Abstract
Many risk factors have been linked to retinal vein occlusions (RVOs) whether central or branch retinal vein occlusion. Ocular risk factors include glaucoma and hypermetropia. Controversy exists to whether short axial length is a risk factor for retinal vein occlusions. We report an extreme case that supports the latter hypothesis. A 33-year-old male presented with decreased visual acuity in the left eye. He turned out to have nanophthalmos with hemiretinal vein occlusion and macular edema with unremarkable systemic work up for retinal vein occlusion except for a glycated hemoglobin (HbA1c) level of 7%. To our knowledge this is the first case report of hemiretinal vein occlusion in the setting of nanophthalmos and suggests that short axial length may be a risk factor for retinal vein occlusion.
Keywords: Nanophthalmos, Retinal vein occlusion, Macular edema, Papillomacular fold
Introduction
Many risk factors have been linked to retinal vein occlusions (RVOs) whether central, hemi or branch retinal vein occlusion. Controversy exists to whether short axial length is a risk factor for retinal vein occlusions. We report an extreme case that may support the latter hypothesis.
Case report
A 33-year-old male with no known medical illness presented to the emergency department with painless decrease in vision in his left eye of 2 week duration. On examination the best-corrected visual acuity was 20/40 in the right eye (OD) and 20/300 in the left eye (OS). Refraction measured +14.00 − 1.00 × 81 in OD and +14.00 + 1.00 × 104 in OS. A +2 grade left afferent pupillary defect was present. The intraocular pressure measured 20 mmHg in both eyes and the anterior chamber angles were open bilaterally. The patient had been maintained on Timolol eye drops twice daily to both eyes for an unclear reason. The horizontal corneal diameter measured 9.5 mm in both eyes and the axial length was 16.35 mm and 16.28 mm in OD and OS respectively thus making the diagnosis of nanophthalmos. Fundus examination of the right eye was unremarkable except for subtle thin retinal wrinkles in the papillomacular area (Fig. 1a). The optic nerve in the right eye had a normal cup to disk ratio. Spectral-domain optical coherence tomography (SD-OCT) of the right eye showed a small dome-shaped papillomacular fold and no macular edema (Fig. 1b). Fundus evaluation of the left eye showed flame-shaped hemorrhages in the superior half of the retina with macular edema and optic disk congestion (Fig. 2a). Fundus fluorescein angiography (FFA) supported the diagnosis of hemiretinal vein occlusion (HRVO) in this eye (Fig. 2b), and SD-OCT confirmed the presence of macular edema with significant neurosensory retinal thickening primarily in the outer plexiform layer accompanied by a few hyporeflective spaces (cysts) in the inner nuclear layer (Fig. 3a). There was no evidence of retinal vasculitis in either eye.
Figure 1.
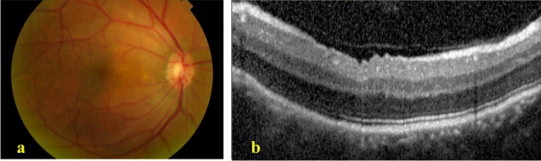
(a) Color photograph of the right fundus shows a thin papillomacular fold. (b) Macular spectral-domain optical coherence tomography of the right eye shows a small dome-shaped papillomacular fold and no macular edema.
Figure 2.
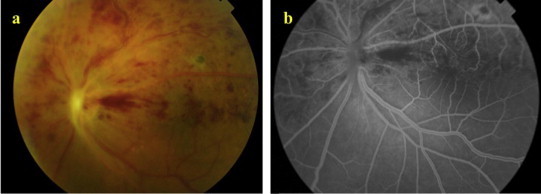
(a) Color photograph of the left fundus shows flame shaped hemorrhages in the superior half of the retina. (b) Fluorescein angiography shows delayed venous filling.
Figure 3.
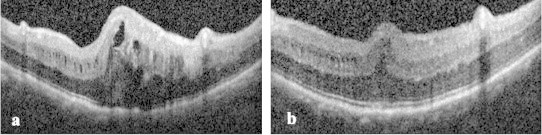
Macular spectral domain optical coherence tomography of left eye before (a) and after (b) bevacizumab injection.
Systemic work up for retinal vein occlusion in a young patient was performed and was normal except for a mild elevation in the glycated hemoglobin (HbA1c) level of 7% suggesting that the patient was diabetic, although he had been unaware of it. These tests included a complete blood count, PT/PTT, lipid profile, fasting blood sugar, glycated hemoglobin, screening for sickle cell anemia, serum homocysteine level, factor V Leiden mutation, protein C and S deficiency, antithrombin deficiency, anticardiolipin antibodies and anti-phospholipid antibodies.
Prior to proceeding with an intravitreal injection of an anti-vascular endothelial growth factor agent (anti-VEGF), a glaucoma service consultation corroborated that the anterior chamber angle was open and no prophylactic iridotomy was needed at the time. An intravitreal injection of bevacizumab 1.25 mg/0.05 ml was administered in the left eye and 1 month later the visual acuity improved to 20/70. In addition, the macular edema decreased dramatically making evident the characteristic papillomacular fold (PMF) typically seen on fundus examination and SD-OCT in eyes with posterior microphthalmos and nanophthalmos (Fig. 3b), as well as the typical features of the PMF previously described in the literature.1–3 These features had been mostly concealed by the macular edema in the pre-treatment OCT and include: (1) a dome-shaped, partial thickness retinal fold, sparing the retinal pigment epithelium, photoreceptors, ellipsoid zone, external limiting membrane and outer nuclear layer; (2) preserved retinal stratification within and beyond the PMF; (3) inner nuclear layer cysts and (4) an accompanying increased posterior pole curvature (Figs. 1b and 3a and b).
Further follow up during the next 15 months showed recurrence of the macular edema requiring 5 subsequent injections of intravitreal anti-VEGF agents. At the last follow up, at 15 months, visual acuity OS was 20/70, the intraretinal hemorrhages and optic nerve congestion had subsided, no new vessels had developed on the retina or optic disk or at the anterior chamber angle, however narrowing of the anterior chamber angle with an increase in the intraocular pressure to 28 mmHg was observed in both eyes and the patient was placed on topical antiglaucoma medications for both eyes. On SD-OCT, the central macular thickness in OS measured 550 microns. The classic features of the PMF were again confounded by the macular edema, the inner nuclear cysts, however, remained visible.
Discussion
Nanophthalmos is a developmental disorder of the eye in which both the anterior and posterior segments are not developed to full dimensions4 without major structural abnormalities. Quantitatively, it is defined as an eye with visibly small anterior segment dimensions, namely a small corneal diameter ranging between 9.5 and 11 mm, a shallow anterior chamber depth, ranging from less than 1 mm to 2.7 mm, an increased crystalline lens to total eye volume ratio,5 and an axial length of 20.5 mm or less,6 however in most cases the axial length is typically less than 18.5 mm.7 Nanophthalmos is clinically distinguished from posterior microphthalmos (PM), by the normal or subnormal dimensions of the anterior segment in PM, although more recent biometric and genetic studies support that PM and nanophthalmos are part of a biometric spectrum instead of distinct entities.7–9 Such extremely short eyes are prone to primary angle closure glaucoma and uveal effusion. In addition, a papillomacular fold (or winkles) is a characteristic finding in nanophthalmos and PM.1–3 We report a case of HRVO associated with nanophthalmos as an illustration of a potentially new retinal complication that may be associated with nanophthalmos. To our knowledge such an association has not been previously reported.
Retinal vein occlusion (RVO) is the second most common retinal vascular disorder after diabetic retinopathy and is considered to be an important cause of visual loss.10,11 Retinal vein occlusions can be classified into central (CRVO), HRVO, or branch retinal vein occlusions (BRVO). HRVO involves the anterior part of a trunk of the central retinal vein.10 Many risk factors have been linked to retinal vein occlusion whether CRVO or BRVO. In the literature, RVO has been strongly associated with hypertension, diabetes mellitus, retinal arteriolar abnormalities, hypertriglyceridemia and renal dysfunction.12,13 Several studies postulate that thrombophilic risk factors may be more prevalent in younger patients especially in a patient without other risk factors (hypertension, hyperlipidemia and diabetes mellitus).14 A meta-analysis of RVO examined the prevalence of hyperhomocysteinemia, methylenetetrahydrofolate reductase gene mutation, factor V Leiden mutation, protein C and S deficiency, antithrombin deficiency, prothrombin gene mutation, anticardiolipin antibodies, and lupus anticoagulant. Only hyperhomocysteinemia and anticardiolipin antibodies had significant effects.15,16 Glaucoma,17 short axial length and hypermetropia have also been described as ocular risk factors for CRVO. There is no consensus in the literature as to whether short axial length is a real risk factor for RVO. Many authors believe that short axial length increases the risk of RVO 18–21 while others do not believe in such an association.22,23 All risk factors were negative in our patient except for significantly short axial length and early diabetes. The case reported herein supports a potential role for a short axial length in RVO and further observation of such association may help in establishing stronger evidence.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
This article was presented the World Ophthalmology Congress meeting held in Tokyo, 2–6 April, 2014.
References
- 1.Nowilaty S.R. The posterior pole and papillomacular fold in posterior microphthalmos: novel spectral-domain optical coherence tomography findings. Ophthalmology. 2013;120(8):1656–1664. doi: 10.1016/j.ophtha.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Yalçındağ F.N., Atilla H., Batıoğlu F. Optical coherence tomography findings of retinal folds in nanophthalmos. Case Rep Ophthalmol Med. 2011;2011:491894. doi: 10.1155/2011/491894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helvacioglu F., Kapran Z., Sencan S., Uyar M., Cam O. Optical coherence tomography of bilateral nanophthalmos with macular folds and high hyperopia. Case Rep Ophthalmol Med. 2014;2014:173853. doi: 10.1155/2014/173853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh O.S., Sofinski S.J. Anomalies in the size of the eye. In: Duke-Elder, editor. vol. 3. CV Mosby; St. Louis: 1963. pp. 488–495. (System of ophthalmology). [Google Scholar]
- 5.Parrish Richard K., II, Donaldson Kendall, Kairala Marianne B. Mellem. Nanophthalmos, relative anterior microphthalmos, and axial hyperopia. In: Steinert Roger F., editor. Cataract surgery. 3rd ed. Saunders; Philadelphia: 2010. [Google Scholar]
- 6.Singh O.S. Nanophthalmos: guidelines for diagnosis and therapy. In: Albert, Jakobiec, editors. 2nd ed. vol. 3. WB Saunders; Philadelphia: 2000. pp. 2846–2859. (Principles and practice of ophthalmology). [Google Scholar]
- 7.Nowilaty S.R. Biometric and molecular characterization of clinically diagnosed posterior microphthalmos. Am J Ophthalmol. 2013;155(2):361–372.e7. doi: 10.1016/j.ajo.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Aldahmesh M.A., Nowilaty S.R., Alzahrani F. Posterior microphthalmos as a genetically heterogeneous condition that can be allelic to nanophthalmos. Arch Ophthalmol. 2011;129:805–807. doi: 10.1001/archophthalmol.2011.129. [DOI] [PubMed] [Google Scholar]
- 9.Orr A., Dubé M., Zenteno J. Mutations in a novel serine protease PRSS56 in families with nanophthalmos. Mol Vis. 2011;17:1850–1861. [PMC free article] [PubMed] [Google Scholar]
- 10.Klein R., Klein B.E., Moss S.E., Meuer S.M. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141. [PMC free article] [PubMed] [Google Scholar]
- 11.Rehak J., Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008;33:111–131. doi: 10.1080/02713680701851902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaulim A., Ahmed B., Khanam T., Chatziralli I.P. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013;33(5):901–910. doi: 10.1097/IAE.0b013e3182870c15. [DOI] [PubMed] [Google Scholar]
- 13.Cheung N., Klein R., Wang J.J. Traditional and novel cardiovascular risk factors for retinal vein occlusion: the multiethnic study of atherosclerosis. Invest Ophthalmol Vis Sci. 2008;49:4297–4302. doi: 10.1167/iovs.08-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehak M., Krcova V., Slavik L. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171–175. doi: 10.3129/i09-273. [DOI] [PubMed] [Google Scholar]
- 15.Janssen M.C., den Heijer M., Cruysberg J.R. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021–1026. doi: 10.1160/TH04-11-0768. [DOI] [PubMed] [Google Scholar]
- 16.Yau J.W., Lee P., Wong T.Y. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008;38:904–910. doi: 10.1111/j.1445-5994.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- 17.Hayreh S.S., Zimmerman M.B., Beri M., Podhajsky P. Intraocular pressure abnormalities associated with central and hemicentral retinal vein occlusion. Ophthalmology. 2004;111(1):133–141. doi: 10.1016/j.ophtha.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein M., 1, Leibovitch I., Varssano D., Rothkoff L., Feitt N., Loewenstein A. Axial length, refractive error, and keratometry in patients with branch retinal vein occlusion. Eur J Ophthalmol. 2004;14(1):37. doi: 10.1177/112067210401400106. [DOI] [PubMed] [Google Scholar]
- 19.Timmerman E.A., 1, de Lavalette V.W., van den Brom H.J. Axial length as a risk factor to branch retinal vein occlusion. Retina. 1997;17(3):196–199. doi: 10.1097/00006982-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Cekiç O., 1, Totan Y., Aydin E., Pehlivan E., Hilmioglu F. The role of axial length in central and branch retinal vein occlusion. Ophthalmic Surg Lasers. 1999;30(7):523–527. [PubMed] [Google Scholar]
- 21.Aritürk N., 1, Oge Y., Erkan D., Süllü Y., Mohajerý F. Relation between retinal vein occlusions and axial length. Br J Ophthalmol. 1996;80(7):633–636. doi: 10.1136/bjo.80.7.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons B.D., 1, Brucker A.J. Branch retinal vein occlusion. Axial length and other risk factors. Retina. 1997;17(3):191–195. doi: 10.1097/00006982-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Kir E., 1, Tülin Berk A., Osman Saatci A., Kaynak S., Ergin M.H. Axial length and hyperopia in eyes with retinal vein occlusions. Int Ophthalmol. 1997–1998;21(4):209. doi: 10.1023/a:1006057226046. [DOI] [PubMed] [Google Scholar]


