Abstract
There are many described surgical techniques for the treatment of recurrent anterior shoulder instability. Numerous authors have performed anterior bone block procedures with good results for the treatment of anterior shoulder instability with glenoid bone loss. The benefits of using arthroscopic procedures for surgical stabilization of the shoulder include smaller incisions with less soft-tissue dissection, better visualization of the joint, better repair accessibility, and the best possible outcome for external rotation. We describe an arthroscopic anteroinferior shoulder stabilization technique with an iliac crest tricortical bone graft and capsulolabral reconstruction. It is an all-arthroscopic technique with the advantage of not using fixation devices, such as screws, but instead using special buttons to fix the bone graft. The steps of the operation are as follows: precise placement of a specific posterior glenoid guide that allows the accurate positioning of the bone graft on the anterior glenoid neck; fixation of the graft flush with the anterior glenoid rim using specific buttons under arthroscopic control; and finally, subsequent capsular, labral, and ligament reconstruction on the glenoid rim using suture anchors and leaving the graft as an extra-articular structure.
Shoulder dislocation can cause a variety of pathologic lesions. Patients with chronic anterior shoulder instability may present with recurrent dislocation, subluxation, or chronic shoulder pain.1 Anteroinferior glenoid bone deficiency has been reported in 22% of initial traumatic anterior shoulder dislocations and in up to 90% of recurrent anteroinferior shoulder instability cases.2
The benefits of using arthroscopic procedures for surgical stabilization of the shoulder include smaller incisions with less soft-tissue dissection, better visualization of the joint, improved repair accessibility, and the best potential outcome for external rotation.3
The aim of this study is to describe a new arthroscopic technique for the treatment of concomitant bony defects with the accurate placement of a tricortical bone graft perfectly flush on the anterior glenoid rim, followed by soft-tissue fixation on the anteroinferior glenoid rim (Tables 1 and 2). This technique allows an anatomic reconstruction of the unstable shoulder fixing, at the same time, bony and soft-tissue lesions. Further benefits are the consequential advantages of minimally invasive surgery, such as easier rehabilitation, faster return to sports, and good cosmetic results.
Table 1.
Indications for Arthroscopic Bone Graft Procedure
| Isolated anterior glenoid bone loss >20% |
| Anterior glenoid bone loss <20% with associated Bankart lesion |
| Anterior glenoid bone loss >10% but <20% with ISIS score of 3-6 points |
| First episode of dislocation ≤3 years earlier |
| ≤5 episodes of dislocation |
ISIS, instability severity index score.
Table 2.
Decision Algorithm for Instable Shoulder
| Arthroscopic Capsuloplasty | Latarjet | Arthroscopic Bone Block |
|---|---|---|
| ISIS score <3 points | ISIS score >6 points | Young age |
| No glenoid bone loss | Chronic instability for >3-5 yr | ≤5 episodes |
| Isolated glenoid bone loss <10% | >10 episodes | First episode of dislocation ≤3 years earlier |
| ≤3 episodes | Glenoid bone loss >25% | Good tissue consistency of capsule and ligaments |
ISIS, instability severity index score.
Surgical Technique
Patient Positioning and Joint Preparation
Under general anesthesia and with the administration of perioperative antibiotics, the patient is placed in the beach-chair position (Video 1). The scapula can be bolstered to rotate the glenoid externally.
A standard posterior portal is created for the insertion of the arthroscope. Viewing from the posterior portal, the surgeon creates an anterosuperior portal and midglenoid portal, and two 5.5-mm cannulas are introduced in the rotator interval. Initially, the labrum is accurately detached from the glenoid rim, and all soft tissues are removed from the anterior glenoid neck using a soft-tissue shaver. We then introduce the arthroscope through the anterosuperior portal, and the anterior glenoid rim is further decorticated and flattened with a motorized burr to create a flat and bleeding bony surface to accommodate the graft (Fig 1). At this point, a spinal needle is inserted from posterior to anterior along, and perfectly parallel to, the face of the glenoid and centered on the anterior glenoid bone defect. Then, a more posteromedial portal is made to provide access to the glenoid guide.
Fig 1.
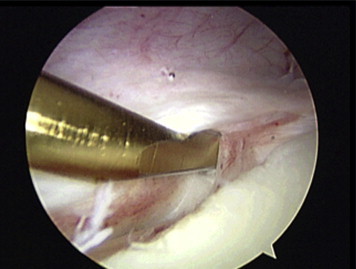
Arthroscopic view from posterior portal. The labrum and capsule are elevated, and the anterior glenoid rim is decorticated to create a flat surface to accommodate the graft.
Glenoid Guide and Drill Pin Placement
The hook end of the glenoid guide is inserted through the specific portal. The hook is passed along the glenoid parallel to the glenoid face to avoid damaging the articular surface, and then, it is passed over the anterior edge (Fig 2A). Once sufficiently advanced, the guide is rotated to capture the anterior edge of the glenoid under the hook. The hook should be placed at the center of the anterior glenoid defect, usually between the 3- and 4-o’clock positions (Fig 2B). It is mandatory that the glenoid guide is aligned with the posterior and anterior glenoid rim.
Fig 2.
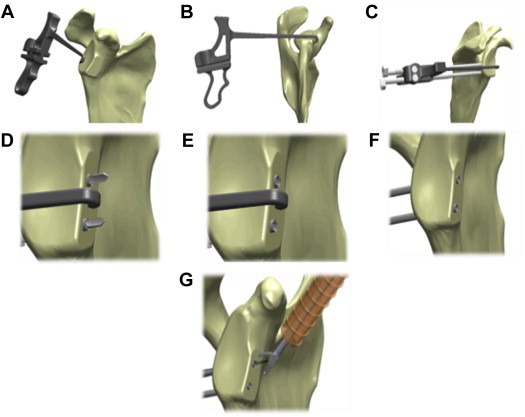
Glenoid guide and drill pin placement. (A) Insertion of hook from posterior portal. (B) Rotation of hook to capture glenoid rim. (C) Insertion and advancement of bullet to posterior border of glenoid. (D) Positioning and advancing of sleeves. (E) Removal of inner drill. (F) Removal of bullet. (G) Introduction of 10-mm cannula through rotator interval and retrieval of wires.
Once the guide is positioned, a bullet is placed in the inferior hole of the guide (Fig 3). A small skin incision is made, and the bullet is advanced until it firmly contacts the posterior aspect of the glenoid neck (Fig 4). The ratchet teeth of the bullet should be aligned with the screws adjacent to the guide handle. The process is repeated for the superior bullet (Fig 2C). A 2.8-mm sleeved drill is placed in each bullet and advanced under power until exiting from the anterior aspect of the glenoid. The drills are placed 5 mm on the center below the cortical edge of the glenoid face, parallel to one another and 10 mm apart (Fig 2D). The inner drill is removed, leaving the cannulated outer sleeve. Arthroscopic fluid exiting from the outer sleeve posteriorly confirms intra-articular positioning (Fig 2E).
Fig 3.
Glenoid guide with special bullets used to create holes from posterior to anterior at level of glenoid rim and two 2.8-mm sleeves (specific instruments created by Smith & Nephew): 1 T-Handle, Dual Glenoid Guide Poster (model 72203340); 1 Bullet, Glenoid Guide, Pos, Long (model 72203341); 1 Bullet, Glenoid Guide, Pos, Short (model 72203342); and 2 MTO guidewires with 2.8-mm sleeve (model 72202973).
Fig 4.
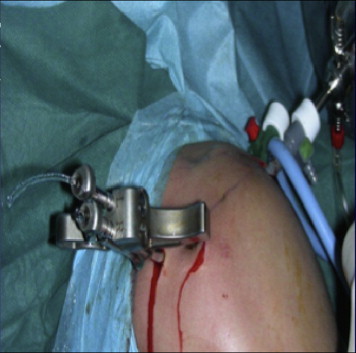
Outside view of proper positioning of glenoid guide and insertion of bullet (right shoulder).
Once drilling is completed, the bullets can be removed by rotating each bullet to disengage the ratcheting teeth and extracting them posteriorly (Fig 2F). The guide can be removed at this stage. Care should be taken to ensure that the sleeves remain firmly positioned in the glenoid neck. Flexible looped guidewires are then introduced into the joint by passing one wire through each sleeve posterior to anterior (Fig 5A). Each guidewire is retrieved using a loop grasper, which is passed through the 10-mm cannula introduced through the rotator interval (Fig 5B). The wires are separated and stored. The drill sleeves should be removed after this step is completed (Fig 2G).
Fig 5.
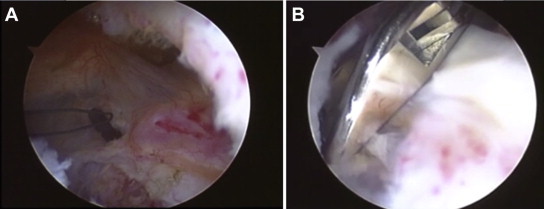
(A) Arthroscopic view from posterior portal of flexible loop guidewires in joint (Looped Guidewire and Extension Pack [model 72203526]; Smith & Nephew). (B) Arthroscopic view from posterior portal of loop grasper used to retrieve flexible loop (Loop Grasper [model 7209494]; Smith & Nephew).
Bone Block Preparation
The tricortical bone graft is harvested from the ipsilateral anterior iliac crest. After a 4-cm skin incision has been made and splitting of the subcutaneous tissue has been performed, the inserting muscles are dissected from the iliac crest and 2.5 to 3 cm of bone is marked with a fine-tip cautery while keeping a minimum safety distance of 4 cm to the anterior superior iliac spine.
The tricortical iliac crest bone block, measuring 20 mm × 8 mm × 8 mm, is fashioned using the graft master preparation board. Two 2.8-mm drill holes are made 10 mm apart and 5 mm from each edge. The drill enters through the cortex and exits the cancellous side of the bone block. The holes created correspond to the distance of the cannulated drill sleeves previously placed in the glenoid neck. With a marking pen, we color the superior aspect of the bone block.
Graft Passage and Loading of Implants (Anterior and Posterior Round EndoButtons)
Before loading the implant onto the guidewires, care is taken to ensure that the looped guidewires are not tangled within the joint. Each looped guidewire is fed through the prepared bone block and exits on the cortical side (Fig 6A). The bone block is oriented so that the cancellous surface is facing the anterior neck of the glenoid. The anterior implant is fed with the preassembled suture through the end of the looped guidewire with a classic slip knot. This can be achieved by passing the lead suture through the looped guidewire and feeding the implant through the lead suture (Fig 6B).
Fig 6.
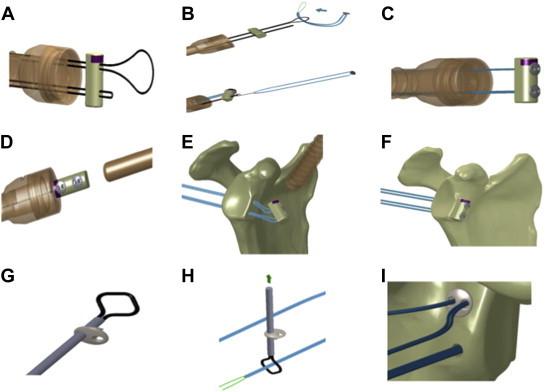
Graft passage and loading of implant. (A) The implant is loaded onto the guidewires. Each guide is fed through the bone block. (B) The implant is fed with the preassembled suture through the end of the looped guidewire with a classic slip knot. (C) The bone block is slid toward the end of the guidewires to lodge the implants. (D) The bone block is slid through the cannula up to the anterior glenoid rim. (E) The bone block is advanced, pulling the suture from posterior. (F) The bone block is made flush with the anterior glenoid rim. (G) The implant is placed on the transporter. (H) The suture is passed through the transporter. (I) The posterior round EndoButton is advanced to sit flush against the posterior face of the glenoid.
The bone block is slid toward the end of the guidewires to lodge the implants. Anterior round EndoButtons (Smith & Nephew, London England) (Fig 7) are advanced until they lie flat on the bone block. Sutures should be taut to allow smooth movement down the cannula (Fig 6C). The bone block is tipped to be inserted into the 10-mm cannula, and care is taken to ensure that the superior end of the bone block enters the cannula first (Fig 6D). The bone block is advanced by pulling the guidewires out posteriorly. Slight tension should be maintained on the sutures throughout this step (Fig 6E). The sutures should advance the implant until the bone block sits flush on the anterior neck of the glenoid, with each implant's lead suture exiting the skin posteriorly (Fig 6F). The posterior implants are placed on the transporter by advancing the instrument through the eyelet of a posterior round EndoButton (Fig 6G). The suture is passed through the transporter. The transporter is retracted to allow the suture to pass through the eyelet of the posterior round EndoButton. The same steps must be performed for the second eyelet with the other side of the suture (Fig 6H). The posterior round EndoButtons are advanced until they sit flush against the posterior face of the glenoid. The knot pusher is used to secure the posterior round EndoButtons. The knot pusher will provide tactile feedback when the posterior round EndoButtons are properly seated (Fig 6I).
Fig 7.
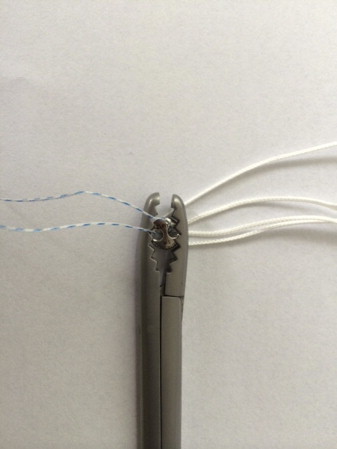
Round EndoButton used to fix bone block to anterior glenoid rim (Round EndoButton SS with No. 2 Suture Loop [model 71934840]; Smith & Nephew).
Nice Knot
The side of the suture that was cut with the remaining lead suture tails will serve as a post (Fig 8A). With the post in hand, we create a figure of 4 by placing the loop over the post (Fig 8B). We then bring the loop underneath the post and through the figure of 4. The loop is opened at the end of the thread (Fig 8C). Then, we place the post through the open loop created previously (Fig 8D). Finally, we build the knot behind the posterior implant by pulling tight on the loop (Fig 8E). Care must be taken to ensure that the knot is fully taut before pulling the post and advancing the posterior implant (Fig 8F).
Fig 8.
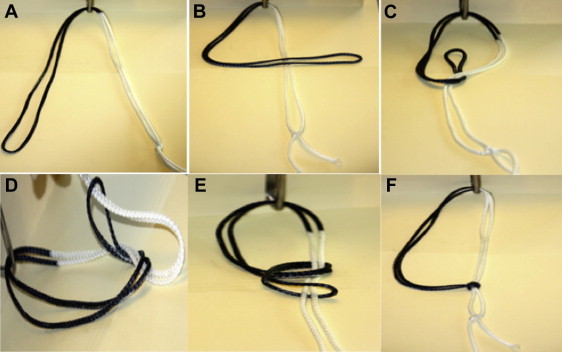
Nice knot. (A) The side of the suture that was cut is the post. (B) A figure of 4 is created by placing the loop over the post. (C) The loop is brought underneath the post, and the loop is opened at the end of the thread. (D) The post is placed through the open loop created before. (E) The knot is built behind the posterior implant by pulling tight on the loop. (F) Care is taken to ensure that the knot is fully taut before pulling the post and advancing the implant.
Secure Nice Knot
We advance the Nice knot to the face of the posterior round EndoButton (Fig 9A). At this point, we use a suture tensioner device (Fig 10) to secure the implant and to provide strong compression of the graft on the anterior glenoid neck. Once the implant has been tensioned, we secure the posterior knots with half-hitches, and we cut the remaining sutures using a blind knot cutter (Figs 9 B and C and 11).
Fig 9.
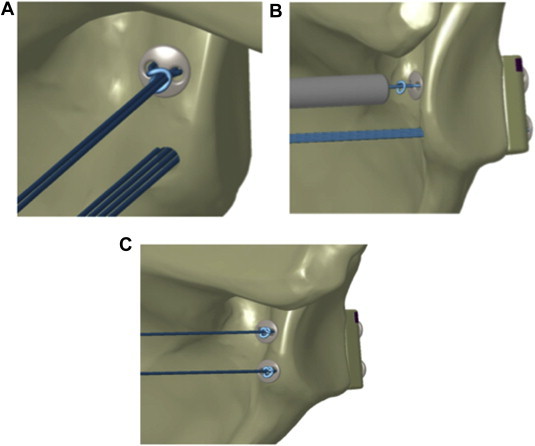
Secure Nice knot. (A) The Nice knot is advanced up to the round button. (B) The implant is secured with the tensioner, and the remaining suture is cut. (C) The same steps are repeated for the inferior implant.
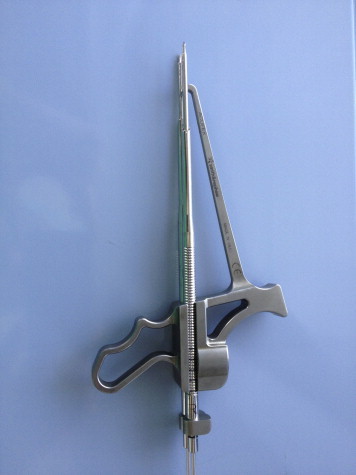
Fig 10.
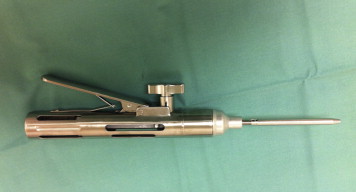
Tensioner device used to fix and secure implant (Suture Tensioner [model 71934995]; Smith & Nephew).
Fig 11.
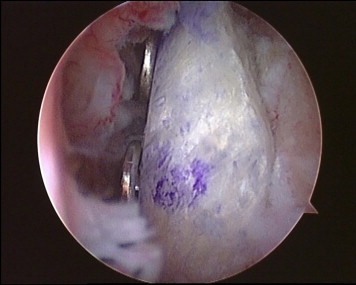
Arthroscopic view from anterior portal. The bone block is perfectly flush with the anterior glenoid rim.
Soft-Tissue Repair
The anterior labrum, capsule, and ligaments are repaired to the glenoid rim with suture anchors and a standard arthroscopic soft-tissue repair technique (Fig 12).
Fig 12.
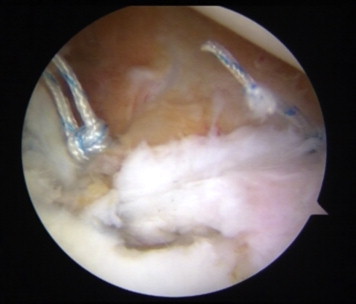
Arthroscopic view from posterior portal. The anterior labrum and capsule are repaired to the glenoid rim with suture anchors and a standard Bankart repair technique.
Rehabilitation Protocol
After surgery, the shoulder is immobilized in a brace in abduction of 10° for 4 weeks. There are no limitations regarding passive movement after immobilization, and patients are subsequently allowed to regain full elevation and external rotation.
After complete healing of the wound, pool exercises and return to work activities are authorized. Progressive stretching exercises are started after 6 to 8 weeks. Return to contact sports and overhead mobility is generally allowed 4 to 6 months after surgery (Figs 13 and 14).
Fig 13.
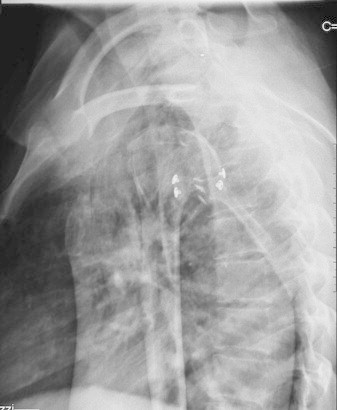
Lateral radiographic projection of a right shoulder the day after surgery. The bone block and buttons are correctly positioned.
Fig 14.
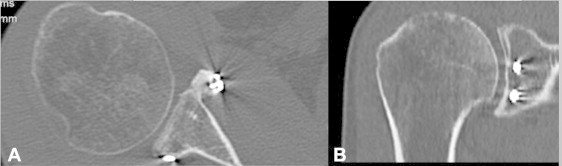
Computed tomography images showing bone graft healing and remodeling after 6 months in a right shoulder: (A) axial view and (B) coronal view.
Risks
The general risks related to our intervention can be compared with the risks that exist during an arthroscopic procedure for shoulder instability repair.4 Our procedure has some specific risks. If correct placement of the graft cannot be achieved, it is necessary to change to an open approach with consequent arthrotomy. This has occurred twice with the previous technique of fixing the bone block with screws but, to date, has never occurred with the newly described procedure.
To obtain perfect placement of the graft, it is mandatory to achieve perfect placement of the guide. If the hook is not perfectly parallel to the glenoid face, there will be a bone graft offset greater than 5 mm, with final placement of the graft medial to the glenoid rim.
The positioning of the anterior portals is performed at the level of the rotator interval, thereby avoiding possible nerve injuries. In the same manner, the glenoid guide is designed to maintain distance from the vascular or neural structures while positioning the sleeves.
Discussion
Operative techniques have recently advanced sufficiently so that the shoulder surgeon can successfully repair anteroinferior glenohumeral instability arthroscopically.5 Optimally, however, the arthroscopic surgeon should be able to address all clinically relevant lesions, including bony defects, by incorporating techniques that allow restoration of both the anatomy and biomechanical function of damaged structures.6
Other authors have described and reported bone block procedures for anteroinferior glenohumeral instability, but our technique is the first performed entirely arthroscopically without the use of screws.7-11 The well-established advantages of using arthroscopy for shoulder stabilization include smaller incisions with less soft-tissue dissection and improved ability to completely inspect the glenohumeral joint and access all areas of the joint for repair, as well as preservation of external rotation. In case of mechanical inconsistency or absence of the soft tissues, it is possible to switch to an arthroscopically assisted Latarjet procedure that we have recently described.12
In conclusion, this new entirely arthroscopic bone grafting procedure with concomitant soft-tissue reconstruction allows an anatomic repair for anterior shoulder instability with bony defects. Moreover, it has shown promising early clinical results, avoiding the use of fixation screws.
Limitations
The most important disadvantage of this procedure is the withdrawal of the iliac crest, which can be very painful in both the short- and long-term.13,14 The use of allograft might solve this inconvenience.
We started to use this technique 9 months ago, and although we have encountered no intraoperative and postoperative complications to date, long-term results are necessary to confirm our findings. Most importantly, the arthroscopic bone block procedure is a challenging intervention, which involves a long learning curve and must be performed by surgeons specializing in arthroscopic shoulder surgery.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article.
Supplementary Data
Main steps of bone block arthroscopic procedure: debridement of the anterior labrum and cartilage; placement of the glenoid guide and progress of the 2 drill sleeves up to the anterior glenoid rim; removal of the 2 sleeves and introduction of the flexible loop retrieved from posterior to anterior with a grasper; bone block preparation; graft passage and loading of implant; advancement of the bone block up to the anterior glenoid rim; passage of the suture through the transporter; progress of the posterior round EndoButtons to sit flush against the posterior face of the glenoid; placement of Nice knot to secure the suture posteriorly; tensioning of sutures with the tensioner; and reconstruction of the anterior labrum.
References
- 1.Provencher M.T., Bhatia S., Ghodadra N.S. Recurrent shoulder instability: Current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92:133–151. doi: 10.2106/JBJS.J.00906. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 2.Sayegh ET, Mascarenhas R, Chalmers PN, Cole BJ, Verma NN, Romeo AA. Allograft reconstruction for glenoid bone loss in glenohumeral instability: A systematic review. Arthroscopy in press, available online 4 July, 2014. doi:10.1016/j.arthro.2014.05.007. [DOI] [PubMed]
- 3.Lafosse L., Boyle S. Arthroscopic Latarjet procedure. J Shoulder Elbow Surg. 2010;19:2–12. doi: 10.1016/j.jse.2009.12.010. (suppl) [DOI] [PubMed] [Google Scholar]
- 4.Moen T.C., Rudolph G.H., Caswell K., Espinoza C., Burkhead W.Z., Jr., Krishnan S.G. Complications of shoulder arthroscopy. J Am Acad Orthop Surg. 2014;22:410–419. doi: 10.5435/JAAOS-22-07-410. [DOI] [PubMed] [Google Scholar]
- 5.Kim S.J., Kim S.H., Park B.K., Chun Y.M. Arthroscopic stabilization for recurrent shoulder instability with moderate glenoid bone defect in patients with moderate to low functional demand. Arthroscopy. 2014;30:921–927. doi: 10.1016/j.arthro.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Burkhart S.S., De Beer J.F. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: Significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16:677–694. doi: 10.1053/jars.2000.17715. [DOI] [PubMed] [Google Scholar]
- 7.Taverna E., Golanò P., Pascale V., Battistella F. An arthroscopic bone graft procedure for treating anterior-inferior glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2008;16:872–875. doi: 10.1007/s00167-008-0541-y. [DOI] [PubMed] [Google Scholar]
- 8.Warner J.J., Gill T.J., O’Hollerhan J.D., Pathare N., Millett P.J. Anatomical glenoid reconstruction for recurrent anterior glenohumeral instability with glenoid deficiency using an autogenous tricortical iliac crest bone graft. Am J Sports Med. 2006;34:205–212. doi: 10.1177/0363546505281798. [DOI] [PubMed] [Google Scholar]
- 9.Scheibel M., Kraus N., Diederichs G., Haas N.P. Arthroscopic reconstruction of chronic anteroinferior glenoid defect using an autologous tricortical iliac crest bone grafting technique. Arch Orthop Trauma Surg. 2008;128:1295–1300. doi: 10.1007/s00402-007-0509-2. [DOI] [PubMed] [Google Scholar]
- 10.Kraus N, Amphansap T, Gerhardt C, Scheibel M. Arthroscopic anatomic glenoid reconstruction using an autologous iliac crest bone grafting technique. J Shoulder Elbow Surg in press, available online 12 June, 2014. doi:10.1016/j.jse.2014.03.004. [DOI] [PubMed]
- 11.Anderl W., Kriegleder B., Heuberer P.R. All-arthroscopic implant-free iliac crest bone grafting: New technique and case report. Arthroscopy. 2012;28:131–137. doi: 10.1016/j.arthro.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Taverna E., Ufenast H., Broffoni L., Garavaglia G. Arthroscopically assisted Latarjet procedure: A new surgical approach for accurate coracoid graft placement and compression. Int J Shoulder Surg. 2013;7:120–123. doi: 10.4103/0973-6042.118912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Neill K.R., Lockney D.T., Bible J.E., Crosby C.G., Devin C.J. Bupivacaine for pain reduction after iliac crest bone graft harvest. Orthopedics. 2014;37:e428–e434. doi: 10.3928/01477447-20140430-52. [DOI] [PubMed] [Google Scholar]
- 14.Singh D., Gombar K.K., Bhatia N., Gombar S., Garg S. Evaluation of analgesic effect of local administration of morphine after iliac crest bone graft harvesting: A double blind study. J Anaesthesiol Clin Pharmacol. 2013;29:356–360. doi: 10.4103/0970-9185.117109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main steps of bone block arthroscopic procedure: debridement of the anterior labrum and cartilage; placement of the glenoid guide and progress of the 2 drill sleeves up to the anterior glenoid rim; removal of the 2 sleeves and introduction of the flexible loop retrieved from posterior to anterior with a grasper; bone block preparation; graft passage and loading of implant; advancement of the bone block up to the anterior glenoid rim; passage of the suture through the transporter; progress of the posterior round EndoButtons to sit flush against the posterior face of the glenoid; placement of Nice knot to secure the suture posteriorly; tensioning of sutures with the tensioner; and reconstruction of the anterior labrum.


