Abstract
Objective
Fibroblast growth factor 21 (FGF21) is a hormone with pleiotropic metabolic activities which, in rodents, is robustly regulated by fasting and ketogenic diets. In contrast, similar dietary interventions have either no or minimal effects on circulating FGF21 in humans. Moreover, no intervention or dietary challenge has been shown to acutely stimulate circulating FGF21 in either humans or animals. Recent animal data suggest that the transcription factor Carbohydrate Responsive-Element Binding Protein (ChREBP) stimulates hepatic FGF21 expression and that fructose may activate hepatic ChREBP more robustly than glucose. Here, we examined whether fructose ingestion can acutely stimulate FGF21 in humans.
Methods
We measured serum FGF21, glucose, insulin, and triglyceride levels in ten lean, healthy adults and eleven adults with the metabolic syndrome following oral ingestion of 75 g of glucose, fructose, or a combination of the two sugars.
Results
FGF21 levels rose rapidly following fructose ingestion, achieved a mean 3.4-fold increase at two hours (P < 0.01), and returned to baseline levels within five hours. In contrast, FGF21 did not increase in the first two hours following ingestion of a glucose load, although more modest increases were observed after three to four hours. Both baseline and fructose-stimulated FGF21 levels were 2–3 fold elevated in subjects with metabolic syndrome.
Conclusions
Fructose ingestion acutely and robustly increases serum FGF21 levels in humans in a pattern consistent with a hormonal response. While FGF21 appears to be critical for the adaptive response to fasting or starvation in rodents, these findings suggest that in humans, FGF21 may play an important role in fructose metabolism.
Keywords: FGF21, Fructose, ChREBP, Metabolic syndrome
1. Introduction
Fibroblast growth factor 21 (FGF21) is a recently discovered hormone produced primarily by key metabolic tissues including the liver and adipose tissue [1–3] which has pleiotropic actions on glucose and lipid homeostasis [4]. Fasting or consumption of ketogenic diets markedly increases circulating FGF21 in rodent models [1,5], and FGF21 has been shown to play a key physiological role in the adaptation to starvation by enhancing hepatic fatty acid oxidation and ketogenesis [1,5,6]. Yet, fasting and ketogenic diets have little or no effect on circulating FGF21 in humans [3,7,8].
In both humans and animals, circulating levels of FGF21 are elevated in association with obesity, insulin resistance, hypertriglyceridemia, nonalcoholic fatty liver disease (NAFLD), and type 2 diabetes [3,9–12] and predict the development of the metabolic syndrome and type 2 diabetes independently of obesity [13]. FGF21 levels have also been reported to be highly elevated in humans with mitochondrial myopathies [14]. The mechanisms leading to increased FGF21 in association with cardiometabolic or mitochondrial disease are unknown. Moreover, to date no stimulus has been identified that can acutely increase serum FGF21 in either humans or animals on the time-scale of minutes to a few hours.
Recent evidence indicates that in rodents, FGF21 also responds to carbohydrate consumption and is regulated by the transcription factor Carbohydrate Responsive-Element Binding Protein (ChREBP) which is activated by products of carbohydrate metabolism [15,16]. However, acutely, an oral glucose challenge either produces no change or a decrease in circulating FGF21 [3,17]. Fructose, which is metabolized rapidly and preferentially over glucose in the liver [18], potently activates hepatic ChREBP in rodents [19]. This suggested the intriguing hypothesis that fructose ingestion might acutely stimulate production of FGF21.
We tested this hypothesis in humans by comparing the acute effects of fructose versus glucose ingestion on serum FGF21 levels. We found that fructose ingestion led to a rapid and robust increase in circulating FGF21 levels which peaked within two hours. Additionally, the FGF21 excursion following fructose was increased in subjects with metabolic syndrome. Fructose loading had no substantial effect on serum glucose or insulin levels. However, the FGF21 response to fructose correlated with indices of glucose intolerance and insulin resistance. We propose that the FGF21 response to a fructose challenge constitutes a “fructose tolerance test” and that in humans FGF21 may play an unanticipated role in fructose metabolism.
2. Materials and methods
2.1. Subjects
Subjects were recruited by advertisement. All study visits occurred in the Harvard Catalyst Clinical Research Center at the Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts between March 2012 and September 2013. Protocols were approved by the BIDMC Institutional Review Board (ClinicalTrials.gov Identifier: NCT00968747). Screening visits included a medical history, physical examination, and baseline laboratory tests. Inclusion criteria were age 18–60 years, BMI 19–38 kg/m2, no significant medical illness, and no medications except oral contraceptives. Subjects had no known history of fructose intolerance. Subjects were categorized as having the metabolic syndrome if they met criteria as defined by the National Cholesterol Education Program Adult Treatment Panel III [20].
2.2. Study protocol
After a 16 h overnight fast, subjects ingested one of the following three beverages: 75 g fructose, 75 g glucose, or a combination of 37.5 g of fructose and 37.5 g of glucose dissolved in 225 ml water. Carbohydrate beverage challenges occurred at least 2 weeks apart. Blood samples were collected every 30 min for 1 h and then every hour for 4 h after the challenge. Ten lean subjects and eleven subjects with metabolic syndrome underwent testing as described above. Four lean subjects had additional blood sampling at 90 and 150 min after fructose challenge to better define the dynamics of the FGF21 excursion following fructose ingestion.
2.3. Biochemical assays
Serum glucose, cholesterol, and triglyceride levels were analyzed in the BIDMC Clinical Laboratory according to standard procedures. Insulin samples were measured by radioimmunoassay (Millipore, Millipore Corp. Billerica, MA, USA). Serum FGF21 levels were measured using a commercially available enzyme-linked immunosorbent assay (BioVendor USA, Candler, NC) from blood collected in aprotinin-pretreated tubes. Blood samples were stored at −80 °C until use.
2.4. Calculations and statistical analyses
Data are presented as mean ± standard error. Post-ingestion hormone and metabolite values were compared to baseline values using a two-tailed paired t-test at each time point with Bonferroni correction for multiple comparisons. Comparisons of hormone and metabolite levels between lean subjects and those with metabolic syndrome were performed by two-tailed t-tests. Area under the curve (AUC) for hormones or metabolite was calculated by the trapezoidal method. Incremental area under the curve (iAUC) was calculated by subtracting the portion of AUC accounted for by the baseline metabolite or hormone level. Correlations were determined by univariate linear regression. Differences among iAUC were calculated using 2-way ANOVA and Tukey's post-hoc test.
3. Results
Baseline characteristics of the lean and metabolic syndrome study groups are summarized in Table 1. Subjects with metabolic syndrome were older and had increased BMI and waist circumference compared to lean counterparts. Metabolic syndrome subjects had lower HDL cholesterol, higher systolic blood pressure, and higher fasting and two hour glycemia during an oral glucose tolerance test. FGF21 levels were elevated in metabolic syndrome subjects consistent with prior reports [3].
Table 1.
Clinical data.
| Lean group | Metabolic syndrome group | |
|---|---|---|
| Subjects (M/F) | 10 (7/3) | 11 (6/5) |
| Age (Years) | 29 ± 2 | 49 ± 3‡ |
| Body mass index (kg/m2) | 25 ± 1 | 32 ± 1‡ |
| Waist circumference (cm) | 89 ± 2 | 108 ± 2† |
| Total cholesterol (mg/dl) | 176 ± 10 | 177 ± 9 |
| LDL cholesterol (mg/dl) | 98 ± 7 | 110 ± 9 |
| HDL cholesterol (mg/dl) | 59 ± 4 | 47 ± 3* |
| Triglycerides (mg/dl) | 94 ± 13 | 103 ± 13 |
| Systolic blood pressure (mm Hg) | 117 ± 4 | 133 ± 4† |
| Diastolic blood pressure (mm Hg) | 74 ± 3 | 80 ± 3 |
| Fasting plasma glucose (mg/dl) | 82 ± 1 | 98 ± 6* |
| 2 h oGTT plasma glucose (mg/dl) | 97 ± 4 | 158 ± 24* |
| Plasma FGF21 (pg/ml) | 127 ± 18 | 278 ± 60* |
Values are means ± SE. *P < 0.05, † for P < 0.01, and ‡ for P < 0.001.
Figure 1A demonstrates the effects of a 75 g oral fructose load on serum FGF21 levels in lean, healthy subjects. FGF21 initially declined by 21% (P = 0.003) at 30 min and returned to baseline at 1 h. This was followed by a rapid, 340% increase above baseline at 2 h (P = 0.002). FGF21 levels measured in four lean subjects with more frequent sampling confirm that the FGF21 peak lies between minutes 90 and 150 (data not shown). All subjects exhibited an increase in FGF21 at 2 h of between 1.5-fold and 6.6-fold. Despite the substantial variation in the magnitude of the FGF21 response and differences in the rate of subsequent decline, serum levels returned to baseline over the subsequent 3 h in all subjects (Figure 1B).
Figure 1.
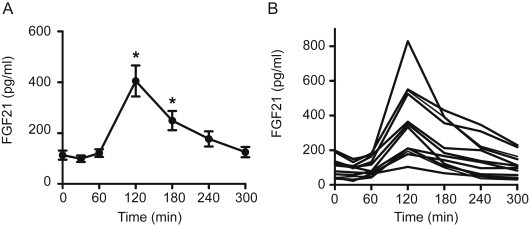
The serum FGF21 response to fructose ingestion in healthy adults. Panel A shows the average serum FGF21 level following oral ingestion of 75 g of fructose in 10 lean, healthy individuals. Panel B shows the serum FGF21 level for each of the individual subjects in Panel A. *P < 0.05 compared to baseline.
Subjects with metabolic syndrome had higher baseline serum FGF21 levels as has been previously described [3,9,11]. Nevertheless fructose also stimulated an acute increase in serum FGF21 in these subjects. Peak FGF21 levels attained in this cohort were 2.5-fold higher (P = 0.001) than in lean subjects (Figure 2A). The FGF21 incremental area under the curve (iAUC) was 2.7-fold greater in metabolic syndrome subjects (lean: 26.2 ± 6.6 min * ng per ml vs metabolic syndrome: 70.6 ± 11, P = 0.002). Despite marked differences in the magnitude of the response, the kinetics were similar between lean and metabolic syndrome subjects (Figure 2A). Fructose ingestion produced a modest increase in serum insulin levels in both groups (Figure 2B). Fructose ingestion produced a small, transient increase in glycemia (7.4 mg/dl, P < 0.05) in lean subjects at 30 min, and a slightly larger increase in glycemia in metabolic syndrome subjects (14 mg/dl at 30 min, P < 0.003; 17 mg/dl at 1 h, P < 0.005) (Figure 2C).
Figure 2.
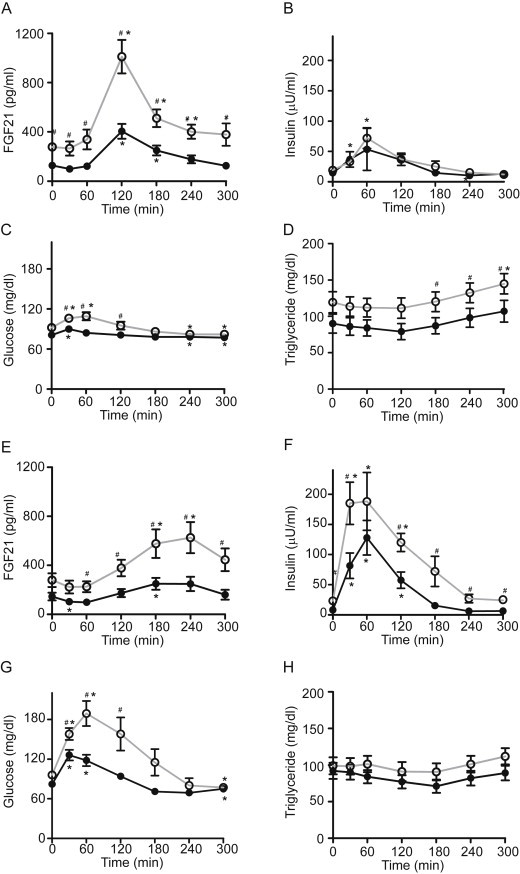
Hormone and metabolite responses to fructose or glucose ingestion. Effects of the ingestion of 75 g of fructose on serum FGF21 (Panels A), insulin (Panels B), glucose (Panels C), and triglyceride (Panels D) in lean healthy subjects (closed circle, black line) or patients with the metabolic syndrome (open circle, gray line). Effects of the ingestion of 75 g of glucose on serum FGF21 (Panels E), insulin (Panels F), glucose (Panels G), and triglyceride (Panels H). *P < 0.05 compared to baseline within group. #P < 0.05 at given time point between groups.
As fructose is lipogenic [21,22], we measured serum triglyceride levels following fructose ingestion (Figure 2D). In both lean and metabolic syndrome subjects, triglyceride levels tended to decline for the first 2 h and then tended to increase over the next 3 h. Triglyceride levels were higher in the metabolic syndrome subjects compared to lean subjects at 3, 4, and 5 h (P < 0.05).
Three lean subjects and one subject with metabolic syndrome experienced gastrointestinal symptoms (diarrhea or nausea) 30–120 min following oral fructose ingestion. The FGF21 response did not correlate with these symptoms (data not shown).
To examine the specificity of the FGF21 response, we compared the FGF21 response to fructose with the response to an equivalent amount of glucose in each subject. Previous studies had reported no increases in FGF21 levels two or three hours after glucose tolerance tests [3,17]. Consistent with this and in contrast to the acute, robust increases in FGF21 noted with fructose ingestion (Figure 2A), changes in FGF21 following glucose ingestion were more modest and delayed (Figure 2E). FGF21 levels decreased for the first hour following glucose ingestion, then rose gradually, peaked at 4 h and subsequently decreased toward baseline. Peak FGF21 levels following glucose ingestion lagged the fructose-induced peak by 2 h and were ∼40% lower (P < 0.01) in both healthy and metabolic syndrome subjects.
FGF21 levels were higher in metabolic syndrome subjects compared to healthy controls from hours 1 through 5 following glucose ingestion (Figure 2E). Incremental AUC calculations confirm differential regulation of FGF21 by glucose versus fructose. One hundred twenty minutes after the fructose challenge, the iAUC for FGF21 was positive in both lean (7.4 ± 2 min * ng/ml) and metabolic syndrome groups (24.3 ± 3.9 min * ng/ml), and was 3-fold higher (P = 0.002) in metabolic syndrome subjects. In contrast, after the glucose challenge the iAUC for FGF21 was negative in both groups at 120 min (lean: −2.5 ± 1.4 min * ng/ml; metabolic syndrome: −1.2 ± 2).
As expected, glucose ingestion produced a larger increase in serum insulin and glucose levels in metabolic syndrome subjects compared to lean counterparts (Figure 2F and G). Glucose ingestion produced no significant changes in serum triglyceride levels in either healthy or metabolic syndrome subjects (Figure 2H).
The magnitude and kinetics of the serum FGF21 response following a glucose-fructose mixture are qualitatively and quantitatively similar to that seen with fructose alone (Figures 2A and 3A). Furthermore the FGF21 excursion following a fructose challenge strongly correlates with the excursion following the mixed glucose-fructose challenge (Figure 3B) (R2 = 0.47, P < 0.001) although there is an 8 fold individual variation.
Figure 3.
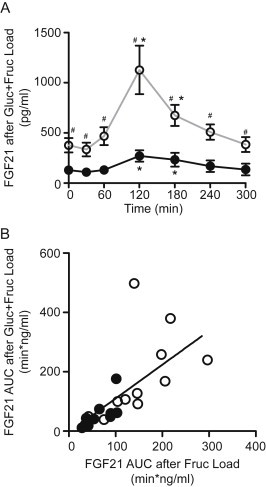
The serum FGF21 response to a mixture of fructose and glucose. Panel A shows the average serum FGF21 level following ingestion of a mixture of 37.5 g of glucose and 37.5 g of fructose in lean healthy subjects (closed circle, black line) and subjects with the metabolic syndrome (open circle, gray line). *P < 0.05 compared to baseline within group. #P < 0.05 at given time point between groups. Panel B shows the correlation between the FGF21 AUC in response to fructose alone versus the mixture of fructose and glucose. Each point represents an individual subject. Closed circles represent lean subjects. Open circles represent metabolic syndrome subjects.
To determine whether the FGF21 response to fructose associates with impaired glucose homeostasis and insulin resistance, we examined the correlation between the FGF21 excursion following an oral fructose load with glucose and insulin excursions following a glucose load (Figure 4A and B). The FGF21 AUC following fructose ingestion correlates strongly with both the glucose (R2 = 0.44, P < 0.001) and the insulin (R2 = 0.56, P < 0.001) AUCs following glucose ingestion.
Figure 4.
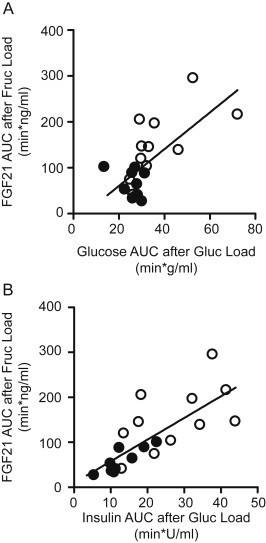
The FGF21 response to fructose ingestion correlates with indices of glucose intolerance and insulin resistance. Panels A and B show the correlations between the FGF21 AUC in response to fructose and the serum glucose AUC (Panel A) or serum insulin AUC (Panel B) following glucose. Each point represents an individual subject. Open circles represent lean subjects. Closed circles represent metabolic syndrome subjects.
4. Discussion and conclusions
This study contributes several findings relevant to the study of human metabolism. For the first time, we demonstrate that circulating FGF21 levels respond acutely to a dietary challenge – ingestion of a fructose load. Importantly, to our knowledge, this is the only known acute hormonal response to fructose ingestion. Baseline levels of FGF21 are elevated in subjects with features of metabolic disease [3,9–11,13], and we show that in this state the FGF21 response to fructose ingestion is further enhanced.
Although glucose and fructose are calorically identical they are metabolized differently [18]. Approximately 90% of an oral fructose load is extracted by the liver first pass [23]. In contrast, only a small fraction of an oral glucose load is taken up by the liver and the vast majority of glucose is disposed of in peripheral tissues [24]. These differences likely contribute to the distinct effects of glucose and fructose on hepatic lipogenesis and other metabolic processes [25]. We find that the effects of fructose and glucose on the dynamic FGF21 response are markedly different. Fructose ingestion produces a sharp, acute increase in circulating FGF21. In comparison, glucose produces a delayed and modest, but prolonged increase in circulating FGF21. These differences are likely related to the differences in hepatic fructose and glucose metabolism and support the concept that glucose and fructose ingestion may have distinct physiological consequences beyond simple caloric content [26]. Regulation of circulating FGF21 may serve as a benchmark of these differences.
The mechanisms by which fructose, and to a lesser degree glucose, increase circulating FGF21 levels remain uncertain. One molecular factor that may be involved is Carbohydrate Responsive-Element Binding Protein (ChREBP). ChREBP is master transcriptional regulator of glycolytic and lipogenic genes highly expressed in key metabolic tissues including the liver [27]. ChREBP is activated by products of carbohydrate metabolism [28], and stimulates expression of FGF21 in vitro and in animal models [15,29]. Because the liver efficiently extracts and metabolizes fructose, we hypothesized that an oral fructose challenge might acutely activate hepatic ChREBP and stimulate FGF21 production. The 60 min delay between fructose ingestion and the time when circulating FGF21 begins to rise is consistent with a transcriptional response and the proposed mechanism. Confirming this proposed mechanism will require further investigation.
The magnitude of the FGF21 response to fructose is highly variable across subjects. Variation in the magnitude of the response may signify individual differences in fructose sensing and signaling mechanisms, including differences in rates of fructose absorption or metabolism and rates of FGF21 secretion or clearance. For example, it is known that the efficiency of intestinal fructose absorption varies widely across individuals and approximately half the population cannot completely absorb a 25 g fructose load [30,31]. Also, the quantity of fructose in the diet can impact the ability to absorb fructose in subsequent meals [32]. We chose to administer 75 g of fructose in order to match the carbohydrate load of a standard oral glucose tolerance test although we recognize that this exceeds the amount of fructose typically ingested in a single meal. Limitations in fructose absorption can contribute to functional gastrointestinal symptoms [30,31,33]. However, few of our subjects reported symptoms and symptoms did not correlate with the FGF21 response. Although we observe large variation across individuals, the magnitude of the FGF21 response to fructose is highly correlated with the FGF21 response to a mixture of glucose and fructose within an individual. The reproducibility of this FGF21 response within subjects, but variation across subjects, is consistent with the possibility that genetics, adaptation to dietary differences, other environmental factors, or the presence of specific medical conditions such as impaired glucose metabolism might regulate the FGF21 response. Future studies are needed to further elucidate the dose-response of FGF21 to incremental oral fructose loads, to assess the impact of differences in fructose absorption on this response, and to determine whether chronic changes in fructose consumption might affect the acute response.
Catalytic amounts of fructose enhance hepatic glucose uptake by activating hepatic glucokinase, a rate-determining step in hepatic glucose metabolism [18]. This is borne out by the fact that small amounts of fructose decrease the glycemic excursion during oral glucose tolerance tests in humans [33]. Based upon this concept, we hypothesized that administering a combination of fructose and glucose might synergistically enhance glucokinase flux and provide more glucose metabolites to activate ChREBP and stimulate FGF21 secretion. In this experiment, the combination of 37.5 grams of glucose and 37.5 grams of fructose had no synergistic effect on fructose-stimulated FGF21 levels compared to 75 grams of fructose. It remains possible that small amounts of fructose could act synergistically with glucose to stimulate FGF21. Detailed dose-response studies will be required to further explore this hypothesis.
The observation that fructose ingestion can induce an FGF21 response reminiscent of the classic response of insulin to glucose ingestion has important implications for potential physiological functions of FGF21 in humans. Based on animal studies, FGF21 was originally found to increase with fasting and regulate the starvation response [1,5]. However, in healthy adult humans, fasting does not appear to increase FGF21 [3]. Several studies suggest that FGF21 may function as an endocrine signal of amino acid restriction [34–37]. In humans, 4 weeks of protein restriction increases circulating FGF21 1.7-fold [35]. These results indicate that FGF21 may play a more nuanced role in macronutrient regulation than previously suspected. Consistent with the notion that FGF21 may have unanticipated effects on macronutrient metabolism, polymorphisms in the FGF21 locus have been shown to associate with increased carbohydrate and lower fat consumption in humans [38,39]. This genetic data in conjunction with our data suggests that FGF21 might play a role in sucrose or fructose metabolism. However, a specific physiological function for FGF21 in carbohydrate metabolism remains to be identified.
Both baseline and fructose-stimulated FGF21 levels are increased in subjects with metabolic syndrome. Moreover, the FGF21 response to fructose ingestion strongly correlates with the glucose and insulin excursions following glucose ingestion. This indicates that the underlying biology that determines the FGF21 response to fructose couples to the biology that defines glucose homeostasis. Expression of ChREBP-β, a recently discovered potent isoform of ChREBP is elevated in the liver of people with impaired glucose metabolism [40–42]. The increased circulating FGF21 in patients with metabolic disease may therefore be due to increased hepatic ChREBP activity. In the future, it will be of interest to determine whether the FGF21 response to fructose predicts type 2 diabetes independently of more traditional risk factors and whether it might play a contributory role in the development of cardiometabolic disease.
In summary, we demonstrate that fructose ingestion acutely and robustly increases circulating FGF21 levels in humans. This response is exaggerated in subjects with metabolic syndrome and is distinct from the FGF21 response to an equivalent amount of oral glucose. The 75 g oral glucose tolerance test is the gold standard for characterizing impaired glucose homeostasis and diabetes, and the insulin response to oral glucose provides further insights into glucose homeostasis. Our findings here identify FGF21 as a measurable circulating biomarker to assess an individual's acute hormonal response to fructose ingestion which may constitute a “fructose tolerance test.” Future studies are needed to further elucidate whether this FGF21 response might contribute to or characterize risk for the development of fructose-associated physiology or metabolic disease.
Conflict of interest
None declared.
Acknowledgments
We thank Jeffrey Flier for insightful review of this manuscript. JRD assisted in the development of the protocol, implementation of the protocol, review of the analysis, and contributed to the manuscript writing. ET assisted in implementing the protocol, data collection, data analysis, and to the manuscript writing. EKM assisted in implementation of the protocol, data collection, and data analysis. ffMF helped in implementation of the study, data collection, data analysis, and contributed to manuscript authoring. MAH and EMF supervised all aspects of the study including study design, study oversight, data analysis and manuscript authoring. This work was supported by the JPB Foundation (E.M.F.) and the Harvard Catalyst/Harvard Clinical and Translational Science Center (8UL1TR000170-05 and 1UL1 TR001102-01). EMF is supported by R01DK028082 and MAH is supported by P30DK057521 and R01DK100425.
Contributor Information
Mark A. Herman, Email: mherman1@bidmc.harvard.edu.
Eleftheria Maratos-Flier, Email: emaratos@bidmc.harvard.edu.
References
- 1.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007 Jun;5(6):426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J. FGF-21 as a novel metabolic regulator. The Journal of Clinical Investigation. 2005 Jun;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dushay J., Chui P.C., Gopalakrishnan G.S., Varela–Rey M., Crawley M., Fisher F.M. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010 Aug;139(2):456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimeno R.E., Moller D.E. FGF21-based pharmacotherapy–potential utility for metabolic disorders. Trends in Endocrinology and Metabolism. 2014 Jun;25(6):303–311. doi: 10.1016/j.tem.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metabolism. 2007 Jun;5(6):415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Potthoff M.J., Inagaki T., Satapati S., Ding X., He T., Goetz R. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proceedings of the National Academy of Sciences of the United States of America. 2009 Jun;106(26):10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christodoulides C., Dyson P., Sprecher D., Tsintzas K., Karpe F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. Journal of Clinical Endocrinology & Metabolism. 2009 Sep;94(9):3594–3601. doi: 10.1210/jc.2009-0111. [DOI] [PubMed] [Google Scholar]
- 8.Gälman C., Lundåsen T., Kharitonenkov A., Bina H.A., Eriksson M., Hafström I. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metabolism. 2008 Aug;8(2):169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Chavez A.O., Molina-Carrion M., Abdul-Ghani M.A., Folli F., Defronzo R.A., Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009 Aug;32(8):1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H., Fang Q., Gao F., Fan J., Zhou J., Wang X. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. Journal of Hepatology. 2010 Nov;53(5):934–940. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Yeung D.C.Y., Karpisek M., Stejskal D., Zhou Z.-G., Liu F. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008 May;57(5):1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 12.Fisher F.M., Chui P.C., Antonellis P.J., Bina H.A., Kharitonenkov A., Flier J.S. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010 Nov;59(11):2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobbert T., Schwarz F., Fischer-Rosinsky A., Pfeiffer A.F.H., Möhlig M., Mai K. Fibroblast growth factor 21 predicts the metabolic syndrome and diabetes type 2 mellitus in caucasians. Diabetes Care. 2012 Aug 28;36(1):145–149. doi: 10.2337/dc12-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suomalainen A., Elo J.M., Pietiläinen K.H., Hakonen A.H., Sevastianova K., Korpela M. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. The Lancet Neurology. 2011 Sep;10(9):806–818. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iizuka K., Takeda J., Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Letters. 2009 Sep;583(17):2882–2886. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez J., Palou A., Picó C. Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: striking effects on fibroblast growth factor-21 induction. Endocrinology. 2009 Dec;150(12):5341–5350. doi: 10.1210/en.2009-0466. [DOI] [PubMed] [Google Scholar]
- 17.Lin Z., Gong Q., Wu C., Yu J., Lu T., Pan X. Dynamic change of serum FGF21 levels in response to glucose challenge in human. Journal of Clinical Endocrinology & Metabolism. 2012 Jul;97(7):E1224–E1228. doi: 10.1210/jc.2012-1132. [DOI] [PubMed] [Google Scholar]
- 18.McGuinness O.P., Cherrington A.D. Effects of fructose on hepatic glucose metabolism. Current Opinion in Clinical Nutrition and Metabolic Care. 2003 Jul;6(4):441–448. doi: 10.1097/01.mco.0000078990.96795.cd. [DOI] [PubMed] [Google Scholar]
- 19.Koo H.-Y., Miyashita M., Cho B.H.S., Nakamura M.T., Simon Cho B.H. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochemical and Biophysical Research Communications Elsevier Inc. 2009 Dec;390(2):285–289. doi: 10.1016/j.bbrc.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection Evaluation and T of HBC in a. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) The Journal of the American Medical Association. 2001 May 16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Hudgins L.C., Parker T.S., Levine D.M., Hellerstein M.K. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. Journal of Clinical Endocrinology & Metabolism. 2011 Mar;96(3):861–868. doi: 10.1210/jc.2010-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parks E.J., Skokan L.E., Timlin M.T., Dingfelder C.S. Dietary sugars stimulate fatty acid synthesis in adults. Journal of Nutrition. 2008 Jun;138(6):1039–1046. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran C., Jacot-descombes D., Lecoultre V., Fielding B.A., Carrel G., Lê K.-A. Sex differences in lipid and glucose kinetics after ingestion of an acute oral fructose load. British Journal of Nutrition. 2010 Jun;1:1–9. doi: 10.1017/S000711451000190X. [DOI] [PubMed] [Google Scholar]
- 24.Ludvik B., Nolan J.J., Roberts A., Baloga J., Joyce M., Bell J.M. A noninvasive method to measure splanchnic glucose uptake after oral glucose administration. The Journal of Clinical Investigation. 1995 May;95(5):2232–2238. doi: 10.1172/JCI117913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox C.L., Stanhope K.L., Schwarz J.M., Graham J.L., Hatcher B., Griffen S.C. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein- 4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutrition & Metabolism (London) 2012 Jul 24;9(1):68. doi: 10.1186/1743-7075-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanhope K.L., Schwarz J.M., Keim N.L., Griffen S.C., Bremer A.A., Graham J.L. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. The Journal of Clinical Investigation. 2009 May;119(5):1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iizuka K., Miller B., Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. American Journal of Physiology. Endocrinology and Metabolism. 2006 Aug;291(2):E358–E364. doi: 10.1152/ajpendo.00027.2006. [DOI] [PubMed] [Google Scholar]
- 28.Poupeau A., Postic C. Cross-regulation of hepatic glucose metabolism via ChREBP and nuclear receptors. Biochimica et Biophysica Acta. Elsevier B.V. 2011 Aug;1812(8):995–1006. doi: 10.1016/j.bbadis.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Benhamed F., Denechaud P., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. The Journal of Clinical Investigation. 2012 Jun 1;122(6):2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishkin D., Sablauskas L., Yalovsky M., Mishkin S. Fructose and sorbitol malabsorption in ambulatory patients with functional dyspepsia: comparison with lactose maldigestion/malabsorption. Digestive Diseases and Sciences. 1997 Dec;42(12):2591–2598. doi: 10.1023/a:1018841402133. [DOI] [PubMed] [Google Scholar]
- 31.Gibson P.R., Newnham E., Barrett J.S., Shepherd S.J., Muir J.G. Review article: fructose malabsorption and the bigger picture. Alimentary Pharmacology & Therapeutics. 2007 Feb 15;25(4):349–363. doi: 10.1111/j.1365-2036.2006.03186.x. [DOI] [PubMed] [Google Scholar]
- 32.David E.S., Cingari D.S., Ferraris R.P. Dietary induction of intestinal fructose absorption in weaning rats. Pediatric Research. 1995 Jun;37(6):777–782. doi: 10.1203/00006450-199506000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Choi Y.K., Johlin F.C., Summers R.W., Jackson M., Rao S.S.C. Fructose intolerance: an under-recognized problem. American Journal of Gastroenterology. 2003 Jun;98(6):1348–1353. doi: 10.1111/j.1572-0241.2003.07476.x. [DOI] [PubMed] [Google Scholar]
- 34.De Sousa-Coelho A.L., Relat J., Hondares E., Pérez-Martí A., Ribas F., Villarroya F. FGF21 mediates the lipid metabolism response to amino acid starvation. Journal of Lipid Research. 2013 Jul;54(7):1786–1797. doi: 10.1194/jlr.M033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laeger T., Henagan T.M., Albarado D.C., Redman L.M., Bray G.A., Noland R.C. FGF21 is an endocrine signal of protein restriction. The Journal of Clinical Investigation. 2014 Aug 18;124(9):3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher F.M., Chui P.C., Nasser I.a, Popov Y., Cunniff J.C., Lundasen T. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology. Elsevier, Inc. 2014 Jul 29;(September):1–11. doi: 10.1053/j.gastro.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lees E.K., Król E., Grant L., Shearer K., Wyse C., Moncur E. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014 Jun 17;(May):1–11. doi: 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu A.Y., Workalemahu T., Paynter N.P., Rose L.M., Giulianini F., Tanaka T. Novel locus including FGF21 is associated with dietary macronutrient intake. Human Molecular Genetics. 2013 May 1;22(9):1895–1902. doi: 10.1093/hmg/ddt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka T., Ngwa J.S., van Rooij F.J.A., Zillikens M.C., Wojczynski M.K., Frazier-Wood A.C. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. American Journal of Clinical Nutrition. 2013 Jul 1;97(6):1395–1402. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herman M.A., Peroni O.D., Villoria J., Schön M.R., Abumrad N.A., Blüher M. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012 May 19;484(7394):333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eissing L., Scherer T., Tödter K., Knippschild U., Greve J.W., Buurman W.A. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nature Communications. 2013 Feb 26;4:1528. doi: 10.1038/ncomms2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kursawe R., Caprio S., Giannini C., Narayan D., Lin A., D'Adamo E. Decreased transcription of ChREBP-α/β isoforms in abdominal subcutaneous adipose tissue of obese adolescents with prediabetes or early type 2 diabetes: associations with insulin resistance and hyperglycemia. Diabetes. 2013 Mar 3;62(3):837–844. doi: 10.2337/db12-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]


