Abstract
Objective
Obesity is often accompanied by hyperactivity of the neuroendocrine stress axis and has been linked to an increased risk of psychiatric disorders. Insulin is reciprocally regulated with the stress hormone corticosterone (CORT), raising the possibility that insulin normally provides inhibitory tone to the hypothalamus-adrenal-pituitary (HPA) axis. Here we examined whether disrupting signaling via the insulin receptor (InsR) in hypothalamic subpopulations impacts the neuroendocrine response to acute psychological stress.
Methods
We used Nkx2.1-Cre, Sim1-Cre and Agrp-Cre transgenic driver lines to generate conditional knockouts of InsR signaling throughout the hypothalamus, paraventricular nucleus of the hypothalamus (PVH) and in neurons expressing Agouti-related peptide (AgRP) in the arcuate nucleus of the hypothalamus (ARH), respectively. We used a combination of molecular, behavioral and neuroendocrine criteria to evaluate the consequences on HPA axis responsiveness.
Results
Endpoints related to body weight and glucose homeostasis were not altered in any of the conditional mutant lines. Consistent with observations in the neuronal Insr knockout mice (NIRKO), baseline levels of serum CORT were similar to controls in all three lines. In male mice with broad disruptions of InsR signals in Nkx2.1-expressing regions of the hypothalamus (IRNkx2.1 KO), we observed elevated arginine vasopressin (AVP) levels at baseline and heightened neuroendocrine responses to restraint stress. IRNkx2.1 KO males also exhibited increased anxiety-like behaviors in open field, marble burying, and stress-induced hyperthermia testing paradigms. HPA axis responsivity was not altered in IRSim1 KO males, in which InsR was disrupted in the PVH. In contrast to observations in the IRNkx2.1 KO males, disrupting InsR signals in ARH neurons expressing Agrp (IRAgrp KO) led to reduced AVP release in the median eminence (ME).
Conclusions
We find that central InsR signals modulate HPA responsivity to restraint stress. InsR signaling in AgRP/NPY neurons appears to promote AVP release, while signaling in other hypothalamic neuron(s) likely acts in an opposing fashion. Alterations in InsR signals in neurons that integrate metabolic and psychiatric information could contribute to the high co-morbidity of obesity and mental disorders.
Keywords: Insulin, Hypothalamus, AgRP, HPA axis, Stress response
Abbreviations: ACTH, adrenocorticotropic hormone; AgRP, agouti-related peptide; ARH, arcuate nucleus of the hypothalamus; AVP, arginine vasopressin; CORT, corticosterone; CRH, corticotropin-releasing hormone; FST, forced swim test; Gr, Glucocorticoid receptor; HPA axis, Hypothalamus–Pituitary–Adrenal axis; InsR, insulin receptor; IRAgrp KO, knockout of InsR using Agrp-Cre; IRNkx2.1 KO, knockout of InsR using Nkx2.1-Cre; IRSim1 KO, knockout of InsR using Sim1-Cre; MB, marble burying test; MBH, mediobasal hypothalamus; ME, median eminence; NPY, neuropeptide Y; NSF, novelty suppressed feeding test; OF, open field test; POMC, pro-opiomelanocortin; SIH, stress-induced hyperthermia test
1. Introduction
Epidemiological studies have identified a link between obesity and psychiatric disorders, particularly those involving stress-related depressive symptoms [1–4]. The well-documented effects of glucocorticoids to promote fuel mobilization and insulin secretion led several groups to hypothesize that dysregulation of the neuroendocrine stress axis contributes to the development of metabolic and cardiovascular diseases [5–7]. However, evidence from some prospective studies supports the idea that obesity promotes risk of depression, but not the reverse [8,9]. HPA axis hyperactivity in obese patients is likely due to alterations in central signaling pathways, as they exhibit normal pituitary sensitivity to negative feedback from glucocorticoids [10]. Progress in elucidating the molecular and neuronal players that could mediate the effects of signals of metabolic status on stress responses have been described in several recent reviews [11–13].
The neuroendocrine stress response axis is strongly influenced by the availability of food and energy stores. Basal activity is lowest and stress responsivity highest in the energy replete state [5]. Conversely, fasting is associated with elevated basal levels of CORT and decreased responsivity to stressors [5,14]. ARH neurons sense nutrient and hormone signals of energy status [15] and project to critical nodes of the HPA axis [16], and thus are well-positioned to serve as the conduit for metabolic influences on stress responses. Inhibition of the activity of the mediobasal hypothalamus (MBH) produces a fasting-like pattern of HPA axis activity in fed animals, with no effect on stress responses in the fasted state [14], consistent with idea that the MBH provides signals of positive energy balance to the HPA axis.
There are several hormone signals of positive energy balance that are sensed by MBH neurons and are also reported to modulate stress responses, including leptin, glucagon-like peptide-1 and cholecystokinin (reviewed in [13]). However, conflicting results from studies using gain- and loss-of function approaches have hindered progress toward elucidating the underlying mechanism. Whereas central administration of these hormones has been reported to activate neurons expressing corticotropin-releasing hormone (CRH, also known as CRF) and promote CORT release [17–19], loss of function mutations in the corresponding receptors are associated with increased HPA tone, consistent with an inhibitory effect of these hormones on stress responses [20–22]. Differences in the type, severity and duration of the experimental stressors applied by different groups likely contribute to these apparent inconsistencies. In addition, most studies lack the temporal resolution to distinguish processes associated with the initiation of the stress response from those that are activated to provide negative feedback. Finally, it is possible that different populations in the brain and periphery that are activated by these signals could influence different aspects of the stress response.
Insulin is reciprocally regulated with CORT during stress, consistent with the idea that insulin normally provides inhibitory tone to HPA axis [5]. However, as is the case for other gain of function studies, there are studies that report effects of brain insulin to suppress [23] and enhance [24] the neuroendocrine stress response. As there is a positive correlation between HPA axis responsivity and homeostasis insulin resistance index (HOMA-IR) in obese patients [7,25], we set out to examine whether impaired central insulin signaling in lean animals impacts stress axis function. To this end, we utilized the Nkx2.1-Cre transgene to broadly disrupt signaling through the insulin receptor (InsR) in the hypothalamus [26–28]. After discovering that the resulting IRNkx2.1 KOs have impaired negative feedback to the HPA axis, we generated additional conditional InsR knockouts to assess the contribution of Agrp-Cre-expressing neurons in the ARH (IRAgrp KOs) and Sim1-Cre-expressing neurons in the PVH and amygdala (IRSim1 KOs) to this phenotype.
2. Material and methods
2.1. Animal husbandry
Mice were maintained in a temperature- (22 ± 1 °C) and light- (12:12h light dark cycle, lights on at 7pm and off at 7pm) controlled environment. Pups were weaned on post-natal day 21. Unless otherwise noted, mice had ad libitum access to chow (9% calories from fat, 5058 Mouse diet 20, Labdiet) and water until the time of sacrifice. Male mice older than 8 weeks of age were used in all studies. Since female sex hormones are known to influence activity of the HPA axis [29], we limited our analysis in this study to male mice. All procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at Columbia University Health Sciences Division.
2.2. Generation of IRNkx2.1, IRSim1 and IRAgRP KO mice
To generate conditional knockouts of Insr in the hypothalamus, we crossed the Nkx2.1-Cre driver line (C57BL/6J-Tg(Nkx2-1-Cre)2Sand/J, provided by S. Anderson, Weill Cornell Medical College) [27] to mice homozygous for a floxed allele of Insr (B6.129S4(FVB)-Insrtm1Khn/J) [26] to generate Nkx2.1-cre;Insrfl/fl mice (IRNkx2.1 KO). Sim1-Cre;Insrfl/fl (IRSim1 KO) and Agrp-Cre;Insrfl/fl (IRAgRP KO) mice were generated by a similar mating scheme using Sim1-Cre (B6.FVB(129X1)-Tg(Sim1-cre)1Lowl/J) [30] and Agrp-Cre (Agrptm1(cre)Lowl) [31] drivers, respectively (provided by Brad Lowell, Beth Israel Deaconness Medical Center). Insrfl/fl littermates served as controls in all studies. To avoid confounding effects of estrus cyclicity on the HPA axis [32], we performed all analyses in males. Mouse genotypes were assessed by PCR on genomic DNA from tail tips using the following primers: Cre: 5′ GCGGTCTGGCAGTAAAAACTATC 3’ (forward), 5′ GTGAAACAGCATTGCTGTCACTT 3’ (reverse); Insr: 5′ TGCACCCCATGTCTGGGACCC 3’ (forward), 5′ GCCTCCTGAATAGCTGAGACC 3’ (reverse).
2.3. Measurement of serum corticosterone levels
We collected serum from tail bleeds on minimally-stressed animals between 10am and noon (AM samples) or 6–7pm (PM samples), or at 10am after an overnight (14–16 h) fast with ad libitum access to drinking water. Blood was clotted at room temperature for one hour and centrifuged to isolate serum. Serum was stored at −20 °C until assessed by CORT radioimmunoassay (MP Biomedicals) or Rat/Mouse CORT ELISA (Alpco).
2.4. Restraint stress response and dexamethasone suppression test
We restrained mice individually in a 50-ml falcon tube for 30 min. We collected tail blood samples at 0, 30 (end of restraint), and 60 min (30 min after restraint) after the beginning of a restraint. For the dexamethasone (DEX) suppression test, we injected mice with saline or DEX (0.1 mg/kg, i.p.) 60 min before a 60 min-long restraint session. We collected tail blood at baseline (before the saline/DEX injections), immediately before restraint (60 min after the saline/DEX injections), at the end of the 60 min restraint period, and 30 min after the end of restraint. We collected and analyzed CORT levels in serum samples as described above.
2.5. Preservation of hypothalamus and pituitary
At the time of sacrifice, animals were anesthetized with 2.5% Avertin, 0.02 ml/g i.p., before cervical dislocation. Hypothalamus (whole hypothalamus, PVH, or median eminence) and pituitary were harvested within 5 min of the avertin injection, and snap-frozen in liquid nitrogen. PVH was dissected out bilaterally from the anterior part (1 mm-thick coronal section) of the hypothalamus and median eminence was dissected from the posterior part (2 mm-thick coronal section) of the basal hypothalamus.
2.6. Quantitative RT-PCR
For IRNkx2.1 KO and IRAgRP KO mice, we isolated total RNA from fresh-frozen hypothalamus and pituitary using the RNeasy Plus Universal Kit (Qiagen) and synthesized cDNA using the Transcriptor First Strand cDNA Synthesis kit (Roche). We used a LightCycler 480 SYBR Green I Master System (Roche) in quantitative PCR experiments. We normalized the expression of target genes against Beta actin. For IRSim1 KO mice, we extracted RNA using the ARCTURUS Picopure RNA Isolation Kit and performed quantitative PCR in an ABI-PRISM 7900 HT Sequence Detection system using fluorogenic Taqman probes specific for the target genes (Applied Biosystems).
Primer Sequences:
B. actin (β-actin): 5′ CGCCACCAGTTCGCCAT 3’ (forward), 5′ CTTTGCACATGCCGGAGC 3′ (reverse); Corticotropin-releasing hormone (Crh): 5′ AGGAGGCATCCTGAGAGAAGT 3’ (forward), 5′ CATGTTAGGGGCGCTCTC 3′ (reverse); Arginine vasopressin (Avp): 5′ ACTACCTGCCCTCGCCCTGC 3′ (forward), 5′ GCCACGCAGCTCTCGTCGCT 3′ (reverse); Glucocorticoid receptor (Gr): 5′ ACTTCGCAGGCCGCTCAGTGTT 3′ (forward), 5′ TGGTCCCGTTGCTGTGGAGGAGC 3′ (reverse); Pro-opiomelanocortin (Pomc): 5′ GCAACCTGCTGGCTTGCATCCG 3′ (forward), 5′ CCGAAGCGGTCCCAGCGGAA 3′ (reverse); Neuropeptide Y (Npy): 5′ TCCGCTCTGCGACACTACAT 3′ (forward), 5′ GGAAGGGTCTTCAAGCCTTGTT 3′ (reverse); Agouti-related peptide (Agrp): 5′ GCAATGTTGCTGAGTTGTGT 3′ (forward), 5′ GACTTAGACCTGGGAACTCTG 3′ (reverse).
2.7. Hypothalamic peptide levels
Hypothalamic and median eminence AVP and corticotropin releasing hormone (CRH, also known as CRF) levels were measured with Vasopressin [Arg8] Chemiluminescent EIA (Phoenix Pharmaceuticals) and Corticotropin Releasing Factor Chemiluminescent EIA (Phoenix Pharmaceuticals) kits, respectively. ACTH levels were measured by RIA in the lab of Sharon Wardlaw, as previously described [33].
2.8. Behavioral tests
All behavioral tests were performed between 10am and 2pm.
Open Field Test (OF): After one-hour acclimation to the testing room, we recorded the activity of each mouse in the open field arena for 30 min. We calculated the total distance traveled, and ratio of time spent in the periphery and center of the field; reduced central activity is considered to be an indicator of increased anxiety [34].
Marble Burying Test (MB): We placed each mouse in a cage filled with 4–5 cm of wood chip bedding, and placed 12 marbles, evenly spaced, on top of the bedding. We recorded the number of marbles buried in the bedding within a 20-minute period. An increase in the number of marbles buried is used as an indicator of anxiety- and obsessive-compulsive-disorder-like behavior [35]. We compared marble burying behavior at baseline and one hour after an i.p. injection with 50 μl saline (stress condition).
Stress-Induced Hyperthermia Test (SIH): Stress associated with handling and insertion of a rectal temperature probe thermometer (Thermoworks) causes an increase in core body temperature, an anxiety response that is suggested to be mediated in part by the effects of CORT to increase sympathetic tone [36]. We measured body temperature at baseline and 60 min after stress.
Novelty Suppressed Feeding Test (NSF): After an overnight fast, we placed each mouse in a brightly lit box (30 cm × 40 cm) with bedding on the floor and an accessible food pellet tied down to a circle of Whatman paper in the center of the box. We recorded the time it took an animal to enter the brightly lit center of the arena and to bite the food pellet. Increased latency to feed in a novel environment is considered to be an indicator of anxiety-like behavior [37].
Forced swimming test (FST): We placed mice individually in beakers (46 cm tall × 32 cm in diameter) containing 23–25 °C water 30 cm deep. We conducted two swim sessions between 12:00 and 18:00 h: an initial 6-minute pretest followed 24 h later by a 6-minute test. We monitored and videotaped mice while swimming and recorded the amount of time immobile. Increased immobility is considered to be an indicator of depressive behavior [38].
2.9. Glucose homeostasis
Baseline glucose levels measurements and glucose tolerance test were performed as previously described [39].
2.10. Statistics
Data are presented as group mean ± SEM. We performed statistical comparisons between groups using a one-way ANOVA, followed by a post-hoc Bonferroni correction. We considered a P value of 0.05 or less to be statistically significant.
3. Results
3.1. Diminished InsR signals in Nkx2.1-expressing neurons leads to HPA axis hyperactivity
To explore the possibility that central insulin signals modulate the HPA axis, we assessed the consequences of disrupting InsR signals in Nkx2.1-expressing neurons in the forebrain on CORT levels at baseline and in response to psychological and physiological stress. We did not observe a difference between IRNkx2.1 KO and control males in the morning (Figure 1A), at the nadir of circadian fluctuations in serum CORT levels [40]. CORT levels were approximately 31% and 56% higher in IRNkx2.1 KO males in the evening and in response to fasting, respectively; however, these differences did not reach significance (Figure 1A). Control and IRNkx2.1 KO males exhibited similar CORT levels at the end of a 30-minute restraint period, but CORT levels 30 min after release from restraint were ∼17% higher in IRNkx2.1 KOs (Figure 1B). Since mice carrying the Nkx2.1-Cre transgene alone did not show an overall change in response to restraint when compared to wild-type animals (data not shown), the altered stress response in IRNkx2.1 KOs can be attributed to the loss of InsR signals.
Figure 1.
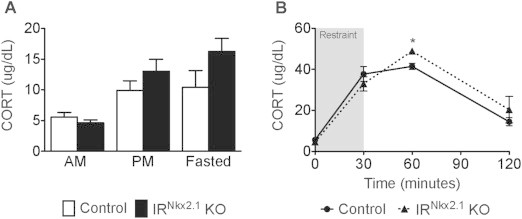
Loss of hypothalamic InsR signals in Nkx2.1 neurons resulted in increased CORT response to restraint. (A) Baseline (AM), peak (PM), and fasting (fasted) serum CORT levels in adult males; n ≥ 7 for all groups at all time points. (B) Serum CORT levels during a restraint test (baseline, at the end of restraint (the period of restraint is indicated by a grey box), and 30 min after restraint) in adult males; n ≥ 12 for IRNkx2.1 KO and controls at all time points. (B) *P < 0.05 control versus IRNkx2.1 KO.
As the neuroendocrine response to restraint was elevated in IRNkx2.1 KOs, we next performed a dexamethasone (DEX)-suppression test to determine whether the deficit in negative feedback occurs at the level of the pituitary. Saline-injected IRNkx2.1 KO males exhibited a 51% increase in CORT levels as compared to controls at the end of the 60 min restraint period (Figure 2A) and a 14% increase in stress responsivity (Figure 2C). IRNkx2.1 KO males were sensitive to DEX suppression (Figure 2B), supporting the idea that negative feedback to the HPA axis via the pituitary is not impaired.
Figure 2.
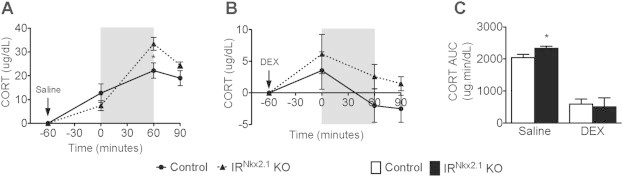
Normal response of IRNkx2.1KOs to dexamethasone-suppression test. (A) Change in serum CORT levels from baseline in saline-treated adult males (the period of restraint is indicated by a grey box) and 30 min after restraint; n ≥ 5 for all groups at all time points. (B) Change in serum CORT levels from baseline in dexamethasone (DEX)-treated adult males; n ≥ 5 for all groups at all time points. (C) Area under the curve for CORT levels in saline- and DEX-treated adult males. *P < 0.05 control versus IRNkx2.1 KO.
3.2. Altered expression of HPA axis components in IRNkx2.1 KOs
To identify the sites of diminished InsR signals that contribute to HPA axis hyperactivity in IRNkx2.1 KO mice, we evaluated gene expression and peptide levels of several major HPA axis components in the hypothalamus, median eminence (ME) and pituitary at baseline, the end of the 30-minute restraint period, and 30 min after restraint (Figure 3A). Baseline levels of Avp mRNA expression (Figure 3F) and AVP protein levels in the ME (Figure 3C) were significantly increased by 53% and 18%, respectively, in IRNkx2.1 KO males. Conversely, baseline levels of pituitary Pomc expression were 40% lower (Figure 3G) and adrenocorticotropic hormone (ACTH) protein levels in the pituitary trended lower (Figure 3D) in IRNkx2.1 KO males. At baseline, hypothalamic expression of Crh, Gr, Pomc, Npy and Agrp mRNA, CRH protein levels in the ME, and pituitary expression of Gr were similar between IRNkx2.1 KO males and controls (Figure 3). At the end of the restraint period, we found that Gr expression was 30% lower in the hypothalamus (Figure 3H), but increased in the pituitary of IRNkx2.1 KOs (Figure 3I), consistent with a centrally-mediated deficit in negative feedback to the HPA axis [41]. Expression of hypothalamic Avp and pituitary Pomc were significantly different in IRNkx2.1 KOs 30 min after release from restraint, but the changes were in the opposite direction as had been observed at baseline (Figure 3F–G).
Figure 3.
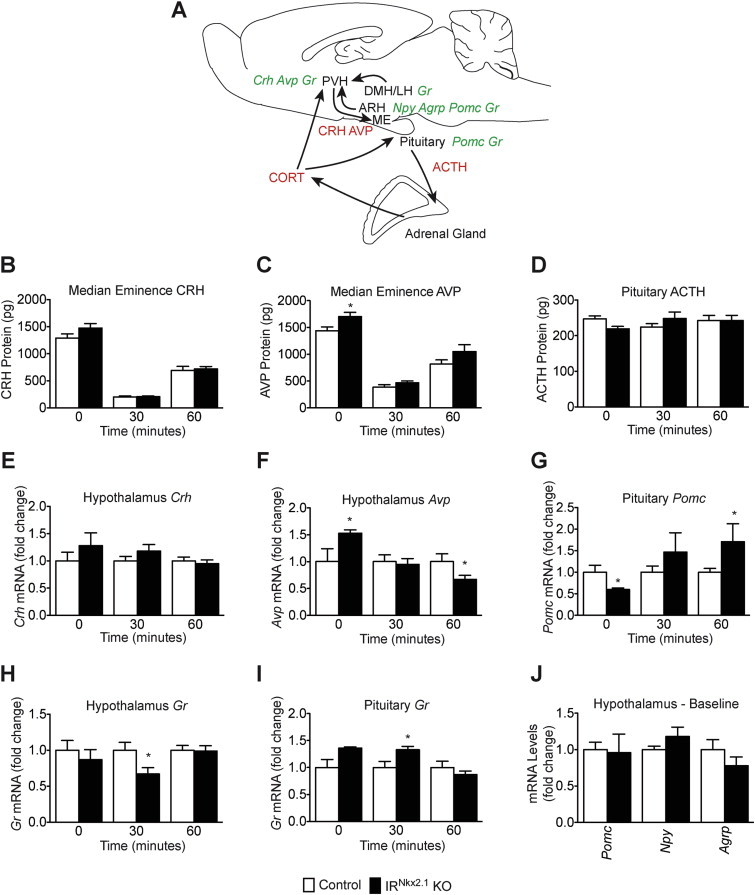
Altered gene expression of HPA axis components at baseline and during restraint test in IRNkx2.1KOs. (A) Key components of HPA axis (black text) that release hormones/neuropeptides (red text) and express genes (green text) assessed in our studies. (B) CRH and (C) AVP protein levels in the median eminence. (D) ACTH protein levels in the pituitary. (E) Crh and (F) Avp mRNA expression levels in the hypothalamus at baseline, at the end of restraint (30 min), and 30 min post-restraint; n ≥ 5 for all groups at all time points. (G) Pomc mRNA in the pituitary; n ≥ 4 for all groups at all time points. (H) Hypothalamic and (I) pituitary Gr mRNA; n ≥ 4 for all groups at all time points. (J) Baseline mRNA levels of Pomc, Npy, and AgRP in the hypothalamus of male IRNkx2.1 KO and controls; n ≥ 4 for all groups. All data are mean ± SEM of control and IRNkx2.1 KO males. *P < 0.05 control versus IRNkx2.1 KO.
3.3. Increased anxiety-like behaviors in IRNkx2.1 KO males following stress
In light of the observed HPA axis hyperactivity in IRNkx2.1 KO males, we evaluated their performance in a series of neuro-behavioral tests. We used open field (OF), marble burying (MB), stress-induced hyperthermia (SIH) and novelty suppressed feeding (NSF) tests to measure anxiety-like behaviors [42]. To evaluate depressive-behavior, we assessed learned helplessness in a forced swim test (FST) [38].
IRNkx2.1 KO males exhibited reduced locomotor activity after 20 min in the OF, but not at earlier timepoints (Figure 4A, left panel). The decrease in locomotor activity observed at later timepoints was not associated with fewer entries to the center of the field (Figure 4A, right panel). Marble burying behavior was the same in IRNkx2.1 KO and control males at baseline, but was roughly 50% higher in IRNkx2.1 KOs in response to stress (5 ± 1 control vs. 8 ± 1 KO, P < 0.05)(Figure 4B). Similarly, core body temperature was not different at baseline, but was roughly 0.3 °C higher in IRNkx2.1 KO males after stress (36.6 ± 0.1 control vs. 36.9 ± 0.1 KO, P < 0.05) (Figure 4C). The latency to feed in the NSF test tended to be higher in IRNkx2.1 KO males as compared to controls, although this difference did not reach significance (Figure 4D). Finally, time spent immobile in the FST, an indicator of depressive-like behavior in mice, was not altered in IRNkx2.1 KO males (Figure 4E). In summary, performance of IRNkx2.1 KO males was the same as controls at baseline, but was increased following exposure to stress.
Figure 4.
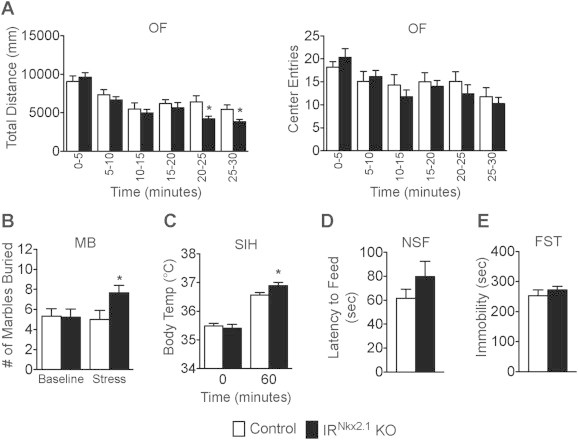
Defective behavioral responses in IRNkx2.1KOs. (A) Total distance traveled (left) and number of entries to the center (right), in each 5-minute bin during the open field test (OF); n ≥ 9 for all groups. (B) Number of marbles buried during a 20-min marble burying test (MB) at baseline and after stress; n ≥ 9 for all groups. (C) Stress-induced hyperthermia (SIH): body temperature at baseline and 60 min after stress; n ≥ 9 for all groups. (D) Latency to feed during novelty-supressed feeding (NSF) test; n ≥ 9 for all groups. (E) Time spent immobile during a 6-min forced-swim test (FST); n ≥ 9 for all groups. All data are mean ± SEM of control and IRNkx2.1 KO males. *P < 0.05 control versus IRNkx2.1 KO.
3.4. HPA axis function is not altered in IRSim1 KO males
Since we observed increased AVP protein levels in the ME and Avp mRNA expression in the hypothalamus of IRNkx2.1 KO males, we explored whether InsR actions directly on AVP neurons could be responsible. To this end, we assessed neuroendocrine and molecular correlates of HPA axis responses in IRSim1 KO males, in which InsR signaling is disrupted in Sim1-expressing cells in the PVH [30]. Adult IRSim1 KO males exhibited similar body weights and fed glucose levels as control littermates (Figure 5A–B). Serum CORT levels at baseline and in response to 30 min restraint were also not altered in IRSim1 KO males (Figure 5C–D). IRSim1 KOs exhibited increased Avp expression in the PVH and decreased Agrp expression in the ARH at baseline, although these differences did not reach significance (Figure 5E). These changes were in the same direction as those observed in IRNkx2.1 KOs (Figure 3F,J). On the other hand, Pomc expression in the hypothalamus was significantly increased at baseline in IRSim1 KOs (Figure 5E), while it was not changed in IRNkx2.1 KOs (Figure 3J). Pomc and Gr expression in the pituitary were not significantly altered in IRSim1 KOs as compared to controls (Figure 5F). In summary, our analyses are consistent with the idea that InsR signals in Sim1 neurons do not exert a major influence on HPA axis responsiveness, although we cannot exclude a minor contribution from these neurons.
Figure 5.
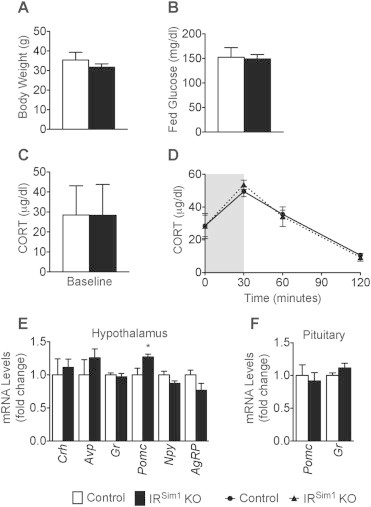
Loss of InsR signals in Sim1 neurons did not lead to changes in stress response. (A) Body weight of male IRSim1 KO and controls; n ≥ 4 for all groups. (B) Fed glucose levels of male IRSim1 KO and controls; n ≥ 4 for all groups. (C) Serum CORT levels during a restraint test (baseline, at the end of restraint, and 30 min after restraint; the period of restraint is indicated by a grey box) in adult males; n ≥ 4 for IRSim1 KO and controls at all time points. (D) Baseline CORT levels of male IRSim1 KO and controls; n ≥ 4 for all groups. (E) Baseline mRNA levels of Crh, Avp, Gr, Pomc, Npy, and AgRP in the hypothalamus of male IRSim1 KO and controls; n ≥ 5 for all groups. (F) Baseline mRNA levels of Pomc and Gr in the pituitary of male IRSim1 KO and controls; n ≥ 5 for all groups. All data are mean ± SEM of control and IRSim1 KO males. *P < 0.05 control versus IRSim1 KO.
3.5. IRAgRP KO mice exhibit reduced AVP levels at baseline
As stress is reported to increase and insulin is reported to decrease ARH expression of Npy and Agrp [43–45], we next examined the consequences of disrupting InsR signals in Agrp-expressing neurons on HPA axis responsiveness. At 13 weeks of age, body weight, fed glucose levels, and glucose tolerance in IRAgRP KO males were similar to control littermates (Figure 6A–C). Serum CORT levels at baseline and at the end of the 30 min restraint period were also not changed in IRAgRP KO males (Figure 6D–E). In contrast to what we observed in IRNkx2.1 KO males, IRAgRP KO males exhibited a 25% decrease in CORT response 30 min after release from restraint, although this difference did not reach significance (Controls 41.5 ± 3.9 vs. IRAgRP KOs 31.2 ± 5, P = 0.07)(Figure 6E). Consistent with decreased HPA axis responsivity, baseline AVP protein levels in the ME were significantly lower (Figure 6F) and Avp mRNA expression in the hypothalamus trended lower in IRAgRP KO mice (Figure 6G). In addition, baseline pituitary Pomc mRNA levels were increased in IRAgRP KO males, while Gr was not changed (Figure 6H). Together, these findings support the idea that diminished InsR signals in AgRP/NPY neurons results in decreased AVP tone and a trend toward reduced HPA axis responsiveness, the opposite of the phenotype produced by widespread disruption of InsR signals in Nkx2.1-Cre-expressing neurons in the forebrain.
Figure 6.

Loss of InsR signals in AgRP neurons resulted in decreased AVP release. (A) Body weight of male IRAgRP KO and controls; n ≥ 5 for all groups. (B) Fed glucose levels of male IRAgRP KO and controls; n ≥ 5 for all groups. (C) Glucose-tolerance test of male IRAgRP KO and controls. 2 mg/kg dextrose was injected i.p. at time 0. n ≥ 6 for all groups. (D) Baseline CORT levels of male IRAgRP KO and controls; n ≥ 5 for all groups. (E) Serum CORT levels during a restraint test (baseline, at the end of restraint, and 30 min after restraint; the period of restraint is indicated by a grey box), and 30 min after restraint in adult males; n ≥ 5 for IRAgRP KO and controls at all time points. (F) Baseline AVP protein levels in the median eminence of male IRAgRP KO and controls. n ≥ 5 for all groups. (G) Baseline mRNA levels of Crh, Avp, Gr, Pomc, Npy, and AgRP in the hypothalamus of male IRAgRP KO mice and controls; n ≥ 5 for all groups. (H) Baseline mRNA levels of Pomc and Gr in the pituitary of male IRAgRP KO and controls; n ≥ 5 for all groups. All data are mean ± SEM of control and IRAgRP KO males. *P < 0.05 control versus IRAgRP KO.
4. Discussion
4.1. Impaired negative feedback to HPA axis in IRNkx2.1 KO males
Our data support the hypothesis that insulin signaling in the hypothalamus modulates negative feedback to the HPA axis following stress. Nkx2.1-Cre-mediated disruption of InsR signaling in the hypothalamus, as well as ventral forebrain-derived neuronal subpopulations in the cortex and amygdala [27,28,39], did not affect plasma CORT levels at baseline or during restraint stress, but delayed the return to baseline (Figure 1B). Similarly, behaviors assessed over a short time period (i.e. NSF, FST) were not altered in IRNkx2.1 KOs, while modest but significant effects were observed in assays lasting 20 min or more (i.e. OF, MB, SIH) (Figure 4). Pituitary Gr expression and the ability of the synthetic glucocorticoid dexamethasone to suppress stress-induced activation of the HPA axis were not altered in IRNkx2.1 KOs (Figures 2C and 3I), suggesting that the pituitary response to stress is not impaired [46]. These observations are consistent with the idea that InsR signals in Nkx2.1-Cre-expressing neurons normally act to provide negative feedback to the HPA axis. This idea is also supported by reports that intranasal administration of insulin lowers stress-induced, but not basal, HPA axis responsiveness in humans [23].
The most prominent change in central components of the HPA axis in IRNkx2.1 KOs was increased Avp expression in the hypothalamus and AVP protein released into the median eminence at baseline (Figure 3). Elevated basal AVP levels are also observed in rodents exposed to chronic stress [47,48] and in patients with major depression [49,50]. It has been proposed that this adaptation maintains HPA axis responsiveness in the face of high circulating glucocorticoid levels during chronic stress [51]. However, it is not clear whether increased AVP in IRNkx2.1 KOs is a direct consequence of diminished hypothalamic InsR signaling, or a secondary response to chronic perturbations in the stress axis. In either case, the failure to observe increased AVP to a similar degree in IRSim1 KOs (Figure 5) argues against a major role for direct effects of insulin on CRH/AVP neurons in the PVH, although a minor contribution cannot be ruled out.
4.2. HPA axis phenotypes of IRAgrp KO males are in opposite direction as those observed in IRNkx2.1 KOs
NPY and AgRP have been reported to activate CRH neurons in vivo and in vitro [52,53], while insulin blunts stress-induced increases in NPY levels [43,54] and inhibits NPY/AgRP neuronal activity [55]. Based on these reports, we predicted that a reduction in inhibitory InsR-mediated inputs to AgRP/NPY neurons would lead to heightened stress responses in IRAgrp KOs. Thus, our finding that IRAgrp KOs exhibit decreased AVP and a trend toward a suppressed HPA axis response was the opposite of the expected result (Figure 6). Consistent with our observations, mice overexpressing an Agrp transgene and global knockouts of Melanocortin receptor 4 also exhibit normal CORT at baseline, but a blunted response to restraint stress [56–58]. The possibility that activation of AgRP neurons can act to dampen HPA responses to psychological stress is also supported by the observation that stress-induced increases in Npy/Agrp occur after the rise in CORT levels and Pomc expression [45,59]. Differences in the timing and/or concentration of exposure to AgRP/NPY following restraint tests in genetic models versus central injections of AgRP/NPY compounds could underlie discordant effects on HPA axis activity. If our hypothesis is correct, activation of AgRP/NPY neurons under conditions of limited nutrient availability could promote survival by limiting stress-induced mobilization of fuels [60].
4.3. Possible sources of impaired InsR signals that contribute to HPA axis hyperactivity in IRNkx2.1 KOs
As Agrp-Cre- and Nkx2.1-Cre-mediated disruption of InsR signaling produce opposite effects on HPA-axis related endpoints, the loss of InsR signals in AgRP/NPY neurons cannot explain the phenotype observed in IRNkx2.1 KOs. Neurons in the dorsomedial hypothalamus are marked by an Nkx2.1-Cre linage trace [39], send heavy projections to the PVH [16] and modulate HPA axis activity [61,62] (Figure 7). However, the lack of information about electrophysiological responses of DMH neurons to insulin and conflicting reports that DMH neurons provide excitatory [61,63] and inhibitory [62] inputs to the HPA axis, hinder the formulation of a hypothesis about the precise mechanism of action. In theory, InsR signaling in ARH POMC neurons could also modulate HPA responses, although projections from these neurons to the neuroendocrine PVH are much sparser than AgRP projections [64,65]. Unfortunately, efforts to explore the possible contribution of InsR in POMC neurons using Pomc-Cre-mediated recombination are complicated by the fact that signaling in pituitary corticotrophs would also be impacted.
Figure 7.

Components of HPA axis impacted by Cre lines in this study. (A) Nkx2.1-Cre-mediated recombination is observed in most of the hypothalamus and posterior pituitary, but not in the PVH (or anterior pituitary). (B) Sim1-Cre-mediated recombination is observed in most neuronal subpopulations in the PVH, including AVP and CRH neurons. (C) Agrp-Cre-mediated recombination is restricted to a subpopulation of neurons in the ARH.
5. Conclusions
There is a high degree of comorbidity of metabolic and psychological disorders, and the severity of depressive symptoms is correlated with insulin resistance [1,3,4]. Our findings support the idea that InsR signaling in hypothalamic neurons provides an important source of crosstalk between energy status and stress reactivity. InsR signaling in AgRP/NPY neurons appears to promote AVP release, while signaling in an unidentified population likely acts in an opposing fashion. Identification of the latter cell type is an important area for future research, because elevated AVP is thought to contribute to depressive disorders [66].
Acknowledgments
We thank the Columbia Diabetes and Endocrinology Research Center Animal Phenotyping Core for help with metabolic cages (P30 DK63608); Sharon Wardlaw (Columbia University) for assistance with CORT assays; Rene Hén for guidance with behavioral assays; Domenico Accili (Columbia University) for Insr floxed mice; S. Anderson (Weill Cornell Medical College) for Nkx2.1-Cre mice; Joel Elmquist (UT Southwestern) and Brad Lowell (Beth Israel Deaconness) for Agrp-Cre mice. This work was supported by R01 DK089038 (LMZ).
Conflicts of interest
The authors do not have any conflicts of interest.
References
- 1.Anderson M.S., Thamotharan M., Kao D., Devaskar S.U., Qiao L., Friedman J.E. Effects of acute hyperinsulinemia on insulin signal transduction and glucose transporters in ovine fetal skeletal muscle. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2005;288:R473–R481. doi: 10.1152/ajpregu.00405.2004. [DOI] [PubMed] [Google Scholar]
- 2.Petry N.M., Barry D., Pietrzak R.H., Wagner J.A. Overweight and obesity are associated with psychiatric disorders: results from the national epidemiologic survey on alcohol and related conditions. Psychosomatic Medicine. 2008;70:288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 3.Shomaker L.B., Tanofsky-Kraff M., Young-Hyman D., Han J.C., Yanoff L.B., Brady S.M. Psychological symptoms and insulin sensitivity in adolescents. Pediatric Diabetes. 2010;11:417–423. doi: 10.1111/j.1399-5448.2009.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson S., Schmidt M., Patton G., Dwyer T., Blizzard L., Otahal P. Depression and insulin resistance: cross-sectional associations in young adults. Diabetes Care. 2010;33:1128–1133. doi: 10.2337/dc09-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dallman M.F., Akana S.F., Strack A.M., Hanson E.S., Sebastian R.J. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Annals of the New York Academy of Sciences. 1995;771:730–742. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- 6.Pasquali R., Vicennati V., Cacciari M., Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Annals of the New York Academy of Sciences. 2006;1083:111–128. doi: 10.1196/annals.1367.009. [DOI] [PubMed] [Google Scholar]
- 7.Gragnoli C. Hypothesis of the neuroendocrine cortisol pathway gene role in the comorbidity of depression, type 2 diabetes, and metabolic syndrome. The Application of Clinical Genetics. 2014;7:43–53. doi: 10.2147/TACG.S39993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts R.E., Deleger S., Strawbridge W.J., Kaplan G.A. Prospective association between obesity and depression: evidence from the Alameda County Study. International Journal of Obesity and Related Metabolic Disorders. 2003;27:514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 9.Faith M.S., Butryn M., Wadden T.A., Fabricatore A., Nguyen A.M., Heymsfield S.B. Evidence for prospective associations among depression and obesity in population-based studies. Obesity Reviews. 2011;12:e438–453. doi: 10.1111/j.1467-789X.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 10.Pasquali R., Ambrosi B., Armanini D., Cavagnini F., Uberti E.D., Del Rio G., E. Study Group on Obesity of the Italian Society of Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose-response study. The Journal of Clinical Endocrinology and Metabolism. 2002;87:166–175. doi: 10.1210/jcem.87.1.8158. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz G.J., Zeltser L.M. Functional organization of neuronal and humoral signals regulating feeding behavior. Annual Review of Nutrition. 2013;33:1–21. doi: 10.1146/annurev-nutr-071812-161125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C., Lee S., Elmquist J.K. Circuits controlling energy balance and mood: inherently intertwined or just complicated intersections? Cell Metabolism. 2014;19:902–909. doi: 10.1016/j.cmet.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulrich-Lai Y.M., Ryan K.K. Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications. Cell Metabolism. 2014;19:910–925. doi: 10.1016/j.cmet.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi S., Horsley C., Aguila S., Dallman M.F. The hypothalamic ventromedial nuclei couple activity in the hypothalamo-pituitary-adrenal axis to the morning fed or fasted state. The Journal of Neuroscience. 1996;16:8170–8180. doi: 10.1523/JNEUROSCI.16-24-08170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cone R.D., Cowley M.A., Butler A.A., Fan W., Marks D.L., Low M.J. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. International Journal of Obesity and Related Metabolic Disorders. 2001;25(Suppl. 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 16.Sawchenko P.E., Swanson L.W. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. The Journal of Comparative Neurology. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- 17.Kamilaris T.C., Johnson E.O., Calogero A.E., Kalogeras K.T., Bernardini R., Chrousos G.P. Cholecystokinin-octapeptide stimulates hypothalamic-pituitary-adrenal function in rats: role of corticotropin-releasing hormone. Endocrinology. 1992;130:1764–1774. doi: 10.1210/endo.130.4.1312423. [DOI] [PubMed] [Google Scholar]
- 18.Larsen P.J., Tang-Christensen M., Jessop D.S. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- 19.van Dijk G., Seeley R.J., Thiele T.E., Friedman M.I., Ji H., Wilkinson C.W. Metabolic, gastrointestinal, and CNS neuropeptide effects of brain leptin administration in the rat. American Journal of Physiology. 1999;276:R1425–R1433. doi: 10.1152/ajpregu.1999.276.5.R1425. [DOI] [PubMed] [Google Scholar]
- 20.Coleman D.L., Burkart D.L. Plasma corticosterone concentrations in diabetic (db) mice. Diabetologia. 1977;13:25–26. doi: 10.1007/BF00996323. [DOI] [PubMed] [Google Scholar]
- 21.MacLusky N.J., Cook S., Scrocchi L., Shin J., Kim J., Vaccarino F. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology. 2000;141:752–762. doi: 10.1210/endo.141.2.7326. [DOI] [PubMed] [Google Scholar]
- 22.Takao T., Tojo C., Nishioka T., Hashimoto K. Increased adrenocorticotropin responses to acute stress in otsuka long-evans tokushima fatty (type 2 diabetic) rats. Brain Research. 2000;852:110–115. doi: 10.1016/s0006-8993(99)02222-2. [DOI] [PubMed] [Google Scholar]
- 23.Bohringer A., Schwabe L., Richter S., Schachinger H. Intranasal insulin attenuates the hypothalamic-pituitary-adrenal axis response to psychosocial stress. Psychoneuroendocrinology. 2008;33:1394–1400. doi: 10.1016/j.psyneuen.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Fruehwald-Schultes B., Kern W., Born J., Fehm H.L., Peters A. Hyperinsulinemia causes activation of the hypothalamus-pituitary-adrenal axis in humans. International Journal of Obesity and Related Metabolic Disorders. 2001;25(Suppl. 1):S38–S40. doi: 10.1038/sj.ijo.0801695. [DOI] [PubMed] [Google Scholar]
- 25.Pasquali R., Vicennati V. The abdominal obesity phenotype and insulin resistance are associated with abnormalities of the hypothalamic-pituitary-adrenal axis in humans. Hormone and Metabolic Research. 2000;32:521–525. doi: 10.1055/s-2007-978680. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni R.N., Bruning J.C., Winnay J.N., Postic C., Magnuson M.A., Kahn C.R. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q., Tam M., Anderson S.A. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. The Journal of Comparative Neurology. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- 28.Chong A.C., Greendyk R.A., Zeltser L.M. Distinct networks of leptin-and insulin-sensing neurons regulate thermogenic responses to nutritional and cold challenges. Diabetes. 2014, Aug 14 doi: 10.2337/db14-0567. pii: DB_140567 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrini M., Piroli G., Frontera M., Falbo A., Lima A., De Nicola A.F. Estrogens normalize the hypothalamic-pituitary-adrenal axis response to stress and increase glucocorticoid receptor immuno-reactivity in hippocampus of aging male rats. Neuroendocrinology. 1999;69:129–137. doi: 10.1159/000054411. [DOI] [PubMed] [Google Scholar]
- 30.Balthasar N., Dalgaard L.T., Lee C.E., Yu J., Funahashi H., Williams T. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Tong Q., Ye C.P., Jones J.E., Elmquist J.K., Lowell B.B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature Neuroscience. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhodes M.E., Balestreire E.M., Czambel R.K., Rubin R.T. Estrous cycle influences on sexual diergism of HPA axis responses to cholinergic stimulation in rats. Brain Research Bulletin. 2002;59:217–225. doi: 10.1016/s0361-9230(02)00868-7. [DOI] [PubMed] [Google Scholar]
- 33.Papadopoulos A.D., Wardlaw S.L. Endogenous alpha-MSH modulates the hypothalamic-pituitary-adrenal response to the cytokine interleukin-1beta. Journal of Neuroendocrinology. 1999;11:315–319. doi: 10.1046/j.1365-2826.1999.00327.x. [DOI] [PubMed] [Google Scholar]
- 34.Walsh R.N., Cummins R.A. The open-field test: a critical review. Psychological Bulletin. 1976;83:482–504. [PubMed] [Google Scholar]
- 35.De Boer S.F., Koolhaas J.M. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. European Journal of Pharmacology. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- 36.Vinkers C.H., van Bogaert M.J., Klanker M., Korte S.M., Oosting R., Hanania T. Translational aspects of pharmacological research into anxiety disorders: the stress-induced hyperthermia (SIH) paradigm. European Journal of Pharmacology. 2008;585:407–425. doi: 10.1016/j.ejphar.2008.02.097. [DOI] [PubMed] [Google Scholar]
- 37.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 38.Castagne V., Moser P., Roux S., Porsolt R.D. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Current Protocols in Neuroscience. 2011 doi: 10.1002/0471142301.ns0810as55. Chapter 8. Unit 8 10A. [DOI] [PubMed] [Google Scholar]
- 39.Ring L.E., Zeltser L.M. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. The Journal of Clinical Investigation. 2010;120:2931–2941. doi: 10.1172/JCI41985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engeland W.C., Arnhold M.M. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine. 2005;28:325–332. doi: 10.1385/ENDO:28:3:325. [DOI] [PubMed] [Google Scholar]
- 41.Tasker J.G., Herman J.P. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14:398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey K.R., Crawley J.N. Anxiety-related behaviors in mice. In: Buccafusco J.J., editor. Methods of behavior analysis in neuroscience. 2009. Boca Raton (FL) [PubMed] [Google Scholar]
- 43.Schwartz M.W., Sipols A.J., Marks J.L., Sanacora G., White J.D., Scheurink A. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. [DOI] [PubMed] [Google Scholar]
- 44.Larsen P.J., Jessop D.S., Chowdrey H.S., Lightman S.L., Mikkelsen J.D. Chronic administration of glucocorticoids directly upregulates prepro-neuropeptide Y and Y1-receptor mRNA levels in the arcuate nucleus of the rat. Journal of Neuroendocrinology. 1994;6:153–159. doi: 10.1111/j.1365-2826.1994.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 45.Chigr F., Rachidi F., Tardivel C., Najimi M., Moyse E. Modulation of orexigenic and anorexigenic peptides gene expression in the rat DVC and hypothalamus by acute immobilization stress. Frontiers in Cellular Neuroscience. 2014;8:198. doi: 10.3389/fncel.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Kloet R., Wallach G., McEwen B.S. Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology. 1975;96:598–609. doi: 10.1210/endo-96-3-598. [DOI] [PubMed] [Google Scholar]
- 47.Ma X.M., Lightman S.L. The arginine vasopressin and corticotrophin-releasing hormone gene transcription responses to varied frequencies of repeated stress in rats. The Journal of Physiology. 1998;510(Pt 2):605–614. doi: 10.1111/j.1469-7793.1998.605bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aubry J.M., Bartanusz V., Jezova D., Belin D., Kiss J.Z. Single stress induces long-lasting elevations in vasopressin mRNA levels in CRF hypophysiotrophic neurones, but repeated stress is required to modify AVP immunoreactivity. Journal of Neuroendocrinology. 1999;11:377–384. doi: 10.1046/j.1365-2826.1999.00338.x. [DOI] [PubMed] [Google Scholar]
- 49.Purba J.S., Hoogendijk W.J., Hofman M.A., Swaab D.F. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Archives of General Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- 50.van Londen L., Goekoop J.G., van Kempen G.M., Frankhuijzen-Sierevogel A.C., Wiegant V.M., van der Velde E.A. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17:284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- 51.Aguilera G., Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regulatory Peptides. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 52.Krysiak R., Obuchowicz E., Herman Z.S. Interactions between the neuropeptide Y system and the hypothalamic-pituitary-adrenal axis. European Journal of Endocrinology. 1999;140:130–136. doi: 10.1530/eje.0.1400130. [DOI] [PubMed] [Google Scholar]
- 53.Dhillo W.S., Small C.J., Seal L.J., Kim M.S., Stanley S.A., Murphy K.G. The hypothalamic melanocortin system stimulates the hypothalamo-pituitary-adrenal axis in vitro and in vivo in male rats. Neuroendocrinology. 2002;75:209–216. doi: 10.1159/000054712. [DOI] [PubMed] [Google Scholar]
- 54.Wilding J.P., Gilbey S.G., Lambert P.D., Ghatei M.A., Bloom S.R. Increases in neuropeptide Y content and gene expression in the hypothalamus of rats treated with dexamethasone are prevented by insulin. Neuroendocrinology. 1993;57:581–587. doi: 10.1159/000126410. [DOI] [PubMed] [Google Scholar]
- 55.Qiu J., Zhang C., Borgquist A., Nestor C.C., Smith A.W., Bosch M.A. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metabolism. 2014;19:682–693. doi: 10.1016/j.cmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham M., Shutter J.R., Sarmiento U., Sarosi I., Stark K.L. Overexpression of Agrt leads to obesity in transgenic mice. Nature Genetics. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- 57.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 58.Ryan K.K., Mul J.D., Clemmensen C., Egan A.E., Begg D.P., Halcomb K. Loss of melanocortin-4 receptor function attenuates HPA responses to psychological stress. Psychoneuroendocrinology. 2014;42:98–105. doi: 10.1016/j.psyneuen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makino S., Baker R.A., Smith M.A., Gold P.W. Differential regulation of neuropeptide Y mRNA expression in the arcuate nucleus and locus coeruleus by stress and antidepressants. Journal of Neuroendocrinology. 2000;12:387–395. doi: 10.1046/j.1365-2826.2000.00451.x. [DOI] [PubMed] [Google Scholar]
- 60.Dallman M.F., Strack A.M., Akana S.F., Bradbury M.J., Hanson E.S., Scribner K.A. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Frontiers in Neuroendocrinology. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 61.Bailey T.W., Dimicco J.A. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2001;280:R8–R15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- 62.Ebner K., Muigg P., Singewald N. Inhibitory function of the dorsomedial hypothalamic nucleus on the hypothalamic-pituitary-adrenal axis response to an emotional stressor but not immune challenge. Journal of Neuroendocrinology. 2013;25:48–55. doi: 10.1111/j.1365-2826.2012.02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boudaba C., Schrader L.A., Tasker J.G. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. Journal of Neurophysiology. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- 64.Wittmann G., Liposits Z., Lechan R.M., Fekete C. Origin of cocaine- and amphetamine-regulated transcript-containing axons innervating hypophysiotropic corticotropin-releasing hormone-synthesizing neurons in the rat. Endocrinology. 2005;146:2985–2991. doi: 10.1210/en.2005-0178. [DOI] [PubMed] [Google Scholar]
- 65.Bouyer K., Simerly R.B. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. The Journal of Neuroscience. 2013;33:840–851. doi: 10.1523/JNEUROSCI.3215-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muller M.B., Landgraf R., Keck M.E. Vasopressin, major depression, and hypothalamic-pituitary-adrenocortical desensitization. Biological Psychiatry. 2000;48:330–333. doi: 10.1016/s0006-3223(00)00886-6. [DOI] [PubMed] [Google Scholar]


