Abstract
Objective
Lipoprotein lipase (LPL) is a key regulator of circulating triglyceride rich lipoprotein hydrolysis. In brain LPL regulates appetite and energy expenditure. Angiopoietin-like 4 (Angptl4) is a secreted protein that inhibits LPL activity and, thereby, triglyceride metabolism, but the impact of Angptl4 on central lipid metabolism is unknown.
Methods
We induced type 1 diabetes by streptozotocin (STZ) in whole-body Angptl4 knockout mice (Angptl4-/-) and their wildtype littermates to study the role of Angptl4 in central lipid metabolism.
Results
In type 1 (streptozotocin, STZ) and type 2 (ob/ob) diabetic mice, there is a ~2-fold increase of Angptl4 in the hypothalamus and skeletal muscle. Intracerebroventricular insulin injection into STZ mice at levels which have no effect on plasma glucose restores Angptl4 expression in hypothalamus. Isolation of cells from the brain reveals that Angptl4 is produced in glia, whereas LPL is present in both glia and neurons. Consistent with the in vivo experiment, in vitro insulin treatment of glial cells causes a 50% reduction of Angptl4 and significantly increases LPL activity with no change in LPL expression. In Angptl4-/- mice, LPL activity in skeletal muscle is increased 3-fold, and this is further increased by STZ-induced diabetes. By contrast, Angptl4-/- mice show no significant difference in LPL activity in hypothalamus or brain independent of diabetic and nutritional status.
Conclusion
Thus, Angptl4 in brain is produced in glia and regulated by insulin. However, in contrast to the periphery, central Angptl4 does not regulate LPL activity, but appears to participate in the metabolic crosstalk between glia and neurons.
Keywords: Angptl4, Lipid metabolism, Lipoprotein lipase
Abbreviations: AgRP, agouti-related protein; Angptl4, angiopoietin-like 4; ARC, arcuate nucleus; CART, cocaine-and-amphetamine-regulated transcript; CNS, central nervous system; FFA, free fatty acid; LPL, lipoprotein lipase; NPY, neuropeptide-Y; POMC, pro-opiomelanocortin; STZ, streptozotocin; TG, triglyceride
1. Introduction
Over the past decade there has been increasing evidence indicating an essential role of the central nervous system in the regulation of energy homeostasis, development of obesity and glucose intolerance [reviewed in Ref. [1]]. Free fatty acids (FFA), and especially long chain fatty acids, have been shown to inhibit food intake and hepatic glucose production [2]. The major pools of FFA within the circulation are contained in triglyceride-rich lipoproteins and or bound to albumin.
Lipoprotein lipase (LPL) is the key enzyme that controls the hydrolysis of triglyceride-rich lipoproteins into FFA, which can then be used for storage or consumption in peripheral tissues. Activity of LPL is governed by a number of different mechanisms, most of which act at the posttranscriptional and posttranslational levels [3]. Angiopoietin-like (Angptl) 4 is a known inhibitor of LPL activity. Angptl4 is primarily expressed in adipose tissue and liver, induced by fasting and secreted into the circulation [4], where it prevents LPL dimerization and thereby preventing LPL activation [5–8]. Studies using genetically modified mouse models of Angptl4 confirm its key role in triglyceride metabolism. Thus, Angptl4 knockout mice have reduced plasma triglycerides, whereas Angptl4 overexpressing mice have increased triglyceride levels [9,10]. Central Angptl4 has been suggested as a modulator of food intake [11] and a protector of brain tissue from ischemia induced alterations [12]. However, its role in central lipid metabolism remains unknown.
The impact of triglyceride hydrolysis and FFAs in the central nervous system is still not completely understood, but inactivation of LPL in neurons of mice affects both food intake and energy expenditure, resulting in obesity [13]. This suggests that regulation of triglyceride metabolism in the brain is important in control of whole body metabolism. LPL is synthesized in various tissues including adipose tissue, skeletal muscle, heart lung, kidney and spread along the vascular endothelium [3]. In addition, LPL is present in most regions of the brain, particularly in areas with high levels of neuronal cell bodies, but to a lesser extent in areas rich in glial cells [14]. The function of LPL in the brain still remains elusive, but it has been implicated in at least two different functions; the regulation of body weight and energy balance as well as cognition [14,15]. In the present study we have explored the regulation and role of Angptl4 in the brain in mouse models of diabetes and through the use of the Angptl4 knockout mice. Since LPL is known to affect whole body glucose homeostasis dependent on expression level in skeletal muscle [16,17], this tissue was included in the analysis to compare differences between central and peripheral regulated lipid metabolism.
2. Material and methods
2.1. Animals
C57Bl/6 and ob/ob (C57Bl/6 background) mice were obtained from Jackson laboratory (Bar Harbor, ME). Angptl4−/− mice (mixed genetic background; C57Bl/6, 129Sv) were provided by Jeffrey Gordon (Washington University) [18]. All mice used for experiments were male. For STZ induced diabetes, seven-week-old C57Bl/6 or 10–14-week-old Angptl4−/− and their wildtype littermates were treated with a single intraperitoneal (i.p.) injection (150 mg/kg bodyweight) of streptozotocin (STZ, Sigma). At day seven mice were sacrificed, and serum and tissues collected. All protocols were in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committees of the Joslin Diabetes Center and Brandeis University. Glucose levels were measured with Infinity glucose monitors (US Diagnostics), insulin (Chrystal Chem) and triglycerides (Abnova) were measured according to the manufacturers' protocols.
2.2. Insulin and Angptl4 intracerebroventricular (i.c.v.) administration
A 26-gauge guide cannula (Plastics One Inc., Roanoke, VA) was inserted into the right lateral cerebral ventricle of seven-week-old C57Bl/6 mice as described previously [19]. At day seven, the mice received a single i.p. injection of STZ to induce diabetes. Twelve days later the mice received three intracerebroventricular (i.c.v.) injections of insulin (3 mU in 2 μl) or 2 μl phosphate buffered saline (PBS) (9 A.M., 7 P.M., and 9 A.M. the following day) [19]. Food intake was measured immediately before the i.c.v. injection. Four hours after the last injection, blood glucose levels were measured, and hypothalami collected. Administration of human recombinant Angptl4 (Axxora, San Diego, CA) or saline was delivered (10 ng/h) using an osmotic pump (Durect Corp., Cupertino, CA) and a 26-gauge guide cannula (Plastics One, Roanoke, VA) located 1.0 mm posterior and 1.0 mm lateral from the bregma.
2.3. Primary culture glia and cortical neurons for insulin and glucose stimulation
Primary glial cells and neurons were isolated as previous described [19]. Glial cells were harvested 14 days after initial plating, while neurons were harvested 18 days after initial plating. For insulin stimulation, glial cells were serum deprived in media containing 0.1% BSA for 6 h before stimulated with 0, 10 or 100 nM insulin in 5.5 or 15 mM glucose for 3, 6 and/or 24 h.
2.4. Quantitative real-time PCR
RNA from mouse tissue and cell samples was extracted using RNeasy Mini Kit (Qiagen) and reverse-transcribed (1 μg) with high-capacity cDNA reverse transcription kit (Applied Biosystems). Real-time PCR was performed in ABI Prism 7900 HT sequence detection system (Applied Biosystems) using a final volume of 10 μl per reaction with SYBR Green PCR Master Mix (Applied Biosystems), 12.5 ng of cDNA and 300 nM sense and antisense primers. Analysis of TATA box-binding protein (TBP) expression was performed in parallel to normalize gene expression and analyzed by using the 2−ΔΔCt method. Primer sequences are given in Supplementary Table 1.
2.5. LPL activity
Mice were either random fed or fasted overnight before sacrificed, and tissues were removed and snap frozen. Heparin-releasable LPL was assayed as described [20]. For skeletal muscle and cerebral cortex, 40–50 mg tissue was used to assess LPL activity, expressed in nmol FFA/min/g tissue, whereas only 10 mg tissue was used for hypothalamus. For glial cells, media was collected and cells were harvested in lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA) containing protease inhibitor mixture and phosphatase inhibitor cocktail 1 and 2 (Sigma). Protein concentration was determined using BCA method (Pierce), and LPL activity was expressed as nmol FFA/min/μg protein. For further details, see Supplementary materials.
2.6. Statistics
All results are expressed as mean ± SEM. Significance was established using the 2-tailed Student's t test and ANOVA when appropriate. Differences were considered significant at p <0.05.
3. Results
3.1. Regulation of hypothalamic Angptl4 in diabetes by insulin
In an attempt to determine how diabetes affects brain metabolism and hypothalamic function, we performed global gene expression on the hypothalamus from animal models of type 1 diabetes (streptozotocin, STZ) and type 2 diabetes/obesity (ob/ob) [19]. As expected, the STZ-induced diabetic mice were hyperglycemic, had low insulin levels and lost weight compared to controls, as opposed to the ob/ob mice, which were hyperglycemic, hyperinsulinemic and obese (Figure 1A–C). One of the most significantly upregulated genes in the hypothalami of both STZ diabetic mice and ob/ob mice compared to controls by microarray analysis was angiopoietin-like 4 (Angptl4). Angptl4 mRNA expression was confirmed by qPCR, which revealed 1.5–3-fold increases in these two models of diabetes, respectively (Figure 1D and F). LPL activity in skeletal muscle is known to be positively correlated with insulin sensitivity and negatively correlated with fasting insulin levels [21]. Indeed, a similar upregulation of Angptl4 was observed in skeletal muscle from the STZ and ob/ob models described above (Figure 1E), suggesting that Angptl4 might be regulated and act the same way centrally as in skeletal muscle. Importantly, in the STZ-diabetic mice, upregulation of Angptl4 in the hypothalamus could be normalized by direct i.c.v. injection of insulin at a dose (3 mU per mouse) which does not affect blood glucose levels (Figure 1F, [19]). Taken together, these data indicate that Angptl4 expression in the brain is highly dysregulated in diabetes and can be normalized by increasing brain insulin levels without normalizing blood glucose.
Figure 1.
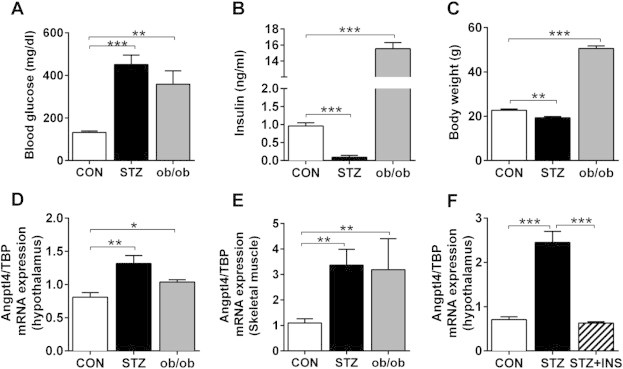
Angptl4 mRNA expression is upregulated in hypothalamus of both type 1 and type 2 diabetic animal models and regulated by insulin. A) Blood glucose levels, B) plasma insulin levels, C) body weight, and E) Angptl4 mRNA expression in hypothalamus (D) and skeletal muscle (E) of control (CON), streptozotocin (STZ) treated and ob/ob mice (n = 5). F) Angptl4 mRNA expression in hypothalamus of control (CON), STZ treated mice and STZ treated mice following three i.c.v. injection of insulin (3 mU) at 9 A.M., 7 P.M., and 9 A.M. the next day, followed by sacrifice at 4 h after last insulin injection. Data are mean ± SEM with 3–5 mice per group, random fed. The asterisks indicate * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001 as assessed by Student's t-test.
3.2. Differential expression of Angptl4 in neurons and glia
It is well known that there is nutritional crosstalk between neurons and glial cells, including transfer of lipid and other metabolites between these two cell types [22]. To determine which cells in the brain might make Angptl4, we isolated primary neurons and glial cells from wildtype mice (Supplementary Figure 1A and B). Interestingly, we found that Angptl4 was almost exclusively expressed in glial cells, with almost none detected in neurons (Figure 2A). By contrast, LPL mRNA was clearly expressed in both neurons and glial cells, although it was also slightly higher (∼37%) in glia. Six hours of insulin treatment in vitro significantly downregulated expression of Angptl4 in glial cells, whereas LPL mRNA was unchanged (Figure 2B). This downregulation was dose-dependent (Figure 2C) and was observed over a 3–24 h time course of insulin treatment, being most significant after 6 h (Figure 2D). By contrast, exposure to high glucose (15 mM) did not influence either Angptl4 or LPL mRNA expression after 3, 6, or 24 h (Figure 2E), supporting our in vivo findings. Interestingly, insulin stimulated LPL activity both intrinsic and in the media from glial cells after 6 h was significantly increased (Figure 2F, Supplementary Figure 1D), at the same time point, where mRNA expression of Angptl4 was at its lowest.
Figure 2.
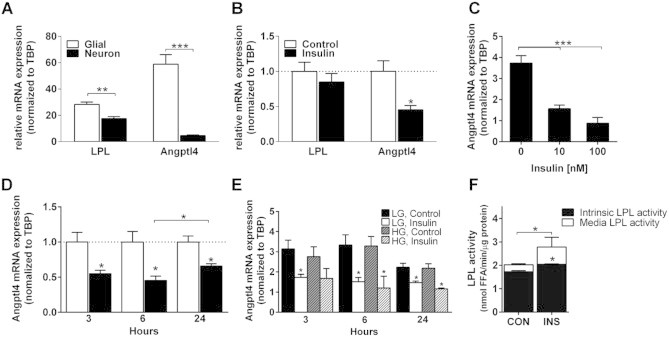
Angptl4 is glial specific and downregulated by insulin. A) mRNA expression of LPL and Angptl4 in glial cells and neurons isolated from cortex of p0 and e16.5 C57Bl/J6, respectively (n = 4). B) mRNA expression of LPL and Angptl4 in glial cells treated with or without 10 nM insulin for 6 h (n = 4). C) Insulin dose dependent downregulation of Angptl4 mRNA expression (n = 3) and D) time dependent downregulation of Angptl4 in glia cells treated with 10 nM insulin (n = 3). E) Angptl4 mRNA expression from glial cells pre-cultured in low glucose (5.5 mM) or high glucose (15 mM) for 24 h and then stimulated with 10 nM insulin for 3, 6 and 24 h (n = 4). F) LPL activity was accessed on both media and the glial cells (intrinsic) treated with or without insulin (10 nM) for 6 h (n = 4). Data are mean ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 by Student's t-test and one-way ANOVA.
3.3. Regulation of LPL activity by Angptl4 and diabetes
As noted above, Angptl4 has been found to regulate lipoprotein lipase (LPL) activity in serum and peripheral tissues [4,6]. To determine if Angptl4 regulates central and peripheral LPL activity in a similar manner and thereby influences nutrient sensing, we utilized global knockout mice of Angptl4 (Angptl4−/−). These mice and their wildtype littermates (Angptl4+/+) were treated with STZ (150 μg/g bodyweight), and as expected, this resulted in a state of insulin deficient (type 1) diabetes, with an ∼80% reduction in plasma insulin levels, a three-fold increase in blood glucose levels, and a 10% loss of body weight in both control and knockout mice (see Supplementary Table 2).
In the STZ-diabetic mice, there was a 3–4 fold increase in Angptl4 mRNA expression in the skeletal muscle of wildtype mice, similar to what was observed above (Figure 1E). As expected, Angptl4 mRNA was totally undetectable in muscle from Angptl4 knockout mice under normal and diabetic conditions (Figure 3A). LPL mRNA expression in muscle was largely unchanged by induction of diabetes, but there was a small increase (∼30%) in the Angptl4 knockout mice, which returned toward control levels in the presence of diabetes (Figure 3A). Consistent with the role of Angptl4 as an LPL inhibitor, Angptl4 knockout mice had a 50% reduction in serum triglycerides levels (Angptl4+/+ 0.96 ± 0.15 vs Angptl4−/− 0.49 ± 0.07 mM, p < 0.001), and LPL activity in skeletal muscle was increased about 3-fold in both control and STZ diabetic Angptl4 knockout mice compared to wildtype mice (Figure 3A). Although STZ diabetes did not significantly change serum triglycerides in the fed state, STZ diabetes did result in a rise in serum triglyceride levels in fasted wildtype mice as expected (1.46 ± 0.12 vs 2.42 ± 0.4 mM; p < 0.01 control vs STZ). There was no change in insulin or blood glucose levels between the genotypes in the fasted state, but, as expected, mice with STZ induced diabetes has significantly higher blood glucose and lower insulin levels (Supplementary Table 2). Consistent with the increased LPL activity, Angptl4 knockout mice exhibited significantly lower serum triglycerides compared to control in both the fed (0.85 ± 0.06 mM, p < 0.001) and fasted states (0.96 ± 0.2 mM, p < 0.001), as well as after induction of diabetes (Supplementary Table 2).
Figure 3.
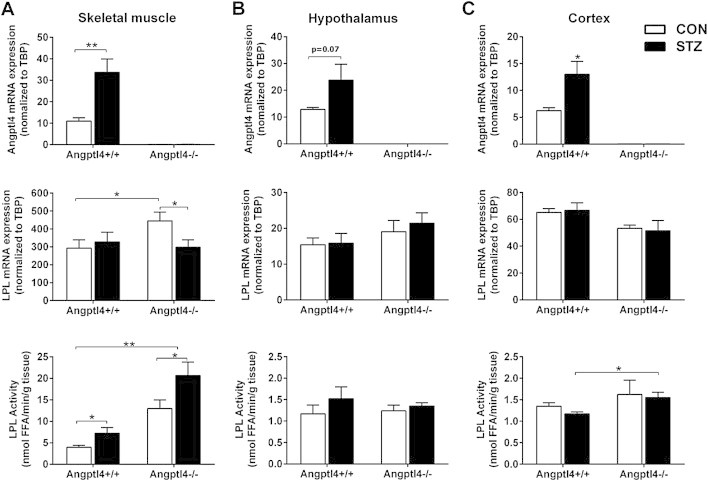
LPL activity in skeletal muscle, hypothalamus and cortex in random fed Angptl4 knockout (Angptl4−/−) mice and their wildtype (Angptl4+/+) littermates. A) Angptl4 and LPL mRNA expression as well as LPL activity in skeletal muscle (n = 3–11 per group). B) Angptl4 and LPL mRNA expression as well as LPL activity in hypothalamus (n = 9–11 per group). C) Angptl4 and LPL mRNA expression as well as LPL activity in hypothalamus (n = 9–11 per group). Open bars represent control mice, and black bars represent STZ-induced diabetic mice. Data are mean ± SEM. * = p ≤ 0.05, ** = p ≤ 0.01, and *** = p ≤ 0.001 by Student's t-test and two-way ANOVA.
3.4. Differential regulation of Angptl4 and LPL in hypothalamus and muscle
STZ diabetes increased mRNA expression of Angptl4 in the skeletal muscle, hypothalamus and cortex of wildtype mice (Figures 1 and 3A–C). In Angptl4 knockout mice LPL mRNA expression was upregulated in skeletal muscle and restored to control levels after induction of STZ diabetes, whereas LPL expression was unchanged in hypothalamus and cortex independent of genotype and diabetes status (Figure 3). Similarly, LPL activity was upregulated in skeletal muscle with STZ induced diabetes in both genotypes, while no differences were detected in either hypothalamus or cortex in response to diabetes or knockout of the Angptl4 gene (Figure 3B–C). To exclude an effect of nutritional status as an explanation for the lack of difference in central versus peripheral LPL activity, the experiment was conducted in random fed as well as overnight fasted mice with similar results (Supplemental Figure 2).
Since LPL in brain has previously been shown to affect appetite and energy expenditure [13], we directly assessed the effect of recombinant Angptl4 delivered i.c.v. (10 ng/h) on food intake and body weight in C57Bl/6J mice fed a chow diet (CD) or high fat diet (HFD). As seen in Figure 4, i.c.v. administration of Angptl4 over 5 days did not affect food intake of mice independent of whether they were on chow or high fat diet, and no change in body weight was observed compared to mice receiving vehicle (Figure 4A–B). Random fed blood glucose was only measured in the chow diet mice, and was not significantly altered after 5 days of saline or Angptl4 i.c.v. treatment (93.8 ± 6.5 vs 97.8 ± 6.8 mg/dl; saline vs Angptl4). In addition, we did not detect any changes on hypothalamic mRNA expression of orexigenic or anorexigenic neuropeptides; NPY, AgRP, POMC, or CART between Angptl4 knockout mice and their control littermates (Figure 4C), consistent with our findings that i.c.v. delivered Angptl4 does not affect food intake.
Figure 4.
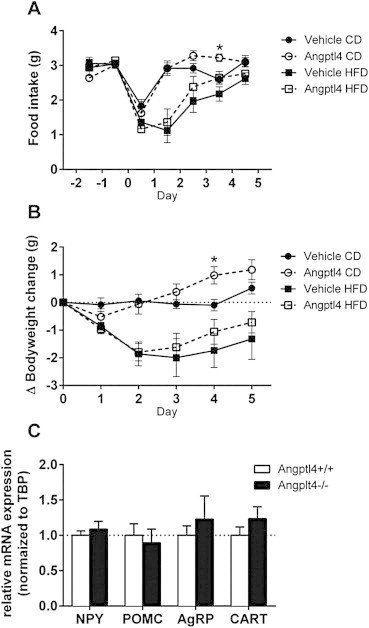
Central infusion of recombinant Angptl4 or vehicle does not change food intake or body weight in mice fed either a chow diet (CD) or high fat diet (HFD). A) Food intake in C57Bl/J6 mice infused with vehicle (closed symbols) or recombinant Angptl4 (10 ng/h) (open symbols) on either CD (circles) or HFD (squares) (n = 5 per group). B) Show the delta change in bodyweight in the C57Bl/J6 mice infused with vehicle or recombinant Angptl4 (10 ng/h) on either CD or HFD (n = 5 per group). C) mRNA expression of NPY, POMC, CART and AgRP in hypothalamus of Angptl4 knockout (Angptl4−/−) mice and their wildtype (Angptl4+/+) controls. (n = 6–8 per group). Data are mean ± SEM. * = p ≤ 0.05 Student's t-test compared to vehicle.
4. Discussion
Alterations in lipid metabolism in periphery and the central nervous system (CNS) are important components of the development of type 2 diabetes and the metabolic syndrome [19,23–25]. Elevated triglyceride-rich lipoproteins are a characteristic of type 2 diabetes and insulin resistance, and a decreased capacity for eliminating triglyceride-rich lipoproteins in the peripheral tissues, as well as in the CNS, may participate in the development of diabetes, as well as neurological diseases, such as Alzheimer's [26,27].
The key enzyme for lipoprotein triglyceride hydrolysis is LPL. As Angptl4 is a known inhibitor of LPL activity in the peripheral tissues [4,6], we explored the role of Angptl4 as a regulator of LPL activity and a potential contributing factor in brain lipid metabolism. We find that Angptl4 expression in hypothalamus is upregulated in both type 1 and type 2 diabetic mice models, paralleling and similar to the increases observed in a classical insulin sensitivity tissues, such as skeletal muscle (current study), adipose tissue and liver [28]. Furthermore i.c.v. injection of insulin reverses the increased expression of hypothalamic Angptl4 observed in STZ diabetes, indicating that this is direct regulation of expression by insulin similar to what occurs in liver and adipose tissue [28].
To our surprise there was no difference in LPL activity in hypothalamus or cortex between control and Angptl4 knockout mice, nor was overall brain LPL activity altered by STZ-induced diabetes or nutritional status, opposite to what we observed in skeletal muscle. On the other hand, serum triglycerides were increased in STZ-induced diabetes, and this increase was lower in Angptl4 deficient mice (Supplementary Table 2), confirming previous reports [29]. In the Angptl4 knockout mice LPL activity was increased as expected in the skeletal muscle, but as shown in Figure 3A, STZ treatment alone induced an increase in LPL activity compared to the control mice in both wildtype and knockout mice. A similar increase has been reported in hearts from STZ-diabetic rats [30]. The increase in LPL activity in wild-type mice made STZ-diabetic occurred despite an increase in Angptl4 mRNA level upon induction of STZ-diabetes and no change in LPL mRNA expression in skeletal muscle, consistent with regulation of LPL activity at the posttranslational level [3]. This increase in LPL activity in skeletal muscle could be a part of a compensatory mechanism to replace the diminished contribution of glucose as an energy source. This could also contribute to increased intramuscular triglyceride accumulation, which is known to occur in diabetes [23]. Consistent with its role as a negative regulator of LPL activity, Angptl4-knockout mice exhibit increased levels of LPL activity in skeletal muscle in both the basal and diabetic state. Although STZ-diabetes itself had little or no effect on serum TG levels in the fed state, the increased LPL activity in Angptl4 knockout mice resulted in a 50% reduction in serum TG levels, and previous studies have shown that this is associated with increased ectopic accumulation of TG in liver [31].
The lack of change in LPL activity in the brain in our study may be due to the relatively short period (overnight) of fasting, since 72 h of fasting is necessary to detect altered brain LPL activity in rats [32]. However, this may also reflect the fact that Angptl4 is almost exclusively expressed in glial cells (Figures 1A and 3A), and thus may act only locally on nearby cells in the brain or hypothalamus. Although it has been suggested that hyperglycemia might regulate Angptl4 expression [33], these previous studies were done with very high levels of glucose, much beyond those that occur in vivo in the CNS, even in diabetes. We find that in glial cells in culture, Angptl4 mRNA expression is regulated directly by insulin, not glucose, confirming our in vivo observations. Importantly, insulin upregulates LPL activity in glial cells at the same time point where the lowest Angptl4 expression was detected, suggesting that Angptl4 may play a role in LPL activity in glial cells and perhaps in the interplay known to exist between glial cells and neurons [34,35].
The notion that diabetes status can affect LPL activity in the nervous system is supported by Ferreira and colleagues, who showed that STZ treatment inhibits LPL activity in the sciatic nerve, and this could be restored by insulin treatment [36]. In addition, recent studies have shown that fatty acid uptake in brain is reduced in the hypothalamus of neuron-specific knockout of LPL compared to control, and this impairment of brain triglyceride metabolism impacts both food intake and energy expenditure resulting in obesity [13]. However, in contrast to Kim et al. [11] who reported that i.c.v. Angptl4 can decrease food intake in mice and that Angptl4 knockout mice exhibited increased body weight compared to controls, we find no role of Angptl4 in regulation of food intake or body weight when delivered i.c.v. The reason for the difference between our study and the study by Kim et al. is not clear, but could be due to the use of a different preparation of recombinant Angptl4, as well as different strains of mice.
It is intriguing that we find Angptl4 upregulated in hypothalamus in both type 1 and type 2 diabetes models. The upregulation in type 1 diabetes is due to lack of insulin at the level of the brain, and i.c.v. injection of insulin can reverse this upregulation. The increase in Angplt4 in type 2 diabetes in hypothalamus of ob/ob mice is most likely due to insulin resistance, since we [37,38] and others [39,40] have shown, obesity is associated with increased inflammation and mitochondrial dysfunction at the level of the hypothalamus and both of these can contribute to an altered insulin response. This upregulation of Angptl4 in the hypothalamus in insulin resistant states could lead to decreased LPL activity in the neurons, and decreased LPL activity in neurons has been shown to lead to hyperphagia and obesity [13] thus creating a viscous cycle of events.
There is increasing evidence that altered insulin action and metabolism in brain may affect both brain function and brain control of metabolism. We have previously shown that insulin regulates cholesterol synthesis and synapse maintenance in both neurons and glia cells in diabetic mice [19], and other studies have suggested that adult neurons may rely on delivery of cholesterol from nearby glial cells for normal synapse formation [22]. Several indicators have shown that central insulin action is associated with cognitive function of the brain. Both patients with type 1 and type 2 diabetes are at higher risk for behavioral changes and accelerated cognitive decline with aging [41]. In humans with either type 1 or type 2 diabetes, imaging studies have shown smaller hippocampi, as well as changes in the functional connectivity between regions of the brain [42–44]. Cholesterol is delivered to the neurons from glial synthesis by ApoE-containing lipoproteins, and this cholesterol is used to support synaptogenesis and maintenance of synaptic connection [22]. The variant of ApoE termed ApoEɛ4 has been linked to the development of Alzheimer's disease by an increased amyloid beta protein (Aβ) deposition [45]. LPL is present in both glial cells and neurons (present study and Refs. [46,47]), and it has been shown that LPL is co-localized with senile plaques containing Aβ [47], which is one of the hallmarks in the development of Alzheimer disease. Since Aβ deposition is crucial for the pathogenesis of Alzheimer's, a potential role for Angptl4 could be to interrupt this interaction and thereby prevent or reduce Aβ LPL mediated uptake. To fully elucidate Angptl4's role in all of these brain function will require further studies.
In summary, Angptl4 expression in hypothalamus is upregulated in both a type 1 and type 2 diabetic animal models, and insulin restores the expression level to normal in the type 1 diabetic mice. This regulation of Angptl4 occurs primarily in glial cells, and in this context, Angptl4 may participate in local regulation of LPL activity, metabolic crosstalk between glial cells and neurons, and function of the brain at both the neuronal level and in brain control of whole body metabolism.
Acknowledgments
This work was supported by National Institutes of Health, United States Grant DK-33201 and the Mary K. Iacocca Professorship. The study was also facilitated by support from the Joslin Diabetes and Endocrinology Research Center Core Laboratories (DK-036836). S.G.V. was supported by the Novo Nordisk STAR International postdoctoral fellowship. A.K. was supported by a German Research Foundation (DFG) project Kl2399-1/1 grant. No potential conflicts of interest relevant to this article were reported.
S.G.V. conceived and designed the experiments, researched data, and wrote the manuscript. A.K. and R.S. researched data, contributed to discussion, and reviewed and edited the manuscript. C.R.K. conceived and designed the experiments, reviewed the data, and wrote the manuscript. C.R.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
None declared.
Appendix A. Supplementary material
The following are the Supplementary material related to this article:
Figure S1. A) Glial fibrillary acidic protein (GFAP) mRNA expression B) Neurofilament-M mRNA expression in primary isolated glial cells and neurons (n = 5). LPL activity was assessed on both media and glial cells (intrinsic) treated with or without insulin (10 nM) for C) 3 h, D) 6 h and E) 24 h (n = 4 for each time point). Data are mean ± SEM. * = p ≤ 0.05 by Student's t-test.
Figure S2. LPL activity in skeletal muscle, hypothalamus and brain cortex of Angptl4 knockout (Angptl4−/−) mice and their wildtype (Angptl4+/+) littermates overnight fasted A) Angptl4 and LPL mRNA expression as well as LPL activity in skeletal muscle (n = 3–11 per group). B) Angptl4 and LPL mRNA expression as well as LPL activity in hypothalamus (n = 9–11 per group). C) Angptl4 and LPL mRNA expression as well as LPL activity in hypothalamus (n = 9–11 per group). Open bars represent control mice, and black bars represent STZ-induced diabetic mice. Data are mean ± SEM. * = p ≤ 0.05, ** = p ≤ 0.01, and *** = p ≤ 0.001 by Student's t-test and two-way ANOVA.
References
- 1.Schwartz M.W., Porte D. Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 2.Obici S., Zhang B.B., Karkanias G., Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nature Medicine. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 3.Wang H., Eckel R.H. Lipoprotein lipase: from gene to obesity. American Journal of Physiology – Endocrinology and Metabolism. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 4.Kersten S., Mandard S., Tan N.S., Escher P., Metzger D., Chambon P. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. Journal of Biological Chemistry. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 5.Ge H., Cha J.Y., Gopal H., Harp C., Yu X., Repa J.J. Differential regulation and properties of angiopoietin-like proteins 3 and 4. Journal of Lipid Research. 2005;46:1484–1490. doi: 10.1194/jlr.M500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Yau Mh, Wang Y., Lam K.S.L., Zhang J., Wu D., Xu A. A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. Journal of Biological Chemistry. 2009;284:11942–11952. doi: 10.1074/jbc.M809802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu A., Lam M.C., Chan K.W., Wang Y., Zhang J., Hoo R.L.C. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6086–6091. doi: 10.1073/pnas.0408452102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin W., Romeo S., Chang S., Grishin N.V., Hobbs H.H., Cohen J.C. Genetic variation in ANGPTL4 provides insights into protein processing and function. Journal of Biological Chemistry. 2009;284:13213–13222. doi: 10.1074/jbc.M900553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köster A., Chao Y.B., Mosior M., Ford A., Gonzalez-DeWhitt P.A., Hale J.E. Transgenic angiopoietin-like (Angptl)4 overexpression and targeted disruption of Angptl4 and Angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 10.Mandard S., Zandbergen F., van Straten E., Wahli W., Kuipers F., Müller M. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. Journal of Biological Chemistry. 2006;281:934–944. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- 11.Kim H.K., Youn B.S., Shin M.S., Namkoong C., Park K.H., Baik J.H. Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes. 2010;59:2772–2780. doi: 10.2337/db10-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouleti C., Mathivet T., Coqueran B., Serfaty J.M., Lesage M., Berland E. Protective effects of angiopoietin-like 4 on cerebrovascular and functional damages in ischaemic stroke. European Heart Journal. 2013;34:3657–3668. doi: 10.1093/eurheartj/eht153. [DOI] [PubMed] [Google Scholar]
- 13.Wang H., Astarita G., Taussig M.D., Bharadwaj K.G., DiPatrizio N.V., Nave K.A. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metabolism. 2011;13:105–113. doi: 10.1016/j.cmet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H., Eckel R.H. Lipoprotein lipase in the brain and nervous system. Annual Review of Nutrition. 2012;32:147–160. doi: 10.1146/annurev-nutr-071811-150703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papassotiropoulos A., Wollmer M.A., Tsolaki M., Brunner F., Molyva D., Lütjohann D. A cluster of cholesterol-related genes confers susceptibility for Alzheimer's disease. Journal of Clinical Psychiatry. 2005;66:940–947. [PubMed] [Google Scholar]
- 16.Kim J.K., Fillmore J.J., Chen Y., Yu C., Moore I.K., Pypaert M. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H., Knaub L.A., Jensen D.R., Young Jung D., Hong E.G., Ko H.J. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes. 2009;58:116–124. doi: 10.2337/db07-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki R., Lee K., Jing E., Biddinger S.B., McDonald J.G., Montine T.J. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metabolism. 2010;12:567–579. doi: 10.1016/j.cmet.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen D.R., Knaub L.A., Konhilas J.P., Leinwand L.A., MacLean P.S., Eckel R.H. Increased thermoregulation in cold-exposed transgenic mice overexpressing lipoprotein lipase in skeletal muscle: an avian phenotype? Journal of Lipid Research. 2008;49:870–879. doi: 10.1194/jlr.M700519-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollare T., Vessby B., Lithell H. Lipoprotein lipase activity in skeletal muscle is related to insulin sensitivity. Arteriosclerosis, Thrombosis, and Vascular Biology. 1991;11:1192–1203. doi: 10.1161/01.atv.11.5.1192. [DOI] [PubMed] [Google Scholar]
- 22.Pfrieger F.W. Cholesterol homeostasis and function in neurons of the central nervous system. Cellular and Molecular Life Sciences. 2003;60:1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krssak M., Petersen K.F., Dresner A., DiPietro L., Vogel S., Rothman D. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Tilve D., Davidson W.S., Tschöp M., Hofmann S.M. CNS regulation of plasma cholesterol. Annals of Medicine. 2011;44:656–663. doi: 10.3109/07853890.2011.590819. [DOI] [PubMed] [Google Scholar]
- 25.Reaven G.M. Insulin resistance: the link between obesity and cardiovascular disease. Medical Clinics of North America. 2011;95:875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Craft S., Cholerton B., Baker L.D. Insulin and Alzheimer's disease: untangling the web. Journal of Alzheimer's Disease. 2013;33:S263–S275. doi: 10.3233/JAD-2012-129042. [DOI] [PubMed] [Google Scholar]
- 27.Burgess B.L., McIsaac S.A., Naus K.E., Chan J.Y., Tansley G.H.K., Yang J. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer's disease mouse models with abundant A beta in plasma. Neurobiology of Disease. 2006;24:114–127. doi: 10.1016/j.nbd.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Mizutani N., Ozaki N., Seino Y., Fukami A., Sakamoto E., Fukuyama T. Reduction of insulin signaling upregulates angiopoietin-like protein 4 through elevated free fatty acids in diabetic mice. Experimental and Clinical Endocrinology & Diabetes. 2012;120:139–144. doi: 10.1055/s-0031-1291258. [DOI] [PubMed] [Google Scholar]
- 29.Adachi H., Kondo T., Koh G.Y., Nagy A., Oike Y., Araki E. Angptl4 deficiency decreases serum triglyceride levels in low-density lipoprotein receptor knockout mice and streptozotocin-induced diabetic mice. Biochemical and Biophysical Research Communications. 2011;409:177–180. doi: 10.1016/j.bbrc.2011.04.110. [DOI] [PubMed] [Google Scholar]
- 30.Sambandam N., Abrahani M.A., Craig S., Al-Atar O., Jeon E., Rodrigues B. Metabolism of VLDL is increased in streptozotocin-induced diabetic rat hearts. American Journal of Physiology – Heart and Circulatory Physiology. 2000;278:H1874–H1882. doi: 10.1152/ajpheart.2000.278.6.H1874. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenstein L., Mattijssen F., de Wit N.J., Georgiadi A., Hooiveld G.J., van der Meer R. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metabolism. 2010;12:580–592. doi: 10.1016/j.cmet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gavin L.A., Cavalieri R.R., Moeller M., McMahon F.A., Castle J.N., Gulli R. Brain lipoprotein lipase is responsive to nutritional and hormonal modulation. Metabolism. 1987;36:919–924. doi: 10.1016/0026-0495(87)90124-7. [DOI] [PubMed] [Google Scholar]
- 33.Sango K., Suzuki T., Yanagisawa H., Takaku S., Hirooka H., Tamura M. High glucose-induced activation of the polyol pathway and changes of gene expression profiles in immortalized adult mouse Schwann cells IMS32. Journal of Neurochemistry. 2006;98:446–458. doi: 10.1111/j.1471-4159.2006.03885.x. [DOI] [PubMed] [Google Scholar]
- 34.Piet R., Vargová L., Syková E., Poulain D.A., Oliet S.H.R. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricci G., Volpi L., Pasquali L., Petrozzi L., Siciliano L. Astrocyte–neuron interactions in neurological disorders. Journal of Biological Physics. 2009;35:317–336. doi: 10.1007/s10867-009-9157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira L.D.M.C., Huey P.U., Pulford B.E., Ishii D.N., Eckel R.H. Sciatic nerve lipoprotein lipase is reduced in streptozotocin-induced diabetes and corrected by insulin. Endocrinology. 2002;143:1213–1217. doi: 10.1210/endo.143.4.8723. [DOI] [PubMed] [Google Scholar]
- 37.Kleinridders A., Ferris H.A., Cai W., Kahn C.R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63:2232–2243. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinridders A., Lauritzen H.P.M.M., Ussar S., Christensen J.H., Mori M.A., Bross P. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. Journal of Clinical Investigation. 2013;123:4667–4680. doi: 10.1172/JCI67615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O. Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pipatpiboon N., Pratchayasakul W., Chattipakorn N., Chattipakorn S.C. PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology. 2011;153:329–338. doi: 10.1210/en.2011-1502. [DOI] [PubMed] [Google Scholar]
- 41.Biessels G.J., Deary I.J., Ryan C.M. Cognition and diabetes: a lifespan perspective. The Lancet Neurology. 2008;7:184–190. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 42.Lyoo I.K., Yoon S., Renshaw P.F., Hwang J., Bae S., Musen G. Network-level structural abnormalities of cerebral cortex in type 1 diabetes mellitus. PLoS ONE. 2013;8:e71304. doi: 10.1371/journal.pone.0071304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antenor-Dorsey J.A., Meyer E., Rutlin J., Perantie D.C., White N.H., Arbelaez A.M. White matter microstructural integrity in youth with type 1 diabetes. Diabetes. 2013;62:581–589. doi: 10.2337/db12-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musen G., Jacobson A.M., Bolo N.R., Simonson D.C., Shenton M.E., McCartney R.L. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes. 2012;61:2375–2379. doi: 10.2337/db11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J., Basak J.M., Holtzman D.M. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldberg I.J., Soprano D.R., Wyatt M.L., Vanni T.M., Kirchgessner T.G., Schotz M.C. Localization of lipoprotein lipase mRNA in selected rat tissues. Journal of Lipid Research. 1989;30:1569–1577. [PubMed] [Google Scholar]
- 47.Nishitsuji K., Hosono T., Uchimura K., Michikawa M. Lipoprotein lipase is a novel amyloid β (Aβ)-binding protein that promotes glycosaminoglycan-dependent cellular uptake of Aβ in astrocytes. Journal of Biological Chemistry. 2011;286:6393–6401. doi: 10.1074/jbc.M110.172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


