Abstract
Aim of work
The aim of our work was to detect any structural or functional visual defects during and in between the attacks in patients with migraine.
Patient and methods
Sixty patients with migraine as well as sixty age and sex matched controls were included. All cases were subjected to full ophthalmological examination. Cases with any previously known optic nerve abnormalities or with history of increased intraocular pressure were excluded from this study. A full threshold 24-2 automated perimetry as well as optical coherence tomography (OCT) were performed for retinal nerve fiber layer (RNFL) thickness. Correlations between results of study group with migraine and controls were analyzed and recorded.
Results
There was a statistically significant difference between the patients with migraine and the controls in visual field analysis which was (P < 0.05) for generalized visual field deficits and (P < 0.001) for localized visual field deficits during the attack with no statistically significant difference in visual field in between the attacks (P > 0.05). OCT RNFL thickness had no statistically significant difference between migraine and control groups (P > 0.05).
Conclusion
Migraine can cause functional ocular disorder without any structural abnormalities.
Keywords: Migraine, Visual field, Optical coherence tomography
Introduction
Headache as the presenting symptom to the ophthalmological clinic is not uncommon and represents an important complaint that should be treated with extreme care.1
Migraine is a common (10–15% of general population), chronic neurovascular disorder typically characterized by recurrent attacks of pulsating headache and autonomic nervous system dysfunction. Most often the headache is unilateral, variable in frequency, and lasts at least for four hours. The pain is usually throbbing or pulsating, moderate to severe, and aggravated by physical activity, bright lights, and loud noises. Associated symptoms are nausea, anorexia, and photophobia.2
Visual symptoms are common in migraine patients, with most of them experiencing photophobia and mild visual disturbances, a subgroup of persons who experience migraine with aura, however, may experience more severe visual symptoms which may be positive such as flashes of light and zigzags or negative such as paracentral scotomata, as well as neurological symptoms such as hemiparesis or dysphasia.3
Patients with migraine have been lately demonstrated to have an increased prevalence of visual field defects that are not of a temporary neural origin.4
There are only a few reports in the literature documenting ocular abnormality in migraine during the attack in a small sample size.
This work attempted to study the visual field changes in an Egyptian sample of patients with migraine, as well as doing, optical coherence tomography (OCT) which is a non invasive, non contact technique for structural imaging of the layers of the retina.
Patients and methods
Prospective non randomized case control study was done for sixty patients with episodes of severe headache recognized and diagnosed by neurologists as migraine and were referred to us from the neurology clinic of Ain-Shams university hospitals and sixty healthy age and sex-matched controls were included in this study.
Written informed consents were taken from all patients and acceptance of medical and ethics committee of the hospital to do this study.
All patients suffered from migraine with aura and migraine without aura diagnosed according to the criteria of the International Headache Society 2004.5
Full ophthalmological examinations were done to all our cases to exclude any abnormalities and to ensure that all patients had normal optic disk appearance, intraocular pressure within normal range, best corrected visual acuity (BCVA) of 6/6 in both eyes and refractive errors of less than 4 diopters (D) Spherical error and 1.5 D astigmatism, with special attention to pupillary response. History was taken to ensure that all patients were free of systemic diseases and not taking any systemic medications known to affect visual functions. None of the migrainous patients were receiving a specific therapy for migraine, but only simple analgesics. The age of onset, duration, frequency, predominant side of headache, presence or absence of aura and family history of migraine if present were recorded.
A full – threshold, central 24-2 automated static perimetry was done to all our cases for both eyes of each patient, using the Humphrey field analyzer II (Humphrey Instruments Inc., CA). These perimetric examinations were done for all patients during the attack of headache and in free periods after one week or more on at least two occasions; the reliability indices must be accepted. The Global indices (mean deviation in decibels (MD), pattern standard deviation (PSD), corrected pattern standard deviation (CPSD) and results of glaucoma hemifield test were recorded. The field was considered beyond normal if; glaucoma hemifield test (GHT) was outside the normal limits and/or presence of a cluster of 3 or more depressed non edge points (P < 1% in one and <5% in the others).4 Right and left eye data were analyzed separately to determine whether unilateral or bilateral involvement was present. We excluded any patient with bad reliability indices or who could not perform the test during the attack.
Optical coherence tomography (OCT-2000; Humphrey zeiss instruments) was used in this study to measure the retinal nerve fiber layer (RNFL) thickness for both eyes of each patient, which was defined as the number of pixels between the anterior and the posterior edges of the RNFL. Each patient underwent three circular scans around the center of the optic disk after pupillary dilatation and the mean of the three scans provided the mean and quadrant RNFL thickness measurements were recorded. The RNFL thickness was differentiated from other retinal layers using edge detection algorithm.
Data were extracted and transposed onto a data sheet, which were entered into a computer programed with the SPSS/PC Software 17 Package (SPSS Inc., Chicago, IL, USA). Record was made of each patient’s name, age, hospital number, visual field, OCT RNFL thickness, duration, side and presenting symptom of migraine, recurrence and follow up for further attacks up to six months. These data were compared using an analysis of variance, P < 0.05 was regarded as significant and highly significant if P < 0.001.
Results
Demographic data of both groups are shown in Table 1. There were no significant differences in the predominant side of migraine in all our cases 28 (46.7%) were predominant right side and 32 (53.3%) were predominant left side.
Table 1.
Comparison of results of migraine group and control group.
| Migraine group | Control group | P value | |
|---|---|---|---|
| Age | 40.43 + 3.33 years | 43.11 + 2.11 years | P > 0.05 |
| Sex | Male/female = 27/33 | Male/female = 24/36 | P > 0.05 |
| Refractive error | Range −2.5 to −3.5 D | Range −1.5 to −3.00 D | P > 0.05 |
| Mean −2.75 + 1.2 D | Mean −1.75 + 1.7 D | ||
| General VFD | 40 (66.7%) | No deficits | P < 0.05 |
| Localized VFD 1 | 47 (78.3%) | No deficits | P < 0.001 |
| Peripheral contraction VFD 1 | 43 (71.7%) | No deficits | P < 0.001 |
| VFD 2 | No deficits | No deficits | P > 0.05 |
| OCT NFLT average | 112.78 + 16.25 um | 114.35 + 12.43 um | P > 0.05 |
VFD = visual field deficits, VFD 1 = visual field deficits during the attack, VFD 2 = visual field deficits after the attack, OCT NFLT = optical coherence tomography nerve fiber layer thickness.
The frequency of episodes varied between one or more attacks a week and once a month and the attack of migraine lasts 4–72 h with a mean of 8.71 ± 1.7 h in patients who had migraine with aura and 9.51 + 1.2 h in patients who had migraine without aura. Frequency and duration of the attacks were not statistically significantly different among both migraine with and without aura (P > 0.05). There was no family history of migraine in all our cases.
The main presenting symptoms were blurring of vision during the attack of migraine in 48 (80%) cases, 5 (8.3%) cases with visual field defects and 7 (11.7%) cases presented with ocular redness, lacrimation and photophobia. Aura was present in 21 (35%) cases and absent in 39 (65%) cases.
Myopia was the most common refractive error in all our cases ranging from −2.5 to −3.5 D. BCVA was 6/6 in all our cases. Anterior segment examination revealed no abnormalities except of moderate ocular congestion in 12 cases. Intraocular pressure was near the high upper normal limit in 3 cases and sluggish pupillary response was recorded in 4 cases during the attack of migraine bilaterally.
Changes in field of vision
During the attack of migraine
Significantly lower general retinal sensitivity across the visual field (P = 0.01) and a high incidence of localized visual field defects in relation to controls were recorded in our study. Localized field defects were recorded in 47 (78.3%) of cases and were unilateral in 36 (60%) of them.
19 (31.7%) of patients had defects simulating the nasal step of glaucoma (Fig. 1), 9 (15%) had the depressed locations clustered in the inferior arcuate nerve fiber bundle region, 18 (30%) had paracentral depression (Fig. 2) and finally 1 (1.7%) had scattered points of low-moderate probability with general reduction in retinal sensitivity (Fig. 3). Peripheral contraction of visual field was present in 43 (71.7%) of our cases.
Figure 1.
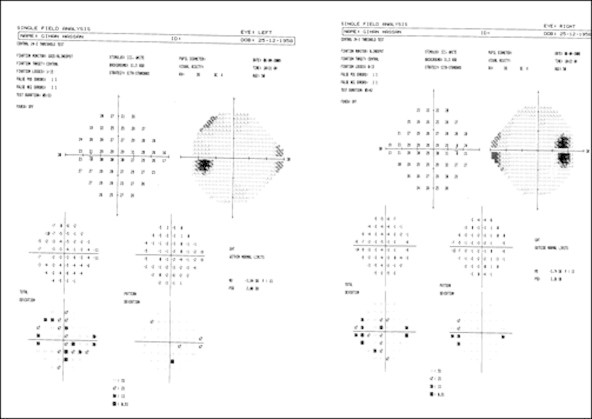
Patient 1-right and left nasal step.
Figure 2.
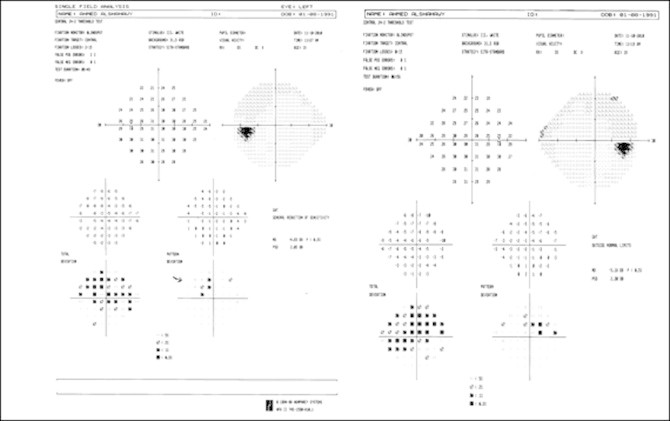
Patient 4-left and right paracentral scotoma.
Figure 3.
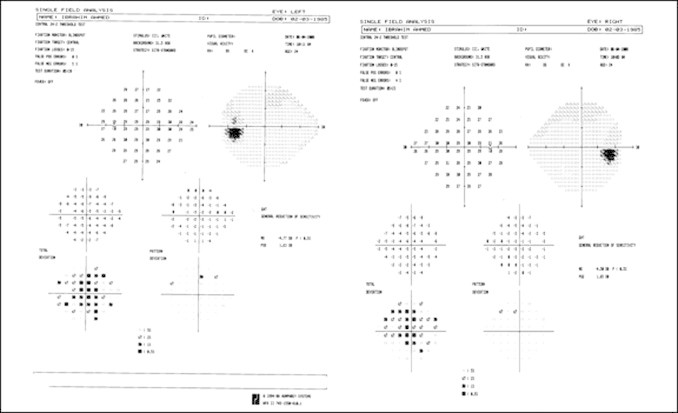
Patient 3-left and right eye generalized depression.
The deficits in the visual field were not correlated with the age or sex of the patients nor with the frequency, duration, presence or absence of the aura, family history or side of migraine.
After the attack of migraine
The visual field deficits disappeared completely in between the attacks (temporary deficit).
For control group there were no visual field deficits.
OCT revealed no abnormalities in the retinal nerve fiber thickness among all our cases; it was within normal limit and no statistically significant difference between migrainous cases and the control group. Table 1 Thus migraine may be considered a functional deficit without any structural abnormalities.
Discussion
Most estimates of migraine prevalence range from 12 to 15% with the prevalence for women being about twice for men6 but there was no significant gender difference in our patients; being 27 (45%) males and 33 (55%) females.
We found visual field deficits of variable extent during the attacks of migraine in 47 (78.3%) of patients which disappeared completely in between the attacks, in agreement with a relatively recent visual field study suggesting that 20% to 48% of migrainous cases demonstrated visual field deficits.4 If these estimates were representative of migraine patients, then there was a possibility that between 2.5% to 6% of the general population might demonstrate some form of visual field abnormalities in association with migraine.
There were several differences between this study and the prior ones,1,4,6 most previous studies did not include a comparable group of non headache control subjects, nor did they perform concurrent OCT and visual field examination on the same group of patients and correlate the findings. To the best of our knowledge OCT was not performed in any of the previous studies.
The visual field deficits in our study were not significantly related to age, sex of patients or to the frequency, duration, presence or absence of the aura, side of migraine or family history.
Although other studies suggested that the visual field deficits are strictly cortical in origin due to changes in blood flow within the cortical areas responsible for vision,7 the collaborative NTG study found that patients with migraine, particularly women, experienced a more rapid course of glaucoma progression than those without a history of migraine.3
In our study, ophthalmological examination revealed normal intraocular pressure in all cases except three showed transient high normal pressure during the attack of headache. Also 19 (31.7%) of our patients had defects simulating the nasal step of glaucoma and 43 (71.7%) showed peripheral contraction of visual field during the attack of headache.
As these visual field deficits were transient and disappeared after the attack so it might have a neural origin.
Several mechanisms may play role in the genesis of neural dysfunction in migraine.
An increase in the aggregation of thrombocytes and the levels of serotonin during the headache period has been demonstrated in migraine patients.8 Chemical transmitters as serotonin might play a role in constriction of large arteries and dilatation of arterioles and capillaries, in fact, a few cases of ischemic optic neuropathy have been reported during the episode of migraine.9
Another study had related the cerebral ischemia found in migraine to ergotamine therapy (which is a vasospastic agent).10 In this study, the selected patients did not receive any specific treatment for migraine, but only simple analgesics, therefore our fundus examination findings could not be related to these medications.
A challenge remains in determining the relationship, if any, between visual dysfunction in migraine and glaucoma. The relationship between these two conditions has not been a simple one, migraine has been suggested as a vascular risk factor for glaucoma.11
Some authors reported a high frequency of migraine in normotensive glaucoma (NTG) patients in comparison to chronic open angle glaucoma, ocular hypertension and normal patients.12
In our study, OCT revealed normal thickness of retinal nerve fiber layer which eliminates the possibility of associated or induced glaucoma. Thus, these visual field deficits may well be cortical in origin.
Our study findings in conjunction with those of previous authors suggest that Migraineurs should be excluded from normative perimetric databases. Claiming that all patients with such deficits had glaucoma will not be an appropriate conclusion, a possible relationship between migraine, normotensive glaucoma, open angle glaucoma and vascular conditions of the eye deserves further investigation.
Conclusion
Migraine may cause functional visual deficits as documented by ophthalmological examination and visual field examination data during the attack without any structural retinal abnormalities as confirmed by OCT. Further studies should be performed to find out if these visual field deficits are of cortical or retinal origin or due to vascular or neural abnormalities.
Financial support
Funded by Ophthalmology Department, Ain Shams University.
Authors declare that there is no financial interest in any of the materials used.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Amer S.A.K., Hassan M.A., Saif Y.S., Samir H. In Headache with ophthalmological correlation in children and young Adolescents at study of 50 cases. Bull-Ophthalmol-Soc Egypt. 2006;99 [Google Scholar]
- 2.Noseda R., Kainz V., Jakubowski M. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goadsby P.J., Lipton R.B., Ferrari M.D. Migraine-current understanding and treatment. N Eng J Med. 2002;364:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 4.McKendrick A.M., Vingrys A.I., Badcock D.R., Heywood J.T. Visual field losses in subjects with migraine headaches. Invest Ophthalmol Vis Sci. 2000;41:1239–1247. [PubMed] [Google Scholar]
- 5.Olesen J., Steiner T.J. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl. 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 6.Russell M.B., Rasmussen B.K., Thorvaldsen P., Olesen J. In Prevalence and Sex ratio of the subtypes of migraine. Int.-J. Epidemiol. 1995;24:612–618. doi: 10.1093/ije/24.3.612. [DOI] [PubMed] [Google Scholar]
- 7.Anderson J.L., Muhr C., Lilja A., Valind S., Lundberg P.O., Langstrom B. Regional cerebral blood. Flow and oxygen metabolism during migraine with and without aura. Cephalalgia. 1997;17:570–579. doi: 10.1046/j.1468-2982.1997.1705570.x. [DOI] [PubMed] [Google Scholar]
- 8.Moskowitz M.A. The neurobiology of vascular head pain. Ann Neurol. 1984;16:157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- 9.O’ Hara M., Connor P.S. Migrainous Optic neuropathy. J Clin Neuroophthalmol. 1984;4(2):85–90. [PubMed] [Google Scholar]
- 10.Tfelt-Hansen P.C., Koehler P.J. History of the use of ergotamine and dihydroergotamine in migraine from 1906 and onward. Cephalalgia. 2008 Aug;28(8):877–886. doi: 10.1111/j.1468-2982.2008.01578.x. [DOI] [PubMed] [Google Scholar]
- 11.Flammer J., Orgul S. Optic nerve blood flow abnormalities in glaucoma. Prog Retin Eye Res. 1998;17:267–289. doi: 10.1016/s1350-9462(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 12.Corbett J.J., Phelps C.D., Eslinger P., Montangue P.R. The neurologic evaluation of patients with low-tension glaucoma. Invest Ophthalmol Vis Sci. 1985;26:1101. [PubMed] [Google Scholar]


