC β- and γsubunits [120]. Down-regulation of the ENaC channel also occurred when endogenous EpETEs and EpDPEs were increased in mice by a combination of an ω-3 PUFA diet was combined with a sEH inhibitor [121]. 11,12-EET directly activates the cardiac KATP channel by reducing its sensitivity to ATP [96], but it activates mesenteric artery KATP channels through the receptor-dependent PKA pathway [122]. EpDPE regioisomers are more potent activators of KATP channels than the corresponding EETs [24]. EETs inhibit the volume-activated chloride channels (VACC) in rat mesenteric arterial smooth muscle cells through a cGMP-dependent mechanism [123].
6. Physiological functions of PUFA epoxides
The effects of EETs on vascular reactivity, renal function and blood pressure regulation have been reviewed in detail [3–5,7,55,56]. This section focuses primarily on other actions of EETs and the effects of ω-3 PUFA epoxides on these processes.
6.1. Angiogenesis
Stimulation of endothelial cell proliferation, migration and tube formation occurred in cerebral microvessel endothelial cells co-cultured with astrocytes which release EETs into the medium, or when incubated in a medium containing 8,9-EET [124,125]. Similar angiogenic effects occurred when CYP2C9 was overexpressed in human lung microvascular endothelial cells or when these cells were incubated with 14,15-EET [126]. 11,12-EET-induced angiogenesis in human umbilical vein endothelial cells occurred through PI3K/Akt mediated phosphorylation of FOXO factors which caused down-regulation of the cyclin dependent kinase inhibitor p27Kip1 [127]. Other mechanisms that have been observed in EET-stimulated angiogenesis include activation of matrix metalloproteinases, MAP kinase, ERK, JNK/cJun, sphingosine kinase-1, EphB4-coupled to PI3K/Akt, and Src kinase coupled to PI3K/Akt or STAT-3 phosphorylation [128–135]. The angiogenic effect of 11,12- and 14,15-EET in a mouse wound healing model occurred through up-regulation of vascular endothelial growth factor [136].
As opposed to EETs, ω-3 PUFA epoxides suppress angiogenesis. 19,20-EpDPE and other EpDPE regioisomers decreased vascular endothelial growth factor-induced angiogenesis in mice and suppressed fibroblast growth factor 2-induced migration and protease production in human umbilical vein endothelial cells [18]. 17,18-EpETE also decreased the proliferation of transformed brain microvessel endothelial cells through down-regulation of the cyclin D1/cyclin dependent kinase 4 complex mediated by the p38 MAK kinase pathway [137].
6.2. Tumor growth and metastasis
EETs and ω-3 PUFA epoxides also have opposite effects on tumor proliferation and metastasis [138]. EETs stimulated escape from tumor dormancy and multi-organ metastasis in mouse tumor models [139], and they increased proliferation and decreased apoptosis of hematologic malignancies by activating AMP kinase, cJun, PI3K/Akt and EGF receptor phosphorylation [140]. 11,12-EET increased motility of prostate cancer cell lines by activating EGF receptor and PI3K pathways [141], and 14,15-EET stimulated the growth of estrogen receptor-positive breast cancer cell lines by promoting STAT-3 phosphorylation [142]. EETs also promote tissue and organ regeneration [143]. Conversely, ω-3 PUFA epoxides decrease tumor growth and metastasis [18]. 19,20-EpDPE combined with a low dose of the sEH inhibitor t-AUCB decreased the growth of the Met-1 breast cancer in mice and reduced metastasis of the Lewis lung carcinoma by 70%, and 16,17-EpDPE alone or combination with t-AUCB reduced Lewis lung carcinoma metastasis. The anti-metastatic effect of the EpDPEs occurred at the site of metastasis, possibly by blocking tumor angiogenesis [15].
6.3. Inflammation
EETs and ω-3 PUFA epoxides have anti-inflammatory effects in the cardiovascular system, bronchi, kidney and nervous system [16,144,145]. The vascular effect is due to attenuation of NF-κB-dependent inflammatory responses [145]. 8,9-EET attenuated lipopolysaccharide-stimulated nuclear translocation of NF-κB in mouse B lymphocytes and decreased basal and activation-induced antibody secretion [146]. 11,12- and 14,15-EET decreased oxidized low density lipoprotein-mediated inflammation in endothelial cells by inhibiting LOX-1 receptor up-regulation and NF-κB activation [147]. TNF-α induced inflammation and apoptosis in rat cardiomyocytes occurred through a mechanism involving PPARγ [148], and 17,18-EpETE attenuated the TNFα-stimulated inflammation in human bronchial explants by targeting PPARγ and reversing the phosphorylation of p38 MAP kinase [149]. Anti-inflammatory effects of PUFA epoxides also occur in the kidney. An orally active stable EET analogue attenuated kidney injury in the Dahl salt-sensitive rat by decreasing oxidative stress, endoplasmic reticulum stress and inflammation [150]. Likewise, increases in EpETEs and EpDPEs produced by administration of a diet rich in ω-3 PUFAs combined with a sEH inhibitor decreased renal markers of inflammation in angiotensin-II-dependent hypertension [121].
Results obtained in mice fed a high-fat diet indicate that sEH deficiency or inhibition attenuates endoplasmic reticulum stress in the liver and adipose tissue. Furthermore, insulin signaling increased when HepG2 cells were incubated with EETs or EpOME [151]. Treatment of mice fed a high-fat diet with the sEH inhibitor TUPS also decreased insulin resistance and glucose concentration [152]. These findings suggest that PUFA epoxides may be physiological modulators of endoplasmic reticulum stress [151].
6.4. Nervous system function and pain
EETs modulate neurohormone and neuropeptide release, integrate cell-cell communication, produce neurogenic vasodilation, and protect against the effects of cerebral ischemia [153]. 14,15-EET stimulates axon growth in primary cortical and sensory neuron cultures [154], and EETs mediate cerebral vasodilation produced by stimulation of astrocyte metabotropic glutamate receptors, possibly by activating K+ channels or TRPV4 channels [114,155,156]. When injected into the rat brain, 14,15-EET produced an anti-nociceptive effect through activation of β-endorphin and met-enkephalin [157], and it reduced the injurious effect of H2O2 in the dopaminergic N27 neuronal cell line [158]. The administration of sEH inhibitors delayed the onset of seizures mediated by GABA, indicating that EET stabilization can suppress pathologic neurotransmission in the brain [159].
EpDPEs are present in brain and spinal cord and have direct anti-hyperalgesic actions in a rat model of pain associated with inflammation [16]. Although 7,8-EpDPE accounts for 85–90 % of the total EpDPE in neural tissues, 13,14-EpDPE has the most potent anti-nociceptive effect. PUFA epoxide levels in the plasma and spinal cord, including EpDPEs and EpETEs, were stabilized by administration of sEH inhibitors, and this augmented their effectiveness in reducing neuropathic pain in a rat model of diabetes [51]. The anti-hyperalgesic effects of EETs result from repression of the COX2 gene and rapid up-regulation of the acute neurosteroid-producing gene StARD1 [160]. It is possible that these mechanisms also may mediate in the anti-nociceptive effect of EpDPEs [16].
6.5. Cardioprotective actions
EETs and the ω-3 PUFA epoxides have protective effects on the heart. Infarct size in the dog heart resulting from coronary occlusion was decreased by administration of 11,12- or 14,15-EET. The protective effect of these EETs was potentiated by the administration of a sEH inhibitor and abolished by the EET antagonist 14,15-EEZE [161]. sEH inhibition also produced cardioprotection by increasing endogenous EET levels in the dog heart [162]. Treatment of mice with sEH inhibitors after myocardial infarction reduced cardiac ischemia-reperfusion injury, cardiac remodeling and fibrosis [163,164]. Addition of 14,15-EET to rat cardiomyocytes decreased mitochondrial dysfunction due to cellular stress by a K+ channel dependent mechanism [165], and treatment with 11,12-EET protected the rat heart following myocardial infarction through a mechanism involving activation of a Gi/o protein coupled to δ- and μ-opioid receptors [166].
Consistent with these EET-dependent protective actions, overexpression of human CYP2J2 in mouse cardiomyocytes protected against doxorubicin-induced cardiotoxicity [167], and endothelial expression of CYP2J2 reduced infarct size and increased left ventricular developed pressure after ischemia-reperfusion [168]. By contrast, endothelial expression of CYP2C8 in mice impaired functional recovery of the heart due to increased production of reactive oxygen species. This caused oxidation of linoleic acid to 9,10-DiHOME which increased coronary resistance [169].
EpETEs and EpDPEs protect the heart because they have potent anti-arrhythmic actions [30]. A negative chronotropic response occurred almost immediately after 30 nM 19,20-EpDPE or 17,18-EpETE was added to rat cardiomyocytes exposed to high concentrations of Ca2+. The effect of 17,18-EpETE was stereoselective and 17(R),18(S)-EpETE, the active enantiomer, was 1000-fold more potent than EPA [30]. The quantity of EpETEs and EpDPEs in left ventricular myocardium increased when rats were fed a high ω-3 PUFA diet, suggesting that the anti-arrhythmic effect of dietary ω-3 PUFAs is due to an increase in the amount of ω-3 PUFA epoxides in the myocardium [17]. Stable epoxide bioisosteric analogues that have a more potent negative chronotropic effect than 17,18-EpETE have been synthesized and may serve as templates for the development of clinically useful anti-arrhythmic agents [72].
7. Conclusions and future directions
Recent findings in animal models indicate that the EETs synthesized from AA have potentially beneficial angiogenic, anti-inflammatory and cardioprotective effects in addition to their well-known actions on vascular and renal function. Likewise, ω-3 PUFA epoxides synthesized from EPA protect against cardiac arrhythmias, and those synthesized from DHA reduce inflammatory and neuropathic pain and prevent tumor metastasis. Translational studies are needed to determine whether these effects of PUFA epoxides are functionally important in humans. Additional studies also are needed to identify the one or more putative EET receptors and to determine whether all of the actions of PUFA epoxides are mediated by receptor-dependent signaling mechanisms. The potent selective sEH inhibitors and stable epoxide bioisosteric analogues now available provide new pharmacological approaches for the treatment of many serious diseases. Furthermore, the possibility of increasing the tissue levels of ω-3 PUFA epoxides to protect against cardiac arrhythmias and tumor metastasis offers a new paradigm for the treatment of heart disease and cancer. The challenge for translational research is to determine whether these pharmacological and dietary interventions targeted to PUFA epoxides actually will produce beneficial outcomes for human health.
Highlights.
PUFA are oxidized to PUFA epoxides by cytochrome P450 epoxygenases
PUFA epoxides function as mediators in the cardiovascular, renal and nervous system
PUFA epoxides activate receptor-mediated signaling pathways and ion channels
Soluble epoxide hydrolase inactivates PUFA epoxides by hydrolysis to PUFA diols
Inhibition of soluble epoxide hydrolase potentiates the action of PUFA epoxides
Footnotes
Abbreviations: CYP, cytochrome P450; AA, arachidonic acid; EET, epoxyeicosatrienoic acid, LA, linoleic acid; EpOME, epoxyoctadecenoic acid; EPA, eicosapentaenoic acid; EpETE, epoxyeicosatetraenoic acid; DHA, docosahexaenoic acid; EpDPE, epoxydocosapentaenoic acid; PLA2, phospholipase A2; BKCa, large-conductance Ca2+-activated K+ channels; sEH, soluble epoxide hydrolase; DHET, dihydroxyeicosatrienoic acid; FABP, cytosolic fatty acid binding protein; EH3, epoxide hydrolase ABHD9; AUDA, 12-(3-adamantan-1-yl-ureido)dodecanoic acid; t-AUCB, trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid; TUPS, 1-(1-methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)urea; 8-HUDE, 12-(3-hexylureido) dodec-8-enoic acid; MS-PPOH, N-methylsulfonyl-6-(2-proparglyoxylphenyl)hexanamide; 14,15-EEZE, 14,15-epoxyeicosa-5(Z)-enoic acid; PPAR, peroxisome proliferator-activated receptor; PKA, protein kinase A; TRP, transient receptor potential channel; ENaC, epithelial sodium channel; VACC, volume-activated chloride channel; KATP, ATP-dependent potassium channel
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation: molecular and functional properties of arachidonic acid monooxygenases. J Lipid Res. 2000;41:163–181. [PubMed] [Google Scholar]
- 2.Oliw EH. Oxygenation of polyunsaturated fatty acids by cytochrome P450 monooxygenases. Prog Lipid Res. 1994;33:329–354. doi: 10.1016/0163-7827(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 3.Roman R. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 4.Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension. 2006;47:629–633. doi: 10.1161/01.HYP.0000208597.87957.89. [DOI] [PubMed] [Google Scholar]
- 5.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfister SL, Gauthier KM, Campbell WB. Vascular pharmacology of epoxyeicosatrienoic acids. Adv Pharmacol. 2010;60:27–59. doi: 10.1016/B978-0-12-385061-4.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeldin DC. Epoxygenase pathways of fatty acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 9.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 10.Fleming I. Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostagl other Lipid Mediat. 2007;82:60–67. doi: 10.1016/j.prostaglandins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Larsen BT, Campbell WB, Gutterman DD. Beyond vasodilation: non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol Sci. 2007;28:32–38. doi: 10.1016/j.tips.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Deng Y, Theken KN, Lee CR. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J Mol Cell Cardiol. 2010;48:331–341. doi: 10.1016/j.yjmcc.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nithipatikom K, Gross GR. Epoxyeicosatrienoic acids: Novel mediators of cardioprotection. J Cardiovsasc Pharmacol Ther. 2010;15:112–119. doi: 10.1177/1074248409358408. [DOI] [PubMed] [Google Scholar]
- 14.Wagner K, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition, epoxygenated fatty acids and nociception. Prostagl other Lipid Mediat. 2011;96:78–83. doi: 10.1016/j.prostaglandins.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Kodani S, Hammock BD. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog Lipid Res. 2014;53:108–123. doi: 10.1016/j.plipres.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morisseau C, Inceoglu B, Schmelzer K, Tsai H-J, Jinks SL, Hegedus CM, et al. Naturally occurring monoexoxides of eicosapentaenoic acid and docosahexaenoic acid are bioselective antihyperalgesic lipids. J Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westphal C, Konkel A, Schunck W-H. CYP-eicosanoids a new link between between omega-3 fatty acids and cardiac disease? Prostagl other Lipid Mediat. 2011;96:99–108. doi: 10.1016/j.prostaglandins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu J-Y, Lee KSS, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci USA. 2013;110:6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Potentiation of endothelium-dependent relaxation by epoxyeicosatrienoic acids. Circ Res. 1997;81:258–267. doi: 10.1161/01.res.81.2.258. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, et al. The anti-inflammatory effect of laminar flow: the role of PPARγ, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 22.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 23.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, et al. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K+ channel activity. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;270:F822–F832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]
- 24.Lu T, VanRollins M, Lee H-C. Stereospecific activation of cardiac ATP-sensitive K+ channels by epoxyeicosatrienoic acids: a structural determinant study. Mol Pharmacol. 2002;62:1076–1083. doi: 10.1124/mol.62.5.1076. [DOI] [PubMed] [Google Scholar]
- 25.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nature Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010;51:2074–2081. doi: 10.1194/jlr.M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanRollins M, Baker RC, Sprecher HW, Murphy RC. Oxidation of docosahexaenoic acid by rat liver microsomes. J Biol Chem. 1984;259:5776–5783. [PubMed] [Google Scholar]
- 28.Fer M, Dréano Y, Lucas D, Corcos J-P, Salaün L, Berthou F, et al. Metabolism of eicosapentaenoic and docosahexaenoic acid by recombinant cytochromes P450. Arch Biochem Biophys. 2008;471:116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Lucas D, Goulitquer S, Marienhagen J, Fer M, Dreano Y, Schwaneberg U, et al. Stereoselective epoxydation of the last double bond of polyunsaturated fatty acids by human cytochromes P450. J Lipid Res. 2010;51:1125–1133. doi: 10.1194/jlr.M003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω-3 fatty acids. J Biol Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanRollins M. Epoxygenase metabolites of docosahexaenoic and eicosapentaenoic acids inhibit platelet aggregation at concentrations below those affecting thromboxane synthesis. J Pharmacol Exp Therap. 1995;274:798–804. [PubMed] [Google Scholar]
- 32.Widstrom RL, Norris AW, Spector AA. Binding of cytochrome P450 monooxygenase and lipooxygenase pathway products by heart fatty acid-binding protein. Biochemistry. 2001;40:1070–1076. doi: 10.1021/bi001602y. [DOI] [PubMed] [Google Scholar]
- 33.Widstrom RL, Norris AW, van der Veer J, Spector AA. Fatty acid-binding proteins inhibit hydration of epoxyeicosatrienoic acids by soluble epoxide hydrolase. Biochemistry. 2003;42:11762–11767. doi: 10.1021/bi034971d. [DOI] [PubMed] [Google Scholar]
- 34.Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostagl Leukot Essent Fatty Acids. 2008;79:215–222. doi: 10.1016/j.plefa.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klett EL, Chen S, Edin ML, Li LO, Ilkayeva O, Zeldin DC, Newgard CB, Coleman RA. Diminished acyl-CoA synthetase isoform 4 activity in INS 832/13 cells reduces cellular epoxyeicosatrienoic acid levels and results in impaired glucose-stimulated insulin secretion. J Biol Chem. 2013;288:21618–21629. doi: 10.1074/jbc.M113.481077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, Capdevila JH, Zeldin DC, Rosenberg RL. Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Mol Pharmacol. 1999;55:288–295. doi: 10.1124/mol.55.2.288. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Chen J-K, Falck JR, Guthi JS, Anjaiah S, Capdevila JH, et al. Mitogenic activity and signaling mechanism of 2-(14,15-epoxyeicosatrienoyl)glycerol, a novel cytochrome p450 arachidonate metabolite. Mol Cell Biol. 2007;27:3023–3034. doi: 10.1128/MCB.01482-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Chen J-K, Imig JD, Wei S, Hackney DL, Guthi JS, et al. Identification of novel endogenous cytochrome p450 arachidonate metabolites with high affinity for cannabinoid receptors. J Biol Chem. 2008;283:24514–24524. doi: 10.1074/jbc.M709873200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman JW, Morriseau C, Hammock BD. Epoxide hydrolases: their role and interactions with lipid metabolism. Prog Lipid res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Marowsky A, Burgener J, Falck JR, Arand MA. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience. 2009;163:646–661. doi: 10.1016/j.neuroscience.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 41.Rawal S, Morriseau C, Hammock BD, Shivachar AC. Differential subcellular distribution and colocalization of the microsomal and soluble epoxide hydrolases in cultured neonatal rat brain cortical astrocytes. J Neurosci Res. 2009;87:218–227. doi: 10.1002/jnr.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bianco K, Agassandian RA, Cassell MD, Spector AA, Sigmund CD. Characterization of transgenic mice with neuron-specific expression of soluble epoxide hydrolase. Brain Res. 2009;1291:60–72. doi: 10.1016/j.brainres.2009.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson JW, Subrahmanyan RM, Summers SA, Xiao X, Alkayed NJ. Soluble epoxide hydrolase dimerization is required for hydrolase activity. J Biol Chem. 2013;288:7697–7703. doi: 10.1074/jbc.M112.429258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cronin A, Mowbray S, Dürk H, Homberg S, Fleming I, Fisslthaler B, et al. The N-terminal domain of mammalian soluble epoxide hydrolase is a phosphatase. Proc Natl Acad Sci USA. 2003;100:1552–1557. doi: 10.1073/pnas.0437829100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman JW, Morriseau C, Harris TR, Hammock BD. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with a novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA. 2003;100:1558–1563. doi: 10.1073/pnas.0437724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enayetallah AE, Grant DF. Effects of human soluble epoxide hydrolase polymorthisms on isoprenoid phosphate hydrolysis. Biochem Biophys Res Commun. 2006;341:254–260. doi: 10.1016/j.bbrc.2005.12.180. [DOI] [PubMed] [Google Scholar]
- 47.Oguro A, Imaoka S. Lysophosphatidic acids are new substrates for the phosphatase domain of soluble epoxide hydrolase. J Lipid Res. 2012;53:505–512. doi: 10.1194/jlr.M022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oguro A, Sakamoto K, Suzuki S, Imaoka S. Contribution of the hydrolase and phosphatase domains in soluble epoxide hydrolase to vascular endothelial growth factor expression and cell growth. Biol Pharm Bull. 2009;32:1962–1967. doi: 10.1248/bpb.32.1962. [DOI] [PubMed] [Google Scholar]
- 49.Zeldin DC, Wei S, Falck JR, Hammock BD, Snapper JR, Capdevila JH. Metabolism of epoxyeicosatrienoic acids by cytosolic epoxide hydrolase: substrate structural determinants of asymmetric catalysis. Arch Biochem Biophys. 1995;316:443–451. doi: 10.1006/abbi.1995.1059. [DOI] [PubMed] [Google Scholar]
- 50.VanRollins M, Kaduce TL, Fang X, Knapp HR, Spector AA. Arachidonic acid diols produced by cytochrome P-450 monooxygenase are incorporated into phospholipids of vascular endothelial cells. J Biol Chem. 1996;271:14001–14009. doi: 10.1074/jbc.271.24.14001. [DOI] [PubMed] [Google Scholar]
- 51.Inceoglu B, Wagner KM, Yang J, Battaieb A, Schebb NH, Hwang SHC, et al. Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model if type 1 diabetes. Proc Natl Acad Sci USA. 2012;109:11390–11395. doi: 10.1073/pnas.1208708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruins MJ, Dane AD, Strassburg K, Vreeken RJ, Newman JW, Salem N, Jr, et al. Plasma oxylipin profiling identifies polyunsaturated vicinal diols as responsive to arachidonic acid and docosahexaenoic acid intake in growing piglets. J Lipid Res. 2013;54:1598–1607. doi: 10.1194/jlr.M034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Decker M, Adamska M, Cronin A, Di Giallonardo F, Burgener J, Markowsky A, et al. EH3 (ABHD9): the first member of a new epoxide hydrolase family with high activity for fatty acid epoxides. J Lipid Res. 2012;53:2038–2045. doi: 10.1194/jlr.M024448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50:S52–S56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiamvimonvat N, Ho C-M, Tsai H-J, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50:225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 56.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular disease. Nature Rev Drug Disc. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Revermann M. Pharmacologic inhibition of the soluble epoxide hydrolase. Curr Op Pharmacol. 2010;10:173–178. doi: 10.1016/j.coph.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 58I.Kim H, Tsai HJ, Nishi K, Kasagami T, Morisseau C, Hammock BD. 1.3-Disubstituted ureas functionalized with ether groups are potent inhibitors of soluble epoxide hydrolase with improved pharmacokinetic properties. J Med Chem. 2007;50:5217–5226. doi: 10.1021/jm070705c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tran KL, Aronov PA, Tanaka HJ, Newman W, Hammock BD, Morisseau C. Lipid sulfates and sulfonates are allosteric competitive inhibitors of the N-terminal phosphatase activity of the mammalian of soluble epoxide hydrolase. Biochemistry. 2005;44:12179–12187. doi: 10.1021/bi050842g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang SHH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable and potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falck JR, Kodela R, Manne R, Atcha KR, Puli N, Dubasi N, et al. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: Influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem. 2009;52:5069–5075. doi: 10.1021/jm900634w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Wang R, Li J, Rao J, Li W, Falck JR, et al. Stable EET urea agonist and soluble epoxide hydrolase inhibitor regulate rat pulmonary arteries through TRPCs. Hypertens Res. 2011;34:630–638. doi: 10.1038/hr.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.la Buscató E, Blöcher RB, Lamers C, Klingler F-M, Hahn S, Steinhilber D, et al. Design and synthesis of dual modulators of soluble epoxide hydrolase and peroxisome proliferator-activated receptors. J Med Chem. 2012;55:10771–10775. doi: 10.1021/jm301194c. [DOI] [PubMed] [Google Scholar]
- 64.Meirer K, Rödl CH, Wisniewska JM, George S, Häfner AK, la Buscató E, et al. Synthesis and structural activity relationship studies of novel dual inhibitors of soluble epoxide hydrolase and 5-lipoxygenase. J Med Chem. 2013;56:1777–1781. doi: 10.1021/jm301617j. [DOI] [PubMed] [Google Scholar]
- 65.Hwang SH, Wecksler AT, Zhang G, Morisseau C, Nguyen LV, Fu SH, Hammock BD. Synthesis and biological evaluation of sorafenib- and regorafenib-like soluble epoxide hydrolase inhibitors. Biorg Med Chem Lett. 2013;23:3732–3737. doi: 10.1016/j.bmcl.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Batchu SN, Lee SB, Samokhvalov V, Chaudhary KR, El-Sikhry H, Weldon SM, Seubert JM. Novel soluble epoxide hydrolase inhibitor protects mitochondrial function following stress. Can J Physiol Pharmacol. 2012;90:811–823. doi: 10.1139/y2012-082. [DOI] [PubMed] [Google Scholar]
- 67.Morisseau C, Pakhomova S, Hwang SH, Newcomer ME, Hammock BD. Inhibition of soluble epoxide hydrolase by fulvestrant and sulfoxides. Biorg Med Chem Lett. 2013;23:3818–3821. doi: 10.1016/j.bmcl.2013.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hwang SH, Wagner KM, Morisseau C, Liu JY, Dong H, Wecksler AT, Hammock BD. Synthesis and structure-activity relationship studiesof urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J Med Chem. 2011;54:3037–3050. doi: 10.1021/jm2001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang X, Hu S, Watanabe T, Weintraub NL, Snyder GD, Yao J, et al. Activation of peroxisome proliferator-activated receptor α by substituted urea-derived soluble epoxide hydrolase inhibitors. J Pharmacol Exp Therap. 2005;314:260–270. doi: 10.1124/jpet.105.085605. [DOI] [PubMed] [Google Scholar]
- 70.Brand-Schrieber E, Falck JR, Schwartzman M. Selective inhibition of arachidonic acid epoxygenation in vivo. J Physiol Pharmacol. 2000;51:655–672. [PubMed] [Google Scholar]
- 71.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlena M, Falck JF, et al. 14,15- Epoxyeicosa-5(Z)enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 72.Falck JR, Wallukat G, Puli N, Goli M, Arnold C, Konkel A, Rothe M, Fischer R, Müller DN, Schunck W-H. 17(R),18(S)-Epoxyeicosatetraenoic acid, a potent eicosapentaenoic acid (EPA) derived regulator of cardiomyocyte contraction: Structure-activity relationships and stable analogues. J Med Chem. 2011;54:4109–4118. doi: 10.1021/jm200132q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bukhari IA, Gauthier KM, Jagadeesh SG, Sangras B, Falck JR, Campbell WB. 14,15-Dihydroxy-eicosa-5(Z)-enoic acid selectively inhibits 14,15-epoxyeicosatrienoic acid-induced relaxations of bovine coronary arteries. J Pharmacol Exp Therap. 2011;336:47–55. doi: 10.1124/jpet.110.169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bukhari IA, Shah AJ, Gauthier KM, Walsh KA, Koduru SR, Imig JD, et al. 11,12,20-Trihydroxy-eicosa-8(Z) enoic acid: a selective inhibitor of 11,12-EET-induced relaxations of bovine coronary and rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2012;302:H1574–H1583. doi: 10.1152/ajpheart.01122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sinal CJ, Myata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble hydroxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 76.Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 77.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, et al. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BKCa channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu T, Katakam PVG, VanRollins M, VanRollins NL, Spector AA, Lee HC. Dihydroxyeicosatrienoic acids are potent activators of Ca++-activated K+ channels in isolated rat coronary arterial myocytes. J Physiol. 2001;534:651–667. doi: 10.1111/j.1469-7793.2001.t01-1-00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang X, Hu S, Xu B, Snyder GD, Harmon S, Yao J, et al. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor α. Am J Physiol Heart Circ Physiol. 2006;290:H55–H63. doi: 10.1152/ajpheart.00427.2005. [DOI] [PubMed] [Google Scholar]
- 80.Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor α. Drug Metab Disp. 2007;35:1126–1134. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- 81.Abukhashim M, Wiebe GJ, Seubert JM. Regulation of forskolin-induced cAMP production by cytochrome P450 epoxygenase metabolites of arachidonic acid in HEK293 cells. Chem Biol Toxicol. 2011;27:321–332. doi: 10.1007/s10565-011-9190-x. [DOI] [PubMed] [Google Scholar]
- 82.Frömel T, Jungblut B, Hu J, Trouvain C, Barbosa-Sicard E, Popp R, et al. Soluble epoxide hydrolase regulates hematopoietic progenitor cell function via generation of fatty acid diols. Proc Natl Acad Sci USA. 2012;109:9995–10000. doi: 10.1073/pnas.1206493109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kundu S, Roome T, Bhattacharjee A, Carnevale KA, Yakubenko VP, Zhang R, et al. Metabolic products of soluble epoxide hydrolase are essential for monocytic chemotaxis to MCP-1 in vitro and in vivo. JLipid Res. 2013;54:436–447. doi: 10.1194/jlr.M031914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fang X, Weintraub NL, Oltman CL, Stoll LL, Kaduce TL, Harmon S, et al. Human coronary endothelial cells convert 14,15-EET to a biologically active chain-shortened epoxide. Am J Physiol Heart Circ Physiol. 2002;283:H2306–H2314. doi: 10.1152/ajpheart.00448.2002. [DOI] [PubMed] [Google Scholar]
- 85.Yi XY, Gauthier KM, Cui K, Nithipatikom K, Falck JR, Campbell WB. Metabolism of adrenic acid to vasodilatory 1α,1β-dihomo-epoxyeicosatrienoic acids by bovine coronary arteries. Am J Physiol Heart Circ Physiol. 2007;292:H2265–H2274. doi: 10.1152/ajpheart.00947.2006. [DOI] [PubMed] [Google Scholar]
- 86.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, et al. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem. 2001;276:14867–14874. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- 87.Fang X, Weintraub NL, McCaw RB, Hu S, Harmon S, Rice JB, et al. Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol. 2004;287:H2412–H2420. doi: 10.1152/ajpheart.00527.2004. [DOI] [PubMed] [Google Scholar]
- 88.Cowart LA, Wei S, Hsu MH, Johnson EF, Krishna MU, Falck JR, et al. The CYP4A isoforms hydroxylate epoxyeicosatrienoic acids to form high affinity peroxisome proliferator-activate receptor ligands. J Biol Chem. 2002;277:35105–35112. doi: 10.1074/jbc.M201575200. [DOI] [PubMed] [Google Scholar]
- 89.Carroll MA, Balazy M, Margiotta P, Falck JR, McGiff JC. Renal vasodilator activity of 5,6- epoxyeicosatrienoic acid depends upon conversion by cyclooxygenase and release of prostaglandins. J Biol Chem. 1993;268:12260–12266. [PubMed] [Google Scholar]
- 90.Snider NT, Kornilov RM, Kent UM, Hollenberg PF. Anamdamide metabolism by human liver and kidney microsomal cytochrome P450 enzymes to form hydroxyeicosatetraenoic and epoxyeicosatrienoic acid ethanolamides. J Pharmacol Exp Therap. 2007;321:590–597. doi: 10.1124/jpet.107.119321. [DOI] [PubMed] [Google Scholar]
- 91.Sridar C, Snider NT, Hollenberg PM. Anandamide oxidation by wild-type and polymorphically expressed CYP2B6 and CYP2D6. Drug Metab Disp. 2011;39:782–788. doi: 10.1124/dmd.110.036707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Snider NT, Walker VJ, Hollenberg PF. Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: Physiological and pharmacological implications. Pharmacol Rev. 2010;62:136–154. doi: 10.1124/pr.109.001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim H-Y, Moon H-S, Cao D, Lee J, Kevala K, Jun SB, et al. N-docosylhexaenoylethanolamide promotes development of hippocampal neurons. Biochem J. 2011;435:327–336. doi: 10.1042/BJ20102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rashid MA, Katakura M, Khavebava G, Kim H-Y. N- docosylhexaenoylethanolamine is a potent neurogenic factor for neural stem cell differentiation. J Neurochem. 2013;125:869–884. doi: 10.1111/jnc.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang R, Fredman G, Krishnamoorthy S, Agrawal N, Irimia D, Piomelli D, et al. Decoding functional metabolomics with docosahexaenoylethanolamide (DHEA) identifies novel bioactive signals. J Biol Chem. 2011;286:31532–31541. doi: 10.1074/jbc.M111.237990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li PL, Chen CL, Bortell R, Campbell WB. 11,12-Epoxyeicosatrienoic acid stimulates endogenous mono-ADP-ribosylation in bovine coronary arterial smooth muscle cells. Circ Res. 1999;85:349–356. doi: 10.1161/01.res.85.4.349. [DOI] [PubMed] [Google Scholar]
- 97.Carroll MA, Doumond AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine 2A receptor vadodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol. 2006;291:F155–F161. doi: 10.1152/ajprenal.00231.2005. [DOI] [PubMed] [Google Scholar]
- 98.Lu T, Hoshi T, Weintraub NL, Spector AA, Lee HC. Activation of ATP-sensitive K+ channels by epoxyeicosatrienoic acids in rat cardiac ventricular myocytes. J Physiol. 2001;537:821–827. doi: 10.1111/j.1469-7793.2001.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TPRV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 100.Wong PY, Lai PS, Falck JR. Mechanism and signal transduction of 14(R),15(S)- epoxyeicosatrienoic acid (14,15-EET) binding in guinea pig monocytes. Prostagl other Lipid Mediat. 2000;62:321–333. doi: 10.1016/s0090-6980(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 101.Yang W, Holmes BB, Gopal VR, Krishna Hishore RV, Sangras B, Yi X-Y, et al. Characterization of 14,15-epoxyeicosatrienoyl-sulfonamides 14,15-epoxyeicosatrienoic acid agonists: use of studies of metabolism and ligand binding. J Pharmacol Exp Therap. 2007;321:1023–1031. doi: 10.1124/jpet.107.119651. [DOI] [PubMed] [Google Scholar]
- 102.Snyder GD, Murali Krishna U, Falck JR, Spector AA. Evidence for a membrane site of action for 14,15-EET on expression of aromatase in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282:H1936–H1942. doi: 10.1152/ajpheart.00321.2002. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y, Falck JR, Manthati VL, Jat JL, Campbell WB. 20-Iodo-14,15-epoxyeicosa-8(Z)-enoyl-3-azidophenylsulfonamide: photoaffinity labeling of a 14,15-epoxyeicosatrienoic acid receptor. Biochemistry. 2011;50:3840–3848. doi: 10.1021/bi102070w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang C, Kwan Y-W, Au AL-S, Poon AC-W, Zhang Q, Chan S-W, et al. 14,15- Epoxyeicosatrienoic acid induces vasorelaxation through prostaglandin EP2 receptors in rat mesenteric arteries. Prostagl other Lipid Mediat. 2010;93:44–51. doi: 10.1016/j.prostaglandins.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J Pharmacol Exp Therap. 2009;328:231–239. doi: 10.1124/jpet.108.145102. [DOI] [PubMed] [Google Scholar]
- 106.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostagl Other Lipid Mediat. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y, Oltman CL, Lu T, Lee H-C, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BKCa channels. Am J Physiol Heart Circ Physiol. 2001;280:H2430–H2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]
- 108.Wang R, Chai Q, Lu T, Lee H-C. Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc Res. 2011;90:344–352. doi: 10.1093/cvr/cvq411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ye D, Zhang D, Oltman C, Dellsperger K, Lee H-C, VanRollins M. Cytochrome P-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large- conductance calcium-activated potassium channels. J Pharmacol Exp Therap. 2002;303:768–776. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- 110.Morin C, Sirois M, Echave V, Rizcallah E, Rousseau E. Relaxing effects of 17(18)-EpETE on arterial and airway smooth muscles in human lung. Am J Physiol Lung Cell Mol Physiol. 2009;296:L130–L139. doi: 10.1152/ajplung.90436.2008. [DOI] [PubMed] [Google Scholar]
- 111.Lauterbach B, Barbosa-Sicard E, Wang M-H, Honeck H, Kargel E, Theuer J, et al. Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension. 2002;39:609–613. doi: 10.1161/hy0202.103293. [DOI] [PubMed] [Google Scholar]
- 112.Hercule HC, Salanova B, Essin K, Honeck H, Falck JR, Sausbier M, et al. The vasodilator 17,18- epoxyeicosatetraenoic acid targets the pore-forming BKα channel subunit in rodents. Exp Physiol. 2007;92:1067–1076. doi: 10.1113/expphysiol.2007.038166. [DOI] [PubMed] [Google Scholar]
- 113.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, et al. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 114.Early J. Cerebral artery dilation mediated by transient receptor potential and KCa channels. J Cardiovas Pharmacol. 2011;57:148–153. doi: 10.1097/FJC.0b013e3181f580d9. [DOI] [PubMed] [Google Scholar]
- 115.Fleming I, Rueben A, Popp A, Fisslthaler B, Schrodt S, Sander A, et al. Epoxyeicosatrienoic acids regulate TRP channel-dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscl Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 116.Loot AE, Fleming I. Cytochrome P450-derived epoxyeicosatrienoic acids and pulmonary hypertension: central role of transient receptor potential C6 channels. J Cardiovasc Res. 2011;57:140–147. doi: 10.1097/FJC.0b013e3181ed088d. [DOI] [PubMed] [Google Scholar]
- 117.Fang X, Weintraub NL, Stoll LL, Spector AA. Epoxyeicosatrienoic acids increase intracellular calcium concentration in vascular smooth muscle cells. Hypertension. 1999;34:1242–1246. doi: 10.1161/01.hyp.34.6.1242. [DOI] [PubMed] [Google Scholar]
- 118.Lee H-C, Lu T, Weintraub NL, VanRollins M, Spector AA, Shibata EF. Effects of epoxyeicosatrienoic acids on sodium channels in isolated rat ventricular myocytes. J Physiol. 1999;519:153–168. doi: 10.1111/j.1469-7793.1999.0153o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pavlov TS, Ilatovskaya DV, Levchenko V, Mattson DL, Roman RJ, Staruschenko A. Effects of cytochrome P-450 metabolites of arachidonic acid on epithelial sodium channel (ENaC) Am J Physiol Renal Physiol. 2011;301:F672–F681. doi: 10.1152/ajprenal.00597.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pidkovka N, Rao R, Mei S, Gong Y, Harris RC, Schunck W-H, et al. Epoxyeicosatrienoic acids (EETs) regulate epithelial sodium channel activity by extracellular signal-regulated kinase ½ (ERK1/2)-mediated phosphorylation. J Biol Chem. 2013;288:5223–5231. doi: 10.1074/jbc.M112.407981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ulu A, Harris TR, Morisseau C, Miyabi C, Inoue H, Schuster G, et al. Anti-inflammatory effects of ω-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol. 2013;62:285–297. doi: 10.1097/FJC.0b013e318298e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu T, Ye D, Wang X, Seubert JM, Graves JP, Bradbury JA, et al. Cardiac and vascular KATP channels in rats are activated by endogenous epoxyeicosatrienoic acids through different mechanisms. J Physiol. 2006;575:627–644. doi: 10.1113/jphysiol.2006.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang C, Kwan YW, Seto SW, Leung GHP. Inhibitory effects of epoxyeicosatrienoic acids on volume-activated chloride channels in rat mesenteric arterial smooth muscle. Prostagl Other Lipid Mediat. 2008;87:62–67. doi: 10.1016/j.prostaglandins.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 124.Munzenmaier DH, Harder DR. Cerebral microvascular endothelial tube formation: role of astrocyte epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278:H1163–H1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- 125.Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic acid. Stroke. 2002;33:2957–2964. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- 126.Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, et al. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H215–H224. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- 127.Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–29625. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- 128.Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial migration and angiogenesis. J Cell Sci. 2005;118:5489–5498. doi: 10.1242/jcs.02674. [DOI] [PubMed] [Google Scholar]
- 129.Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, et al. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesisi via mitogen-activated protein kinase and phosphatidylinositol-3-kinase/Akt signaling pathways. J Pharmacol Exp Therap. 2005;314:522–532. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- 130.Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Aent R, et al. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- 131.Ma J, Zhang L, Han W, Shen T, Ma C, Liu Y, et al. Activation of JNK/cJun is required for the proliferation, survival and angiogenesis induced by EET in pulmonary artery endothelial cells. J Lipid Res. 2012;53:1093–1105. doi: 10.1194/jlr.M024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan C, Chen S, You B, Sun J. Activation of sphingosine kinase-1 mediates induction of endothelial cell proliferation and angiogenesis by epoxyeicosatrienoic acids. Cardiovasc Res. 2008;78:308–314. doi: 10.1093/cvr/cvn006. [DOI] [PubMed] [Google Scholar]
- 133.Webber AC, Popp R, Thomas Korff U, Mechaelis R, Urbich C, Busse R, et al. Cytochrome P450 2C9-induced angiogenesis is dependent on EphB4. Arterioscler Thromb Vasc Biol. 2008;28:1123–1129. doi: 10.1161/ATVBAHA.107.161190. [DOI] [PubMed] [Google Scholar]
- 134.Zhang B, Cao H, Rao GN. Fibroblast growth factor-2 is a downstream mediator of phosphatidylinositol 3-kinase-Akt in 14,15- epoxyeicosatrienoic acid-induced angiogenesis. J Biol Chem. 2006;281:905–914. doi: 10.1074/jbc.M503945200. [DOI] [PubMed] [Google Scholar]
- 135.Chenova SY, Karpurapu M, Wang D, Zhang B, Venema RC, Rao GN. An essential role for Src-activated STAT-3 in 14,15-EET-induced VEGF expression and angiogenesis. Blood. 2008;111:5581–5591. doi: 10.1182/blood-2007-11-126680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sander AL, Jakob H, Sommer K, Sadler C, Fleming I, Marzi I, et al. Cytochrome P450-derived epoxyeicosatrienoic acids accelerate wound epithelialization and neovascularization in the hairless mouse ear wound model. Langerbecks Arch Surg. 2011;396:1245–1253. doi: 10.1007/s00423-011-0838-z. [DOI] [PubMed] [Google Scholar]
- 137.Cui PH, Petrovic N, Murray M. The ω-3 epoxide of eicosapentaenoic acid inhibits endothelial cell proliferation by p38 MAP kinase activation and cyclin D1/CDK4 down-regulation. Br J Pharmacol. 2011;162:1143–1155. doi: 10.1111/j.1476-5381.2010.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. EET signaling in cancer. Cancer Metastasis Rev. 2010;30:525–540. doi: 10.1007/s10555-011-9315-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest. 2012;122:178–191. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen C, Wei X, Wu J, Yang S, Chen F, Ma D, et al. Cytochrome P450 2J2 is highly expressed in hematologic malignant diseases and promotes tumor cell growth. J Pharmacol Exp Therap. 2011;336:344–355. doi: 10.1124/jpet.110.174805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nithipatikom K, Brody DM, Tang AT, Mathati VJ, Williams CL, Campbell WB. Inhibition of carcinoma cell motility by epoxyeicosatrienoic acid (EET) antagonists. Cancer Sci. 2010;101:2629–2636. doi: 10.1111/j.1349-7006.2010.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mitra R, Milani M, Mesaros C, Rodriguez M, Nguyen J, Luo X, et al. Cyp3A4 mediates growth of estrogen receptor-positive breast cancer cells in part by inducing nuclear translocation of phosphor-Stat3 through biosynthesis of (±)-14,15-epoxyeicosatrienoic acid (14,15-EET) J Biol Chem. 2011;286:17543–17559. doi: 10.1074/jbc.M110.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Panigrahy D, Kalish BT, Huang S, Bielenberg DR, Le HD, Yang J, et al. Epoxyeicosanoids promote organ and tissue regeneration. Proc Natl Acad Sci USA. 2013;110:13528–13533. doi: 10.1073/pnas.1311565110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Node K, Huo Y, Ruan X, Yang Y, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Deng Y, Edin ML, Theken KN, Schuck RN, Flake GP, Kannon MA, et al. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011;25:703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gao Y, Feng J, Ma K, Zhou Z, Zhu Y, Xu Q, et al. 8,9-Epoxyeicosatrienoic acid inhibits antibody production of B-lymphocytes in mice. PLoS One. 2012;7:e40258. doi: 10.1371/journal.pone.0040258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jiang J-X, Zhang S-J, Liu Y-N, Lin XX, Sun YH, Shen HJ, et al. EETs alleviate ox-LDL-induced inflammation by inhibiting LOX-1 receptor expression in rat pulmonasry arterial endothelial cells. Eur J Pharmacol. 2014;727:43–51. doi: 10.1016/j.ejphar.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 148.Zhao G, Wang J, Xu X, Jing Y, Tu L, Li X, et al. Epoxyeicosatrienoic acids protect rat hearts against tumor necrosis factor-α-induced injury. J Lipid Res. 2012;53:456–466. doi: 10.1194/jlr.M017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Morin C, Sirois M, Echave V, Albadine R, Rousseau E. 17,18-Epoxyeicosatetraenoic acid targets PPARγ and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung. Am J Resp Cell Mol Biol. 2010;43:564–575. doi: 10.1165/rcmb.2009-0155OC. [DOI] [PubMed] [Google Scholar]
- 150.Khan MAH, Necka J, Manthati V, Errabelli R, Pavlov TS, Staruschenko A, et al. Orally active epoxyeicosatrienoic acid analog attenuates kidney injury in hypertensive Dahl salt-sensitive rat. Hypertension. 2013;62:905–913. doi: 10.1161/HYPERTENSIONAHA.113.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bettaieb A, Nagata N, AbouBechara D, Chahed S, Morisseau C, Hammock BD, Haj FG. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem. 2013;288:14189–14199. doi: 10.1074/jbc.M113.458414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Luria A, Battaieb A, Xi Y, Shieh G-J, Liu H-C, Inoue H, et al. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA. 2011;108:9038–9043. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, Alkayed NJ. Epoxyeicosanoid signaling in CNS function and disease. Prostgl Other Lipid Mediat. 2010;91:68–84. doi: 10.1016/j.prostaglandins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Abdu E, Bruun DA, Yang D, Inceoglu B, Hammock BD, Alkayed NJ, et al. Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J Neurochem. 2011;117:632–642. doi: 10.1111/j.1471-4159.2010.07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu X, Li C, Gebremedhin D, Hwang SH, Harder BD, Falck JL, Harder DR, Koehler RC. Epoxyeicosatrienoic acid-dependent cerebral vasodilation evoked by metabotropic glutamate receptor activation in vivo. Am J Physiol Heart Circ Physiol. 2011;301:H373–H381. doi: 10.1152/ajpheart.00745.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Higashimori H, Blanco VM, Tuniki VR, Falck JR, Filosa JA. Role of epoxyeicosatrienoic acids as autocrine metabolites in glutamate-mediated K+ signaling in perivascular astrocytes. Am J Physiol Cell Physiol. 2010;299:C1068–C1078. doi: 10.1152/ajpcell.00225.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Terashvili M, Tseng LT, Wu H, Narayanan J, Hart LM, Falck JR, et al. Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of β-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Therap. 2008;326:614–622. doi: 10.1124/jpet.108.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Terashvili M, Sarkar P, Nostrand MV, Falck JR, Harder BD. The protective effect of astrocyte-derived 14,15-epoxyeicosatrienoic acid on hydrogen peroxide-induced cell injury in astrocyte-dopaminergic neuronal cell line co-culture. Neurosci. 2012;223:68–76. doi: 10.1016/j.neuroscience.2012.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Inceoglu B, Zolkowska D, Yoo HJ, Wagner KM, Yang J, Hackett E, et al. Epoxy fatty acids and inhibition of soluble epoxide hydrolase selectively modulate GABA neurotransmission to delay onset of seizures. PLoS One. 2013;8:e80922. doi: 10.1371/journal.pone.0080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesics pathways. Proc Natl Acad Sci USA. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gross GR, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, et al. Effect of the selective EET antagonist 14,15-EEZE on cardioprotection produced by exogenous or endogenous epoxyeicosatrienoic acids in the canine heart. Am J Physiol Heart Circ Physiol. 2008;294:H2838–H2844. doi: 10.1152/ajpheart.00186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gross GR, Gauthier KM, Moore J, Campbell WB, Falck JR, Nithipatikom K. Evidence for role of epoxyeicosatrienoic acids in mediating ischemic preconditioning and postconditioning in dog. Am J Physiol Heart Circ Physiol. 2009;297:H47–H52. doi: 10.1152/ajpheart.01084.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chaudhary KR, Abukhashim M, Hwang SH, Hammock BD, Seubert JM. Inhibition of soluble hydroxide hydrolase by trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is protective against ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2010;55:67–73. doi: 10.1097/FJC.0b013e3181c37d69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sirish P, Li N, Liu J-Y, Lee KSS, Hwang SHH, Qui H, et al. Unique mechanistic insights into the beneficial effects of soluble epoxide hydrolase inhibitors in the prevention of cardiac fibrosis. Proc Natl Acad Sci USA. 2013;110:5618–5623. doi: 10.1073/pnas.1221972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Katragadda D, Batchu SN, Cho WJ, Chaudhary KR, Falck JR, Seubert JM. Epoxyeicosatrienoic acids limit damage to mitochondrial function following stress in cardiac cells. J Mol Cell Cardiol. 2009;46:867–875. doi: 10.1016/j.yjmcc.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 166.Gross GR, Baker JE, Hsu A, Wu H, Falck JR, Nithipatikom K. Evidence for a role of opioids in epoxyeicosatrienoic acid-induced cardioprotection in rat hearts. Am J Physiol Heart Circ Physiol. 2010;298:H2201–H2207. doi: 10.1152/ajpheart.00815.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang Y, El-Sikhry H, Chaudhary KR, Batchu SN, Shaylgaupour A, Jukar TO, et al. Overexpression of CYP2J2 provides protection against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2009;297:H37–H46. doi: 10.1152/ajpheart.00983.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, et al. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathways. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 169.Edin ML, Wang Z, Bradbury JA, Graves JP, Lih FB, DeGraff LM, et al. Endothelial expression of human CYP epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 2011;25:3436–3447. doi: 10.1096/fj.11-188300. [DOI] [PMC free article] [PubMed] [Google Scholar]