Abstract
Objective
Introduction of a high-fat diet to mice results in a period of voracious feeding, known as hyperphagia, before homeostatic mechanisms prevail to restore energy intake to an isocaloric level. Acute high-fat diet hyperphagia induces astrocyte activation in the rodent hypothalamus, suggesting a potential role of these cells in the homeostatic response to the diet. The objective of this study was to determine physiologic role of astrocytes in the acute homeostatic response to high-fat feeding.
Methods
We bred a transgenic mouse model with doxycycline-inducible inhibition of NFkappaB (NFκB) signaling in astrocytes to determine the effect of loss of NFκB-mediated astrocyte activation on acute high-fat hyperphagia. ELISA was used to measure the levels of markers of astrocyte activation, glial-fibrillary acidic protein (GFAP) and S100B, in the medial basal hypothalamus.
Results
Inhibition of NFκB signaling in astrocytes prevented acute high-fat diet-induced astrocyte activation and resulted in a 15% increase in caloric intake (P < 0.01) in the first 24 h after introduction of the diet.
Conclusions
These data reveal a novel homeostatic role for astrocytes in the acute physiologic regulation of food intake in response to high-fat feeding.
Keywords: Astrocyte, Glia, High-fat diet, Nuclear factor-kappaB, Food intake, Hypothalamus
Abbreviations: CNS, central nervous system; GFAP, glial-fibrillary acidic protein; HFD, high-fat diet; MBH, medial basal hypothalamus; NFκB, nuclear factor kappa B
1. Introduction
Obesity is a leading public health problem worldwide and is caused by dysregulation of energy homeostasis, the balance between food intake and energy expenditure. The central nervous system (CNS) controls body weight by homeostatic regulation of food intake and energy expenditure. Genetic and pharmacologic studies in rodents have identified a number of CNS circuits critical for the regulation of energy homeostasis [1,2]. However, to date the vast majority of research on the central regulation of energy homeostasis has focused on the contribution of neurons, with the potential contribution of non-neuronal CNS cells, including glia, only beginning to be appreciated [3–6].
Studies from our laboratory and others demonstrate that in rodents high-fat feeding resulting in obesity causes CNS inflammation [5,7,8] and astrocyte activation, known as reactive astrogliosis [5,9–11]. Astrocytes are the most abundant glial cell type in the CNS and are essential for regulation of the CNS microenvironment and neuronal synaptic plasticity [12]. In the arcuate nucleus of the medial basal hypothalamus (MBH) chronic obesity-associated reactive astrogliosis has been implicated in modulating synaptic organization of the melanocortin circuitry [10], thus contributing to obesity by modulating the tone of melanocortin neurons. In addition to being elevated by chronic high-fat feeding, CNS inflammation and astrocyte activation are also evident in the rodent hypothalamus at only 24 h after the introduction of a high-fat diet [5]. During the initial 24 h period after the introduction of a highly-palatable high-fat diet mice undergo a period of voracious food intake, known as hyperphagia, before homeostatic mechanisms prevail to restore energy intake to an isocaloric level. The increase in hypothalamic astrocyte activation during this period suggests a potential contribution of these cells in this homeostatic response; however, the physiologic significance of acute astrocyte activation after high-fat feeding is not clear.
The objective of this study was to determine the contribution of astrocytes to the acute homeostatic response to high-fat feeding. In other CNS disorders, inflammation is both protective and detrimental depending on the context [13]. We hypothesized that high-fat diet-induced activation of inflammatory signaling pathways in astrocytes is part of a protective homeostatic response, which restrains food intake in response to the diet. If this hypothesis is correct then inhibiting inflammatory signaling in astrocytes should attenuate this homeostatic response resulting in increased food intake during the initial acute hyperphagic response to the high-fat diet. In CNS injury, the nuclear-factor kappa B (NFκB) transcription pathway in astrocytes plays a key role in the production of proinflammatory cytokines and the development of reactive astrogliosis [14,15]. To determine the contribution of astrocytes to the acute homeostatic response to high-fat feeding we bred a mouse model with doxycycline-inducible inhibition of the NFκB transcription pathway under the control of the astrocyte specific glial-fibrillary acidic protein (GFAP) promotor. Using this model we examined how preventing astrocyte activation by inhibiting the NFκB transcription pathway specifically in astrocytes of adult mice altered the acute homeostatic response to a high-fat diet.
2. Materials and methods
2.1. Animals
Animal studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Vanderbilt University. Animals were housed at 21 ± 2 °C and fed a standard chow diet (13% kcal from fat; Purina 5010, PMI Nutrition International, MO) unless stated otherwise. Male C57BL/6J mice (Stock # 000664) were purchased from the Jackson Laboratory (Bar Harbor, ME). Double transgenic mice with astrocyte targeted expression of a tetracycline-inducible inhibitor of NFκB signaling were generated by crossing IκBα-dominant negative mice [16–21] on the FVB background with C57BL/6J mice expressing the reverse tetracycline-controlled transactivator under the control of the human glial-fibrillary acidic protein (GFAP) promotor [GFAP-rtTA*M2; Jackson Laboratory, ME (stock number 014098)]. Hereafter these animals will be referred to as IκB-DN+ mice. Single transgenic littermates expressing only the IκBα-dominant negative transgene (IκB-DN-) were used as controls. F1 hybrid mice were used for all studies with transgenic animals. Male mice (8–10 weeks of age) were used for all feeding studies and, to reduce wastage, female mice were used for verification of transgene expression. The tetracycline-inducible IκBα-dominant negative mouse has been extensively characterized and has previously been successfully used in studies examining the importance of NFκB transcription pathway in disease pathology in mouse models of cancer [16,19,20] and lung inflammation [17,18].
2.2. Acute high-fat feeding studies
Mice were individually housed and their food intake measured daily for 7 days to obtain basal caloric intake before being switched to a high-fat diet (HFD; 60% of total calories from fat; Research Diets Inc., NJ). Food intake was measured every 24 h for the next 7 days. For the molecular studies (including immunohistochemistry), the animals were euthanized 24 h after introduction of the HFD. For the studies with the IκB-DN+ mice, the animals were switched to water containing 2 g/L doxycycline hyclate (Sigma–Aldrich, MO) and 20 g/L Splenda® brand sweetener (to mask the bitter taste of the doxycycline) 7 days before the introduction of the HFD to induce expression of the transgene.
2.3. Tissue collection for molecular analyses
Animals were deeply anesthetized and transcardially perfused with 0.9% saline. The brain was removed and sectioned using a 1-mm acrylic brain block. The MBH was dissected from a single 2 mm coronal slice Coordinates for the MBH slice were: +1.0 to +3.0 mm interaural, as referenced by Paxinos and Franklin [22], with the medial basal section of the slice corresponding to the MBH being dissected. All tissues were rapidly frozen on dry ice and placed at −80 °C until used for molecular analyses.
2.4. Ex vivo molecular analyses
2.4.1. Primary cell culture
Primary neural cells were isolated from hypothalamii dissected from adult IκB-DN− and IκB-DN+ mice using a trypsin based neural tissue dissociation kit, according to the manufacturer's instructions (Miltenyi Biotech Inc., CA). The culture procedure was modified from one described in the literature for culture from adult animals [23]. Cells isolated from one hypothalamus were distributed equally across three wells of a 6-well culture dish containing poly l-lysine (Sigma–Aldrich, MO) coated glass coverslips. Cells were maintained in an incubator at 37 °C in 5% CO2 in culture media [Dulbecco's Modified Eagle Medium (DMEM), high-glucose, containing 1% penicillin-streptomycin and fetal bovine serum (FBS)]. For the first 1-week after culture the cells were maintained in culture media containing 20% FBS before being switched to media containing 15% FBS in week 2 and 10% FBS in week 3. After 2-weeks of culture, 1 μg/ml doxycycline hyclate (Sigma–Aldrich, MO) was added to the culture media to induce transgene expression. The cells were maintained in culture for a total 3-weeks before use.
2.4.2. In vitro cell stimulation and immunocytochemistry
Primary neural cells were switched to culture media containing 1% FBS 24 h prior to stimulation with 5 μg/ml lipopolysaccharide (LPS), a potent activator of NFκB signaling. After 1 h of stimulation, the media was removed and the cells fixed with cold 100% methanol. After washing in 0.01M phosphate buffered saline (PBS; pH 7.4) cells were incubated with 1.5% FBS diluted in PBS containing 0.01% Triton-X100 (PBS-T) for 1 h at room temperature to block non-specific binding. The cells were then incubated overnight at 4 °C with antibodies against the p65 subunit of NFκB (cat # sc-372; Santa Cruz Biotechnology Inc., CA) and GFAP (cat # MAB360; Millipore Inc, MA), diluted 1:200 and 1:1,000 respectively, in 1.5% FBS in PBS-T. After washing with PBS the primary antibody binding was detected after incubation with the following secondary antibodies for 1 h at room temperature: donkey anti-rabbit Alexa 488 (p65) and donkey anti-mouse Alexa 594 (GFAP) (Life Technologies, CA), both diluted 1:500 in PBS-T. After washing with PBS, the coverslips were mounted onto glass slides with mounting media containing the nuclear marker DAPI (Pro-long Gold, Life Technologies, CA) and the staining visualized under fluorescence using a Zeiss AxioImager Z1 (Zeiss, Germany). Activation of NFκB signaling was assessed by the ability of LPS to induce translocation of p65-immunoreactivity from the cytoplasm to the nucleus. The images shown are representative of two independent experiments.
2.4.3. Verification of transgene induction using RT-PCR
RNA was extracted from brain, liver, and pancreas using Trizol according to the manufacturer's instructions (Life Technologies, CA). After DNase treatment (Life Technologies, CA), cDNA was synthesized from 1 μg of RNA using the iScript kit according to the manufacturer's instructions (BioRad Inc., CA). Expression of the IκB-DN transgene was detected using PCR with the following primer set: Forward – 5ʹ CCTGGCTGTTGTCGAATACC 3ʹ; Reverse - 5ʹ GGTGATGGTGATGATGACCGG 3ʹ. As a positive control for the integrity of the cDNA, GAPDH expression was detected using the following primer set: Forward – 5ʹ CCATGACAACTTTGGCATTG 3ʹ; Reverse – 5ʹ CCTGCTTCACCACCTTCTTG 3ʹ.
2.4.4. Glial-fibrillary acidic protein (GFAP) immunohistochemistry
After 24 h of HFD access mice were deeply anaesthetized before undergoing transcardial perfusion with 0.9% saline followed by 4% paraformaldehyde in PBS. Control animals were maintained on standard laboratory chow. Immunohistochemistry for GFAP was performed as previously described [9]. The images shown are representative of three animals per group.
2.4.5. Measurement of medial basal hypothalamus protein levels by ELISA
MBH tissues were homogenized on ice in RIPA buffer (Sigma–Aldrich, MO) containing protease inhibitor cocktail (Cat no. P8340, Sigma–Aldrich, MO). S100B and GFAP protein concentrations from MBH homogenates were measured using commercially available ELISAs according to the manufacturer's instructions (Millipore Inc., MO).
2.5. Statistical analyses
Data are expressed as mean ± standard error of the mean (S.E.M.). An unpaired t-test was used to assess differences between two groups (animals on standard chow vs. high-fat diet). Two-way ANOVA was used to assess differences between groups of animals of different genotypes on different diets. All data analyses were performed using GraphPad Prism version 5.04 (GraphPad Software, San Diego, CA), and a significance level of 0.05 was used for statistical inference.
3. Results
3.1. Astrocyte activation was acutely induced in the medial basal hypothalamus of wild-type mice following introduction of a high-fat diet
Initially, we examined changes in markers of astrocyte activation in the MBH of wild-type mice 24 h after HFD was introduced to the animals; the time point at which the peak hyperphagic response is documented. As predicted, switching wild-type mice from standard chow to highly-palatable HFD resulted in hyperphagia; in the first 24 h after introduction of the HFD the mean caloric intake of the mice increased by 75% (Figure 1A; P < 0.001). This increased caloric intake was accompanied by statistically significant increases in MBH levels of markers of astrocyte activation, S100B (Figure 1B; 40% increase, P < 0.01) and GFAP (Figure 1C; 102% increase, P < 0.05). Immunohistochemistry for GFAP reinforced the data from the ELISA, indicating an increased intensity and subtle changes in the morphology of GFAP-immunoreactive cells in the MBH 24 h after introduction of a HFD (Supplementary Figure 1). These findings support published work demonstrating acute hypothalamic astrocyte activation in rodents after 24 h of high-fat feeding [5].
Figure 1.
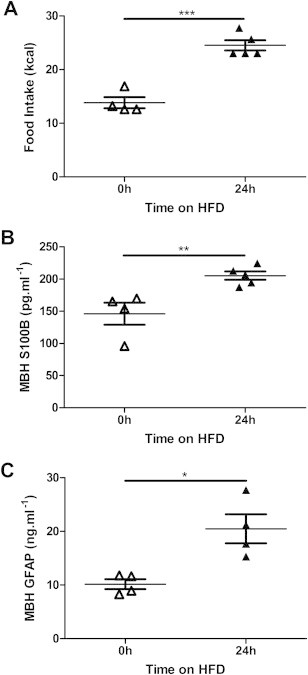
Astrocyte activation was acutely induced in the medial basal hypothalamus of wild-type mice following introduction of a high-fat diet – 24 h of high-fat feeding resulted in hyperphagia (A) accompanied by increased levels of S100B (B) and glial-fibrillary acidic protein (GFAP; C) in the medial basal hypothalamus (MBH). *P < 0.05, **P < 0.01, ***P < 0.001. n = 4–5/group.
3.2. Inhibition of astrocyte activation increased high-fat diet induced hyperphagia
To determine the physiologic significance of MBH astrocyte activation in response to acute high-fat feeding in mice, we blocked NFκB mediated astrocyte activation using a transgenic mouse model. We bred mice with doxycycline-inducible expression of a dominant-negative form of the NFκB inhibitor IκBα under the control of the astrocyte-specific GFAP promotor. We verified that transgene expression was present only in the brains in the IκB-DN+ animals after treatment with doxycycline using PCR (Figure 2A). No expression was detected in either control IκB-DN− mice or IκB-DN+ mice in the absence of doxycycline. Furthermore, the expression of the dominant-negative transgene was not detected in cDNA from the liver or pancreas of any animals (Figure 2A and Supplementary Figure 2).
Figure 2.
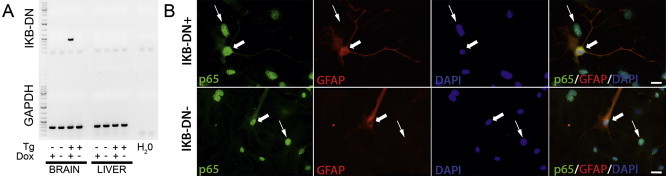
Brain IκB-DN transgene expression was induced upon exposure to doxycycline and inhibited NFκB signaling only in glial-fibrillary acidic protein (GFAP) immunoreactive cells – (A) Expression of the IκB-DN transgene was only detectable in cDNA made from brains of IκB-DN+ mice treated with doxycycline. Transgene expression was absent in liver cDNA from the same animals. Transgene expression was not detected in brain or liver cDNA from IκB-DN- mice regardless of doxycycline treatment. As a positive control, GAPDH was detected in all samples verifying the integrity of the cDNA. (B) Lipopolysaccharide (LPS) failed to induce translocation of p65 from the cytoplasm to the nucleus in GFAP-immunoreactive cells isolated from IκB-DN+ mice (upper panels – wide arrows). LPS induced nuclear translocation of p65 in non-GFAP immunoreactive cells isolated from IκB-DN+ mice (upper panels – narrow arrows). LPS induced nuclear translocation of p65 in all cell types in IκB-DN- mice regardless of GFAP-immunoreactivity (lower panels – all arrows). Tg = transgene; Dox = doxycycline exposure. Scale bars = 20 μm.
An experimental indicator of activation of the NFκB signaling cascade is translocation of the p65 subunit of the NFκB complex from the cytoplasm to the nucleus. LPS is a potent activator of NFκB signaling. To verify the functional activity of our transgene, we used immunocytochemistry to examine whether induction of transgene expression in primary neural cells cultured from IκB-DN+ mice prevented LPS-induced nuclear translocation of p65 in GFAP-immunoreactive cells. Following LPS treatment, p65-immunoreactivity remained localized in the cytoplasm of all GFAP-immunoreactive cells isolated from IκB-DN+ mice (Figure 2B, upper panels), indicating successful inhibition of NFκB signaling in these cells. The inhibition of NFκB signaling was specific to GFAP-immunoreactive cells isolated from IκB-DN+ mice as LPS induced nuclear localization of p65 immunoreactivity in all non-GFAP immunoreactive cells. In the absence of transgene expression, in neural cells isolated from IκB-DN- mice, LPS treatment resulted in localization of p65-immunoreactivity to the nucleus in all cells, including those that were positive for GFAP-immunoreactivity (Figure 2B, lower panels). Together these data verify that the ability of the transgene to inhibit the activity of the NFκB signaling cascade in our model is restricted to GFAP-immunoreactive cells.
S100B and GFAP expression are positively regulated by activation of the NFκB signaling cascade [24,25]. We verified the successful inhibition of astrocyte activation in vivo in our transgenic mouse model by measuring levels of S100B and GFAP in the MBH. IκB-DN+ mice did not show the HFD-induced increase in MBH S100B (Figure 3A; P > 0.05) and GFAP (Figure 3B; P > 0.05) seen in control IκB-DN− littermates 24 h after introduction of the HFD (S100B - Figure 3A; P < 0.05, GFAP - Figure 3B; P < 0.01). There was a statistically significant effect of genotype on baseline S100B (Figure 3A; P(genotype) < 0.01) and GFAP (Figure 3B; P(genotype) < 0.001) expression in IκB-DN+ mice on standard chow; however, the reduction in S100B and GFAP levels in IκB-DN+ animals was not associated with any overt differences in standard chow intake prior to introduction of the HFD (Figure 3C). Inhibiting the NFκB transcription pathway in astrocytes resulted in a 15% increase in peak caloric intake 24 h after introduction of HFD in IκB-DN+ animals, compared with littermate controls (IκB-DN-; Figure 3D; P < 0.01). The increased food intake was only seen at 24 h after introduction of the HFD after which point there was no statistically significant difference between the groups (Figure 3C). Together these data support our hypothesis that acute astrocyte activation 24 h after introduction of a HFD is part of a homeostatic response that restrains food intake.
Figure 3.
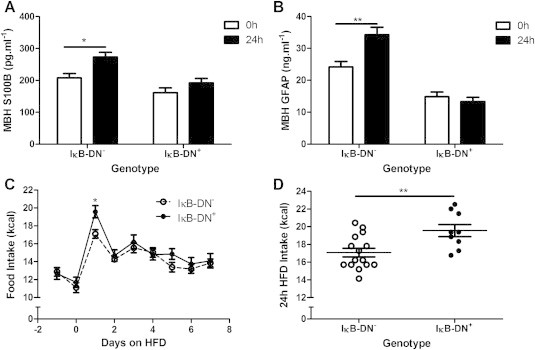
Inhibition of astrocyte activation increased high-fat diet induced hyperphagia – Inhibiting astrocyte activation by inducing expression of a dominant negative form of the inhibitor of NFκB signaling, IκBα, under the control of the glial-fibrillary acidic protein (GFAP) promotor (IκB-DN+ mice) prevented the high-fat diet induced increase in markers of astrocyte activation, S100B (A) and GFAP (B) in the medial basal hypothalamus (MBH) 24 h after introduction of the diet. This was associated with an increase in high-fat diet intake at 24 h (C and D). *P < 0.05, **P < 0.01. n = 3–15/group.
The IκB-DN+ mice and their IκB-DN- littermate controls showed lower overall caloric intake on both standard chow and in response to introduction of the HFD (Figure 3C and D), compared with the wild-type mice used to generate the data in Figure 1. To test whether this was due to doxycycline exposure we performed an acute high-fat feeding study in IκB-DN- mice that were not exposed to the drug. In the absence of doxycycline treatment the peak HFD intake of IκB-DN- mice at 24 h was 17.2 ± 0.6 kcal (Figure 4) compared with 17.1 ± 0.49 kcal in the IκB-DN- mice exposed to doxycycline (Figure 3C and D). This suggests that the genetic background of the animals (C57BL6/J in the case of the wild-type mice used to generate the data in Figure 1, compared with C57BL6/J X FVB F1 hybrids in the case of the transgenic mice used to generate the data in Figure 3) likely contributed to the differences in caloric intake seen between the studies.
Figure 4.
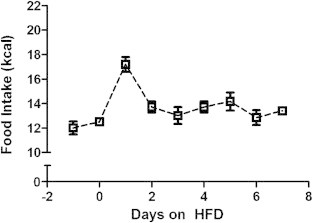
Doxycycline treatment did not influence the peak hyperphagic response to high-fat diet in IκB-DN-mice – In order to control for potential effects of doxycycline on food intake we examined the response to introduction of a high-fat diet in control IκB-DN- mice in the absence of doxycycline. The pattern and magnitude of food intake was similar to the IκB-DN- mice treated with doxycycline (see Figure 3C). n = 6.
4. Discussion
Dysfunction in the homeostatic regulation of food intake is a key contributor to the pathogenesis of obesity. As such, an improved understanding of how food intake is regulated, particularly in response to highly-palatable calorically dense foods, is necessary for the development of effective therapeutic interventions. The presence of hypothalamic inflammation and astrocyte activation acutely after initial exposure to a highly-palatable calorically dense HFD [5] suggests a potential contribution of these cells to the homeostatic response to the diet; however, the physiologic significance of acute astrocyte activation after high-fat feeding is not clear. In this study, we provide evidence that astrocyte activation is part of an acute homeostatic response to introduction of a HFD that restrains food intake and that the absence of HFD-induced astrocyte activation is associated with exaggerated hyperphagia. These findings are significant as they are amongst the first to provide direct experimental evidence for a role of astrocytes in the regulation of food intake.
We focused on astrocyte activation in the MBH in this study, as it is likely to be one of the principal brain regions mediating the effects seen. It has previously been shown that the hypothalamus exhibits rapid changes in glial cell activity in response to high-fat feeding [5] and the presence of a circumventricular site, the median eminence, within this area makes it highly sensitive to changes in hormonal and nutritional inputs. Furthermore, the MBH contains critical neuronal circuitry mediating feeding behavior including the well-characterized melanocortin neurons in the arcuate nucleus. Indeed, astrogliosis associated with chronic high-fat feeding has been implicated in modulating the synaptic organization of melanocortin neurons in this brain region [10]. Like the MBH, caudal brainstem areas such as the nucleus of the solitary tract and dorsal vagal complex also mediate feeding behavior [26] and are in close proximity to another circumventricular organ, the area postrema. To date no one has reported inflammation or glial activation in the brainstem in response to high-fat feeding; but the involvement of this or indeed other brain areas, such as nuclei of the mesolimbic dopaminergic system that mediate reward, cannot be excluded in our study as the transgenic mouse model used targeted all GFAP-expressing cells and not those exclusively in the MBH.
While GFAP is principally expressed in the central nervous system, peripheral sites of expression including the liver [27], pancreas [28] and enteric nervous system [29] have been documented. We did not detect any expression of the IκB-DN transgene in the liver or pancreas in our doxycycline-treated IκB-DN+ animals, likely due to the low level of expression of GFAP in these tissues relative to the brain where astrocytes constitute up to 50% of the cellular mass; however, a potential contribution of non-CNS GFAP-positive cells in mediating the altered acute feeding response to HFD cannot be completely ruled out.
Alterations in synaptic plasticity, exemplified by rapid rewiring of melanocortin neurons in the arcuate nucleus, have been observed acutely in response to introduction of a HFD [30]. In other neuroendocrine axes, astrocytes modulate synaptic plasticity contributing to the regulation of tone within neuronal circuits [31]. A likely mechanism by which astrocytes contribute to a reduction in acute HFD-induced food intake is via modulation of the activity of neurons that regulate food intake in the arcuate nucleus or another brain region. This neuronal modulation may occur via: 1) the action of astrocyte-derived cytokines or other biochemicals such as ketone bodies; 2) astrocyte ensheathment of neurons physically hindering synaptic connectivity; 3) astrocyte mediated alterations in neurotransmitter reuptake at the synapse; and/or 4) astrocyte induced alterations in blood–brain barrier permeability which change humoral and nutritional inputs to the CNS from the periphery. In a recent study Le Foll and colleagues presented evidence that astrocytes in the ventral medial hypothalamus play a role in modulating food intake in response to a high-fat diet via the action of ketone bodies [4]. An important mechanistic question that remains unresolved in the field relates to what is causing the high-fat diet induced inflammation and astrocyte activation in the CNS. Candidates include saturated fatty acids [32], endoplasmic reticulum stress [33], neuronal injury [5] or neurogenic neuroinflammation [34].
5. Conclusion
In summary, we have discovered that astrocytes mediate a previously undescribed homeostatic response to consumption of a high-fat diet that functions to acutely restrain food intake. This has implications for our understanding of the cellular mechanisms by which food intake is regulated by the CNS.
Acknowledgments
This work was supported this work was supported in part by a Young Investigator Award from the Vanderbilt Digestive Disease Research Center (DDRC; NIH P30 DK058404) and start-up funds from Vanderbilt University to KLJE. MMT was supported, in part, by a supplement to the Vanderbilt Diabetes Research Training Center Training Grant (NIH T32 DK007061). We would like to thank Dr. Craig Beall for his advice regarding primary astrocyte culture.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Figure 1.
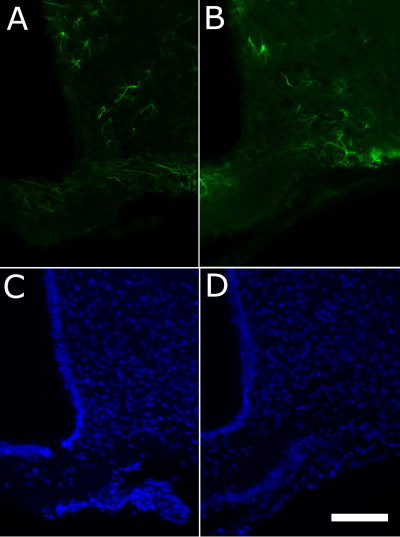
Acute high-fat feeding resulted in subtle changes in astrocyte morphology in the medial basal hypothalamus (MBH) – Immunohistochemistry for glial-fibrillary acidic protein (GFAP) revealed a subtle change in morphology of GFAP-immunoreactive cells in the MBH in mice fed a high-fat diet (HFD) for 24 h (A) compared with controls maintained on standard chow (B). Lower panels show DAPI staining for the HFD (C) and control (D) animals respectively. Scale bar = 100 μm. Images shown are representative from three animals per group.
Supplementary Figure 2.
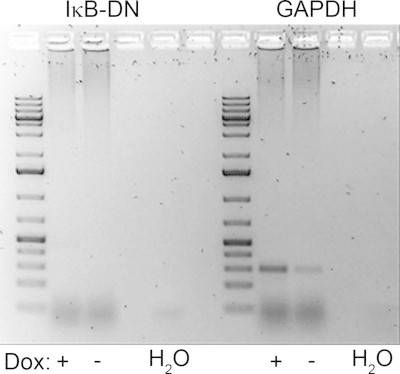
IκB-DN transgene expression was not detected in the pancreas upon exposure to doxycycline - Expression of the IκB-DN transgene was absent in pancreas cDNA from doxycycline treated IκB-DN+ and IκB-DN- animals. As a positive control, GAPDH was detected in all samples verifying the integrity of the cDNA. Dox = Doxycycline exposure.
References
- 1.Williams K.W., Elmquist J.K. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nature Neuroscience. 2012;15(10):1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeltser L.M., Seeley R.J., Tschop M.H. Synaptic plasticity in neuronal circuits regulating energy balance. Nature Neuroscience. 2012;15(10):1336–1342. doi: 10.1038/nn.3219. [DOI] [PubMed] [Google Scholar]
- 3.Chowen J.A., Argente J., Horvath T.L. Uncovering novel roles of nonneuronal cells in body weight homeostasis and obesity. Endocrinology. 2013;154(9):3001–3007. doi: 10.1210/en.2013-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Foll C., Dunn-Meynell A.A., Miziorko H.M., Levin B.E. Regulation of hypothalamic neuronal sensing and food intake by ketone bodies and fatty acids. Diabetes. 2014;63(4):1259–1269. doi: 10.2337/db13-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O. Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi C.X., Habegger K.M., Chowen J.A., Stern J., Tschop M.H. A role for astrocytes in the central control of metabolism. Neuroendocrinology. 2011;93(3):143–149. doi: 10.1159/000324888. [DOI] [PubMed] [Google Scholar]
- 7.De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 8.Nerurkar P.V., Johns L.M., Buesa L.M., Kipyakwai G., Volper E., Sato R. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. Journal of Neuroinflammation. 2011;8:64. doi: 10.1186/1742-2094-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckman L.B., Thompson M.M., Moreno H.N., Ellacott K.L. Regional astrogliosis in the mouse hypothalamus in response to obesity. Journal of Comparative Neurology. 2013;521(6):1322–1333. doi: 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath T.L., Sarman B., Garcia-Caceres C., Enriori P.J., Sotonyi P., Shanabrough M. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14875–14880. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsuchou H., He Y., Kastin A.J., Tu H., Markadakis E.N., Rogers R.C. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009;132(Pt 4):889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathologica. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czeh M., Gressens P., Kaindl A.M. The yin and yang of microglia. Developmental Neuroscience. 2011;33(3–4):199–209. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- 14.Lian H., Shim D.J., Gaddam S.S., Rodriguez-Rivera J., Bitner B.R., Pautler R.G. IkappaBalpha deficiency in brain leads to elevated basal neuroinflammation and attenuated response following traumatic brain injury: implications for functional recovery. Molecular Neurodegeneration. 2012;7:47. doi: 10.1186/1750-1326-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brambilla R., Bracchi-Ricard V., Hu W.H., Frydel B., Bramwell A., Karmally S. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. The Journal of Experimental Medicine. 2005;202(1):145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connelly L., Barham W., Onishko H.M., Chen L., Sherrill T.P., Zabuawala T. NF-kappaB activation within macrophages leads to an anti-tumor phenotype in a mammary tumor lung metastasis model. Breast Cancer Research: BCR. 2011;13(4):R83. doi: 10.1186/bcr2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng D.S., Han W., Chen S.M., Sherrill T.P., Chont M., Park G.Y. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. The Journal of Immunology. 2007;178(10):6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 18.Chen S.M., Cheng D.S., Williams B.J., Sherrill T.P., Han W., Chont M. The nuclear factor kappa-B pathway in airway epithelium regulates neutrophil recruitment and host defence following Pseudomonas aeruginosa infection. Clinical and Experimental Immunology. 2008;153(3):420–428. doi: 10.1111/j.1365-2249.2008.03707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connelly L., Barham W., Onishko H.M., Sherrill T., Chodosh L.A., Blackwell T.S. Inhibition of NF-kappa B activity in mammary epithelium increases tumor latency and decreases tumor burden. Oncogene. 2011;30(12):1402–1412. doi: 10.1038/onc.2010.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connelly L., Barham W., Pigg R., Saint-Jean L., Sherrill T., Cheng D.S. Activation of nuclear factor kappa B in mammary epithelium promotes milk loss during mammary development and infection. Journal of Cellular Physiology. 2010;222(1):73–81. doi: 10.1002/jcp.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connelly L., Robinson-Benion C., Chont M., Saint-Jean L., Li H., Polosukhin V.V. A transgenic model reveals important roles for the NF-kappa B alternative pathway (p100/p52) in mammary development and links to tumorigenesis. The Journal of Biological Chemistry. 2007;282(13):10028–10035. doi: 10.1074/jbc.M611300200. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G., Franklin K.B.J., Franklin K.B.J. 2nd ed. Academic Press; San Diego: 2001. The mouse brain in stereotaxic coordinates. [Google Scholar]
- 23.Xie Z., Morgan T.E., Rozovsky I., Finch C.E. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Experimental Neurology. 2003;182(1):135–141. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 24.Lam A.G., Koppal T., Akama K.T., Guo L., Craft J.M., Samy B. Mechanism of glial activation by S100B: involvement of the transcription factor NFkappaB. Neurobiology of Aging. 2001;22(5):765–772. doi: 10.1016/s0197-4580(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 25.Morga E., Mouad-Amazzal L., Felten P., Heurtaux T., Moro M., Michelucci A. Jagged1 regulates the activation of astrocytes via modulation of NFkappaB and JAK/STAT/SOCS pathways. Glia. 2009;57(16):1741–1753. doi: 10.1002/glia.20887. [DOI] [PubMed] [Google Scholar]
- 26.Berthoud H.R. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterology & Motility. 2008;20(Suppl. 1):64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gard A.L., White F.P., Dutton G.R. Extra-neural glial fibrillary acidic protein (GFAP) immunoreactivity in perisinusoidal stellate cells of rat liver. Journal of Neuroimmunology. 1985;8(4–6):359–375. doi: 10.1016/s0165-5728(85)80073-4. [DOI] [PubMed] [Google Scholar]
- 28.Apte M.V., Haber P.S., Applegate T.L., Norton I.D., McCaughan G.W., Korsten M.A. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43(1):128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jessen K.R., Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980;286(5774):736–737. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- 30.Benani A., Hryhorczuk C., Gouaze A., Fioramonti X., Brenachot X., Guissard C. Food intake adaptation to dietary fat involves PSA-dependent rewiring of the arcuate melanocortin system in mice. The Journal of Neuroscience. 2012;32(35):11970–11979. doi: 10.1523/JNEUROSCI.0624-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tasker J.G., Oliet S.H., Bains J.S., Brown C.H., Stern J.E. Glial regulation of neuronal function: from synapse to systems physiology. Journal of Neuroendocrinology. 2012;24(4):566–576. doi: 10.1111/j.1365-2826.2011.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta S., Knight A.G., Keller J.N., Bruce-Keller A.J. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. Journal of Neurochemistry. 2012;120(6):1060–1071. doi: 10.1111/j.1471-4159.2012.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xanthos D.N., Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nature Reviews Neuroscience. 2014;15(1):43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]


