Abstract
We describe a new technique of platelet-rich plasma (PRP) infiltration for the treatment of severe knee osteoarthritis. PRP intra-articular infiltration is a promising treatment for knee osteoarthritis, but it still has some limitations in high-degree osteoarthritis. Diagnosis of osteoarthritis is based on clinical and radiographic findings, and patients with grade III or IV knee tibiofemoral osteoarthritis based on the Ahlbäck scale are considered candidates for this technique. The technique consists of performing intraosseous infiltration of PRP into the subchondral bone, which acts on this tissue and consequently on cartilage-bone communication. Although the intraosseous injection hinders the conventional knee intra-articular infiltration, it allows an extension of the range of action of the PRP, which acts directly on the subchondral bone, which is involved in the progression of osteoarthritis. Thus this technique involves a new administration of PRP that can delay knee arthroplasty; moreover, it can be applied for not only severe osteoarthritis but also other pathologies in which the subchondral bone is critical in the etiology, such as necrosis and osteochondral lesions.
Osteoarthritis (OA) is a disease of the synovial joints that evolves with pain, loss of motion, and deformation of affected joints. Initially, the pathogenesis of OA was focused almost exclusively on the cartilage; however, nowadays, it is considered a disease that involves all tissues of the joint, all of which are crucial for maintaining articular homeostasis. Both genetic and acquired or environmental factors can disrupt this anabolic-catabolic balance, resulting in cartilage degeneration, osteophyte formation, and inflammation of the synovial membrane and becoming a clinical problem.1 Currently, no treatment can stop the progression of OA or reverse the damage, making joint replacement the only solution for these patients. Conservative treatment include oral pharmacologic treatment, such as analgesics, nonsteroidal anti-inflammatory drugs, or symptomatic slow-acting drugs for OA, and intra-articular infiltrations of steroids and hyaluronic acid, focused on relieving the symptoms but not on stopping the disease.2
In recent years intra-articular infiltrations of platelet-rich plasma (PRP) have emerged as an alternative to current treatments. This biological therapy uses the patient's own platelets and plasma, which mainly convey fibrin and growth factors as effectors. These growth factors act on the entire joint and may well have an influence on the development of OA; they promote restoration of joint homeostasis, have inductive and protective effects on chondrocytes, and stimulate the production of hyaluronic acid by synoviocytes. All these properties help to promote a generative biological environment and to slow down joint and cartilage degeneration, thereby relieving symptomatology.3 Several clinical trials showing promising results have been published; however, there are still some doubts about whether this form of administration is able to reach the deeper layers of the cartilage and subchondral bone, thereby possibly limiting the growth factors' therapeutic potential especially in severe OA.4,5
In light of recent studies showing the importance of the subchondral bone in the pathogenesis of OA and showing cartilage–subchondral bone communication,6 we propose a combination of intra-articular and intraosseous injections to treat severe OA. With this combination, it is possible to expand the effective range of PRP by acting not only on the subchondral bone and, consequently, on its cartilage communications but also on mesenchymal stem cells to modulate the affected tissue regeneration.7
Surgical Technique
Diagnosis
Diagnosis of OA is based on clinical and radiographic findings. The radiographs used are the weight-bearing anteroposterior view of the knee, the lateral view at 30° of knee flexion, and the axial view at 20° of knee flexion. Patients with grade III or IV knee tibiofemoral OA based on the Ahlbäck scale are considered candidates for our technique, which consists of a PRP intra-articular infiltration and 2 PRP intraosseous infiltrations into the medial femoral condyle and into the medial tibial plateau (Fig 1).
Fig 1.
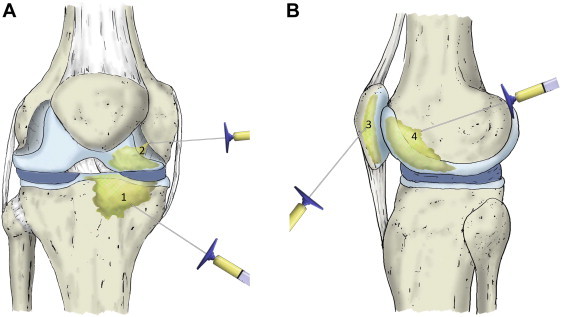
(A) The platelet-rich plasma (PRP) intraosseous infiltration of a knee with severe femorotibial osteoarthritis is performed into the medial tibial plateau (1) and medial femoral condyle (2). (B) If the patient presents with femoropatellar osteoarthritis, the approach is external and the patella (3) and trochlea (4) are infiltrated. Before these intraosseous injections are performed, conventional knee intra-articular infiltration of PRP is conducted.
Patient Preparation
Before sedation is induced, about 80 mL of venous blood is extracted from the patient to prepare the PRP according to PRGF-Endoret technology (Biotechnology Institute, Vitoria-Gasteiz, Spain).4 Sedation is performed by infusing a single dose of normal saline solution, as well as a single dose of midazolam (0.03 to 0.05 mg/kg) and fentanyl (3.2 mg/kg), in the peripheral vein; a single or repeated dose of propofol is also administered (1 to 2 mg/kg), depending on the duration of the infiltration. The degree of sedation is −4 or −5 on the Richmond Sedation Scale. The patient is monitored according to the standards of the American Society of Anesthesiologists. The patient is positioned supine on the operating room table; the infiltration area is prepared with a povidone-iodine solution, covering a region 10 cm proximal and 10 cm distal to the infiltration zone. Sterile drapes defining the treatment zone (proximal, distal, medial, and lateral) are placed (Video 1).
Once the patient has been sedated and prepared and the PRP has been obtained, 2 marks are drawn in the medial region of the knee, one located 2 cm proximal and the other located 2 cm distal to the medial joint line and centered in the midline sagittal plane. Next, a 24-gauge needle is used to anesthetize the area of infiltration; it is introduced through the mark and moved into contact with the femoral condyle. Without retraction of the needle, the periosteum of the femoral condyle is infiltrated with 2 mL of 2% mepivacaine. Then, the needle is withdrawn and moved into contact with the inner face of the tibial plateau (through the other mark), and without retraction of the needle, the periosteum of the medial tibial plateau is infiltrated with 2 mL of 2% mepivacaine.
Intra-articular Infiltration
After application of local anesthesia, intra-articular infiltration is conducted first. We penetrate a 21-gauge needle into the joint through the external patellar wing, centered in the central region of the patella in the craniocaudal plane. Lateralization of the patella during infiltration facilitates this process (Fig 2A). After placement of the needle into the joint space, synovial fluid arthrocentesis can be performed if it is necessary. Once arthrocentesis has been carried out and without removal of the needle, 8 mL of PRP is infiltrated. The infiltration is directed into the midpoint area of the femoropatellar region using an external approach to prevent infiltration into the synovial membrane, which would cause pain (Fig 2B).
Fig 2.
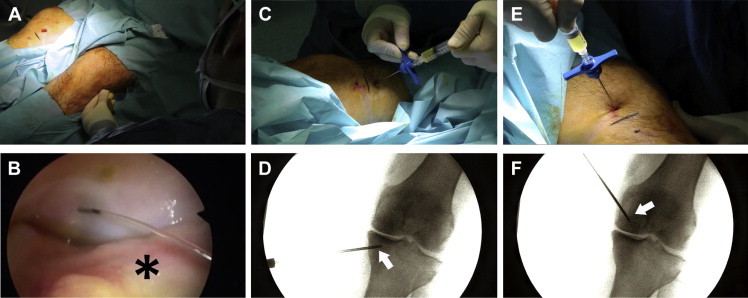
After the patient is positioned supine on the operating room table, (A) intra-articular infiltration is performed into the joint through the external patellar wing, centered in the central region of the patella in the craniocaudal plane; (B) the infiltration is directed into the midpoint area of the femoropatellar region using an external approach and preventing infiltration into the synovial membrane (asterisk). (C, D) Intraosseous tibial plateau infiltration is conducted into the medial tibial plateau, just to its middle area. The arrow indicates the trocar. (E, F) Concerning intraosseous femoral condyle infiltration, a trocar (arrow) is applied to the thickness of the medial femoral condyle, as far as the middle area of the medial condyle.
Intraosseous Tibial Plateau Infiltration
Once the area is anesthetized, PRP is infiltrated into the tibial plateau. A 13-gauge trocar used for bone biopsy (CareFusion, San Diego, CA) is introduced into the bone through the mark previously made. The trocar is placed 2 cm distal to the joint line, leaning on the periosteum; the trocar is then introduced 2 cm into the thickness of the medial tibial plateau (to the middle area of the medial tibial plateau), following a parallel direction to the articular surface. Once the trocar has been placed in the desired position, 5 mL of PRP is infiltrated through the trocar (Fig 2 C and D).
Intraosseous Femoral Condyle Infiltration
Next, PRP is injected into the femoral condyle. A 13-gauge trocar used for bone biopsy is introduced into the bone through the mark previously made. The trocar is placed 2 cm proximal to the joint line, leaning on the periosteum. Then, the trocar is introduced 2 cm into the thickness of the medial femoral condyle (to the middle area of the medial condyle), following a parallel direction to the articular surface of the condyle. Once the trocar has been placed in the desired position, 5 mL of PRP is infiltrated through the trocar (Fig 2 E and F).
Finally, after completion of the infiltrations and removal of the sterile drapes, the skin is cleaned with an alcohol solution, with application of wound dressings at the infiltration points. After infiltration is completed, ice is applied to the site. In the days after surgery, the patient can bear weight and take analgesics (acetaminophen) as required for pain.
Patellofemoral OA
If patients present with severe patellofemoral OA, the same procedure as described earlier will be performed, but in these cases the local anesthesia and PRP are infiltrated into the patella and into the trochlear zone of the condyle. Both approaches will be conducted in the middle area, from the external side of the knee, with introduction of the trocar 2 cm into the thickness. In such cases 3 mL of PRP is infiltrated into the patella and 5 mL into the condyle.
Discussion
The frequency and chronicity of OA make it a challenge for the health and social systems of all developed countries. In affluent countries such as the United States, the numbers are staggering; estimates suggest that about 46 million patients have OA, with OA in more than 50% of adults older than 50 years. By 2030, this figure may reach 70 million.8 Current treatments focus exclusively on relieving the symptoms but not on curing the disease, making joint arthroplasty the definitive option for patients.2 The results obtained with new therapies such as PRP and the use of stem cells are promising but still have some limitations, such as the mode of administration. The most commonly used form of administration is intra-articular injection, which is effective in patients with mild degrees of OA but is not so effective in those with severe OA.4 With this new administration technique for PRP, in which the intra-articular injection is combined with intraosseous infiltrations, treating patients with higher grades of OA is a possibility, giving them an alternative to knee arthroplasty or at least delaying this more radical intervention (Fig 3).
Fig 3.
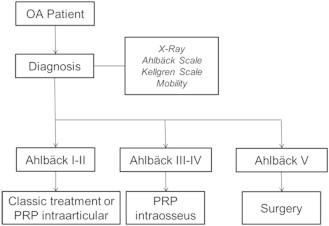
Patients are diagnosed with osteoarthritis (OA) based on physical examination findings and imaging techniques, using scales such as the Ahlbäck scale or Kellgren-Lawrence scale. Depending on the osteoarthritis grade, different treatments can be applied. If the patient presents with Ahlbäck grade I or II, we propose a classic treatment or intra-articular infiltration of platelet-rich plasma (PRP). If the patient presents with grade III or IV, we apply intraosseous infiltration with 2 intra-articular infiltrations in the subsequent weeks. If the patient presents with grade V, he or she undergoes a total knee arthroplasty.
The main limitation of intraosseous infiltration is related to patient preparation, involving sedation and local anesthesia because of the subchondral bone injection. These factors, in addition to training of the medical team, make this technique take more time and make it more expensive than a conventional intra-articular injection. The pressure increment inside the bone could entail pain 48 hours after treatment, so the patient should be advised of this possibility. Sometimes, the use of fluoroscopic control (FMControl, Vitoria-Gasteiz, Spain) is also necessary for proper administration (Tables 1-3).
Table 1.
Benefits
| Stimulates subchondral bone |
| Reaches deeper layers of cartilage |
| Acts on molecules and mesenchymal stem cells of subchondral bone |
| Is applicable to high grades of osteoarthritis, necrosis, and osteochondral lesions |
Table 2.
Technical Pearls
| Intra-articular infiltration is performed into the midpoint of the femoropatellar region using an external approach to prevent infiltration into the synovial membrane. |
| The use of a fluoroscope can facilitate trocar placement. |
| Although the patient is under sedation, local anesthesia is recommended. |
| After infiltration, it is advisable to apply ice to the area. |
Table 3.
Pitfalls
| The technique requires training and practice; therefore the infiltration time is increased. |
| The technique requires patient sedation. |
| In the 48 hours after treatment, the patient has more pain than with conventional infiltration. |
| Treatment will be more complicated in case of an infection due to infiltration. |
The aforementioned disadvantages are not present during intra-articular infiltration, but this form of infiltration does not reach the deeper layers of the cartilage and subchondral bone, thereby limiting its therapeutic potential. Recent studies have shown the importance of the subchondral bone in the pathogenesis of OA, and subchondral bone–cartilage communication has been shown in multiple experiments.6,9 When homeostasis is disrupted because of biochemical and biomechanical offenders, all the tissues of the joint are involved in restoring biological balance. These efforts to restore homeostasis entail cellular and extracellular matrix responses in all tissues. Thus communications occur between the deeper layers of the subchondral bone and cartilage and, on the other hand, between these and the synovial fluid that surrounds the entire joint. This bone-cartilage communication has been described in studies showing channels that reach the cartilage from the subchondral bone, which are more abundant in the cartilage of patients with OA.6
Intraosseous infiltration exploits the communication between the cartilage and subchondral bone such that PRP reaches the deeper layers of cartilage. There is a viscous consistency of PRP and the cellular material of subchondral bone that coagulates and remains in the areas of injured cartilage from which it has come (Fig 4). In addition, infiltrating PRP directly into the subchondral bone could act on this tissue and its mesenchymal stem cells; these cells would be maintained in the PRP matrix and modulate the repair process of subchondral bone, which has a direct impact on halting the progression of OA.6 Therefore, with our technique, PRP could achieve a more extensive range of action and, thereby, higher effectiveness and could be useful not only in severe OA but also in other pathologies, such as necrosis of the condyle or tibial plateau, and during surgical treatment of osteochondral lesions.
Fig 4.
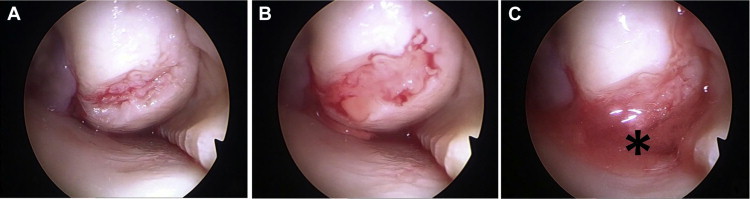
(A) Communications between cartilage and subchondral bone are more pronounced in degenerated cartilage. (B) The platelet-rich plasma infiltrated into subchondral bone flows through the degenerated zones, and because of its viscous consistency, (C) it remains in the area, creating a matrix (asterisk).
Footnotes
The authors report the following potential conflict of interest of source of funding: E.A. and S.P. are researchers at BTI Biotechnology Institute.
Supplementary Data
After sedation of the patient, 2 marks are drawn in the medial region of the knee, one located 2 cm proximal and the other located 2 cm distal to the medial joint line. The patient is positioned supine on the operating room table; the infiltration area is prepared with a povidone-iodine solution. Local anesthesia is conducted by injecting 2 mL of 2% mepivacaine into the periosteum of the condyle and tibial plateau. After evacuation of the synovial fluid, 8 mL of platelet-rich plasma (PRP) is infiltrated into the midpoint area of the femoropatellar region using an external approach. Trocars are introduced into the bone through the logistical marks and inserted 2 cm into the thickness of the medial tibial plateau and condyle. Once the trocars are placed in the desired position, 5 mL of PRP is infiltrated into the subchondral bone. The PRP moves to the articular space from the subchondral bone through the cartilage.
References
- 1.Lories R.J., Luyten F.P. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–49. doi: 10.1038/nrrheum.2010.197. [DOI] [PubMed] [Google Scholar]
- 2.Jevsevar D.S. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21:571–576. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 3.Andia I., Sánchez M., Maffulli N. Joint pathology and platelet-rich plasma therapies. Expert Opin Biol Ther. 2012;12:7–22. doi: 10.1517/14712598.2012.632765. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez M., Fiz N., Azofra J. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28:1070–1078. doi: 10.1016/j.arthro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Khoshbin A., Leroux T., Wasserstein D. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: A systematic review with quantitative synthesis. Arthroscopy. 2013;29:2037–2048. doi: 10.1016/j.arthro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Yuan X.L., Meng H.Y., Wang Y.C. Bone-cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthritis Cartilage. 2014;22:1077–1089. doi: 10.1016/j.joca.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Murphy M.B., Moncivais K., Caplan A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabay O. Osteoarthritis: New perspectives. J Spine. 2012;1:e101. doi: 10.4172/2165-7939.1000e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan J., Wang B., Li W. Elevated cross-talk between subchondral bone and cartilage in osteoarthritic joints. Bone. 2012;51:212–217. doi: 10.1016/j.bone.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
After sedation of the patient, 2 marks are drawn in the medial region of the knee, one located 2 cm proximal and the other located 2 cm distal to the medial joint line. The patient is positioned supine on the operating room table; the infiltration area is prepared with a povidone-iodine solution. Local anesthesia is conducted by injecting 2 mL of 2% mepivacaine into the periosteum of the condyle and tibial plateau. After evacuation of the synovial fluid, 8 mL of platelet-rich plasma (PRP) is infiltrated into the midpoint area of the femoropatellar region using an external approach. Trocars are introduced into the bone through the logistical marks and inserted 2 cm into the thickness of the medial tibial plateau and condyle. Once the trocars are placed in the desired position, 5 mL of PRP is infiltrated into the subchondral bone. The PRP moves to the articular space from the subchondral bone through the cartilage.


