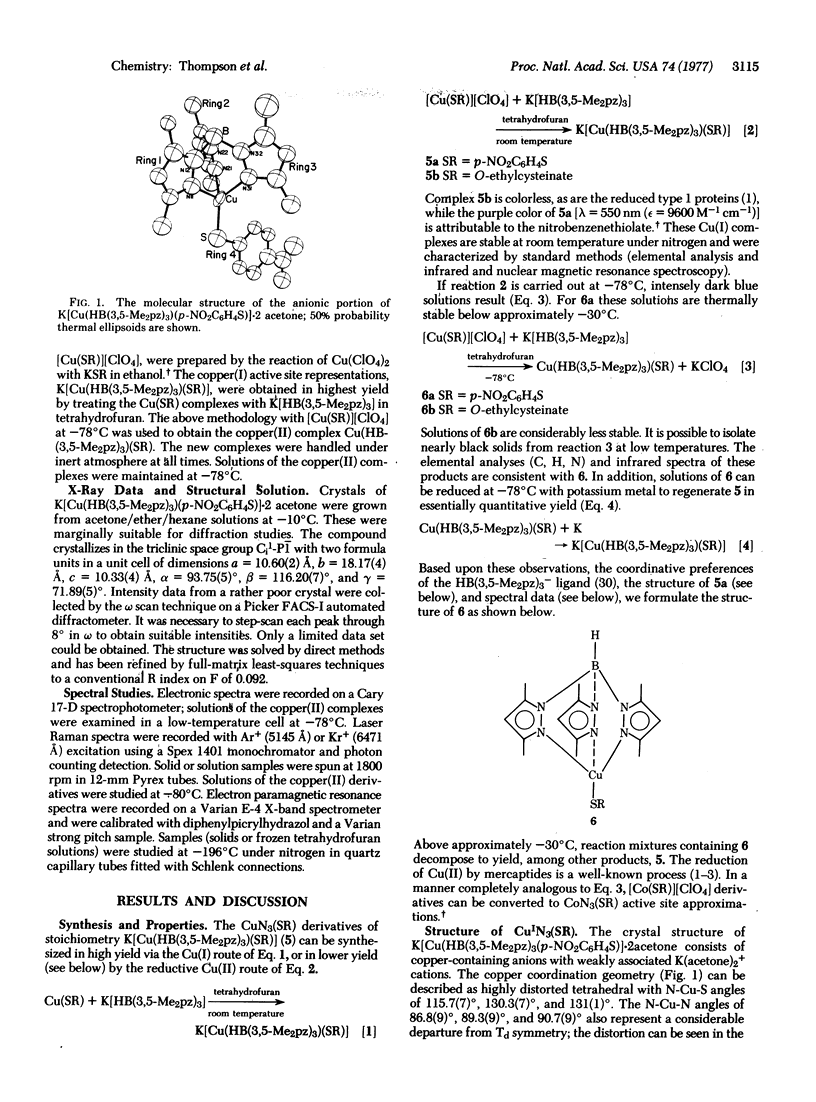
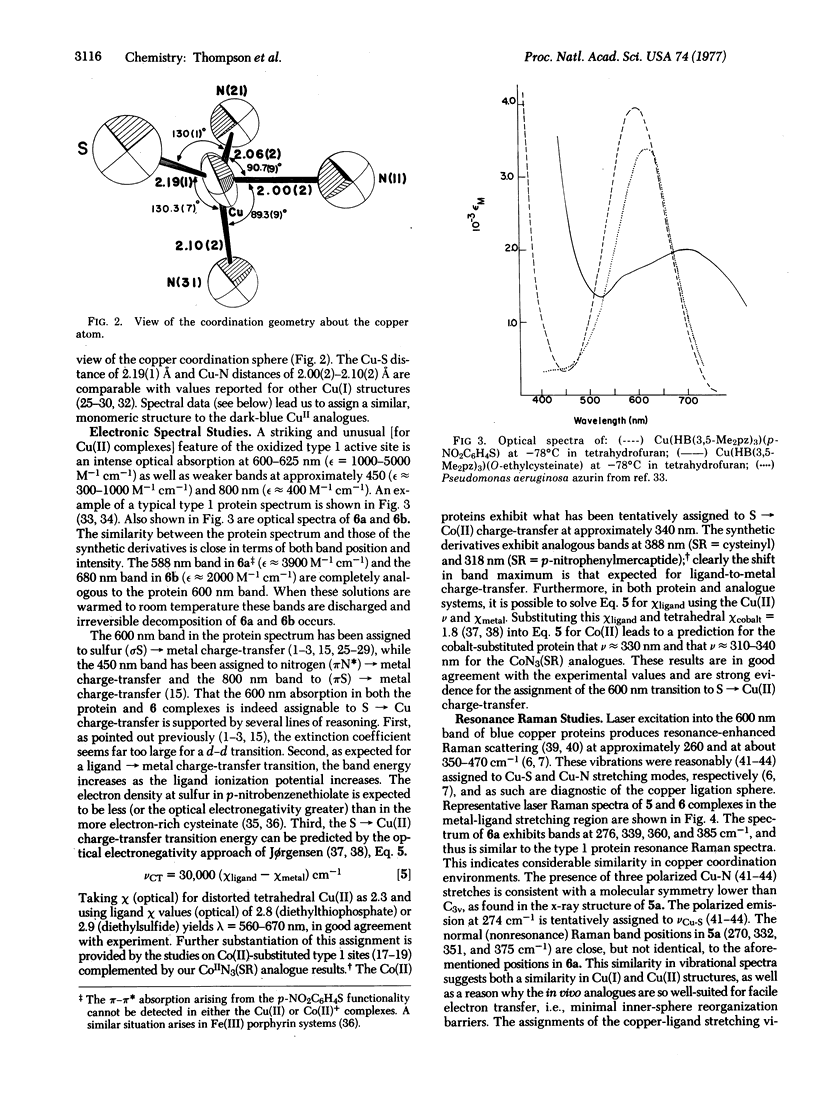
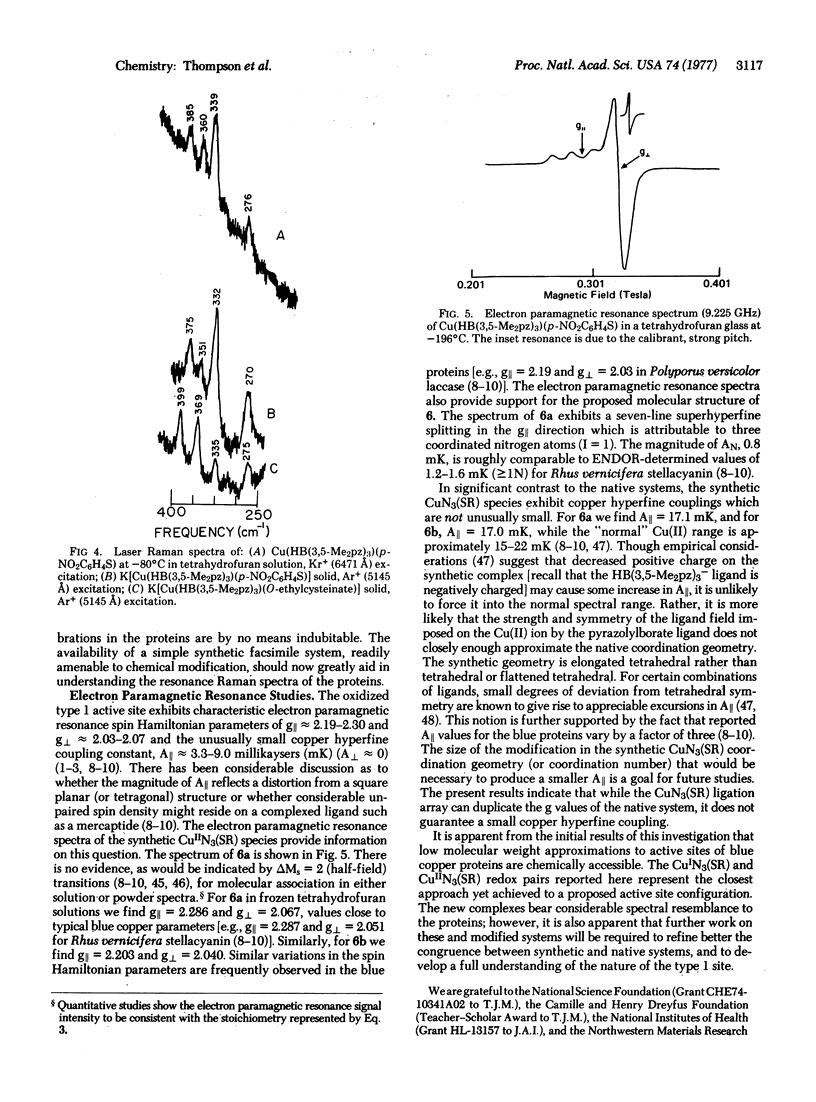
Abstract
The reaction of Cu(SR) or [Cu(SR)][ClO4] derivatives (SR = p-nitrobenzenethiolate or O-ethylcysteinate) with potassium hydrotris(3,5-dimethyl-1-pyrazolyl)borate produces redox pairs of the stoichiometry CuIN3(SR) and CuIIN3(SR). These complexes are well-defined synthetic approximations to the proposed N3S binding sites of blue (type 1) copper electron transfer proteins. The compounds were investigated by a variety of chemical and spectral (optical, resonance Raman, and electron paramagnetic resonance) techniques; the complex K[Cu(hydrotris(3,5-dimethyl-1-pyrazolyl)borate)(p- NO2C6H4S]-2 acetone was also studied by single-crystal x-ray diffraction methods. The spectrochemical characteristics of the CuIIN3(SR) species are in large part similar to the native system and thus provide some perspective regarding the origin of the unique type 1 spectral parameters and electron transfer properties.
Keywords: type 1 copper proteins, N3S coordination, charge transfer transitions, resonance Raman spectra, electron paramagnetic resonance spectra
Full text
PDF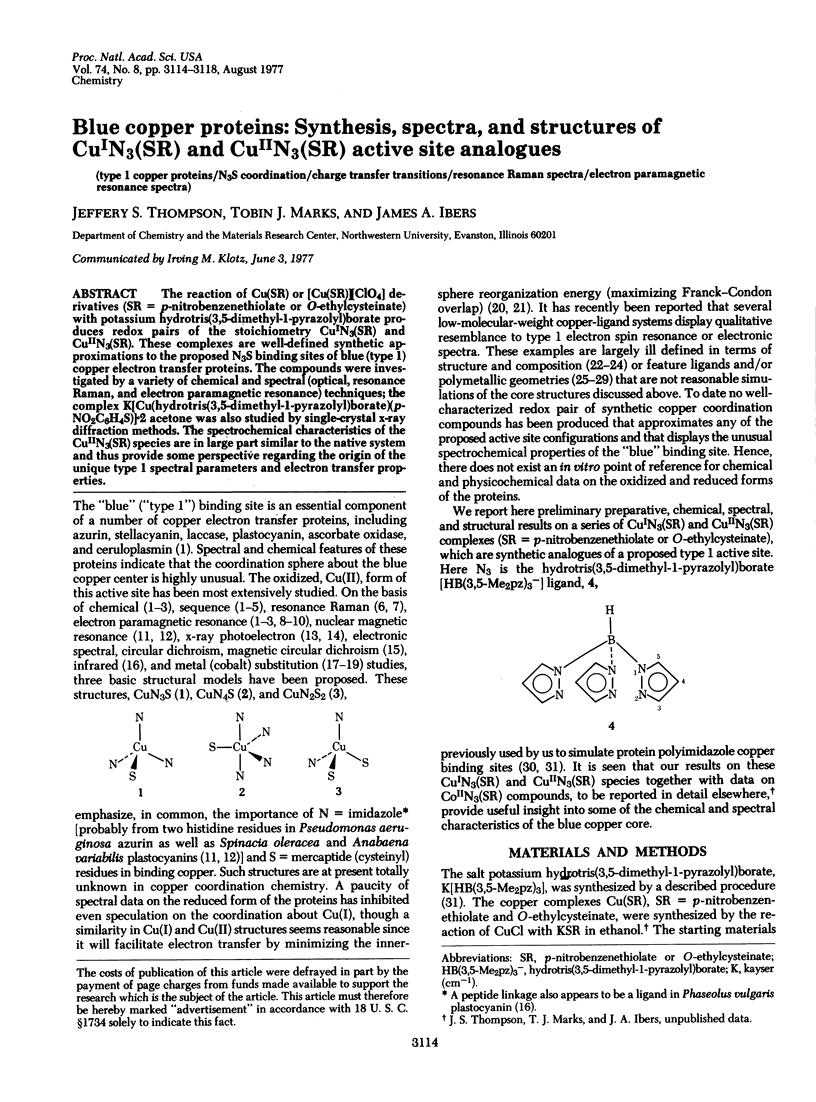

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bereman R. D., Wang F. T., Najdzionek J., Braitsch D. M. Stereoelectronic properties of metalloenzymes. 4. Bis(imidotetraphenyldithiodiphosphino-S,S')copper(II) as a tetrahedral model for type I copper(II). J Am Chem Soc. 1976 Nov 10;98(23):7266–7268. doi: 10.1021/ja00439a025. [DOI] [PubMed] [Google Scholar]
- Brill A. S., Bryce G. F., Maria H. J. Optical and magnetic properties of Pseudomonas azurins. Biochim Biophys Acta. 1968 Feb 19;154(2):342–351. doi: 10.1016/0005-2795(68)90048-2. [DOI] [PubMed] [Google Scholar]
- Gould D. C., Ehrenberg A. Cu2+ in non axial-field: a model for Cu2+ in copper enzymes. Eur J Biochem. 1968 Sep 24;5(4):451–455. doi: 10.1111/j.1432-1033.1968.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Hare J. W., Solomon E. I., Gray H. B. Infrared spectral studies of metal binding effects on the secondary structure of bean plastocyanin. J Am Chem Soc. 1976 May 26;98(11):3205–3209. doi: 10.1021/ja00427a025. [DOI] [PubMed] [Google Scholar]
- Hill H. A., Leer J. C., Smith B. E., Storm C. B. A possible approach to the investigation of the structures of copper proteins: H N.M.R. spectra of azurin. Biochem Biophys Res Commun. 1976 May 17;70(2):331–338. doi: 10.1016/0006-291x(76)91050-0. [DOI] [PubMed] [Google Scholar]
- Jones T. E., Rorabacher D. B. Letter: Simple models for "blue" copper proteins. The copper-thiaether complexes. J Am Chem Soc. 1975 Dec 24;97(26):7485–7486. doi: 10.1021/ja00859a016. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. The state of copper in stellacyanin and laccase from the lacquer tree Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):48–57. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Ulrich E. L., Berg S. P., Krogmann D. W. Nuclear magnetic resonance studies of the copper binding sites of blue copper proteins: oxidized, reduced, and apoplastocyanin. Biochemistry. 1975 Oct 7;14(20):4428–4433. doi: 10.1021/bi00691a014. [DOI] [PubMed] [Google Scholar]
- McMillin D. R., Holwerda R. A., Gray H. B. Preparation and spectroscopic studies of cobalt(II)-stellacyanin. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1339–1341. doi: 10.1073/pnas.71.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin D. R., Rosenberg R. C., Gray H. B. Preparation and spectroscopic studies of cobalt(II) derivatives of blue copper proteins. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4760–4762. doi: 10.1073/pnas.71.12.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowski V., Tang S. P., Spiro T. G., Shapiro E., Moss T. H. The copper coordination group in "blue" copper proteins: evidence from resonance Raman spectra. Biochemistry. 1975 Mar 25;14(6):1244–1250. doi: 10.1021/bi00677a024. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch Biochem Biophys. 1974 Dec;165(2):691–708. doi: 10.1016/0003-9861(74)90298-7. [DOI] [PubMed] [Google Scholar]
- Rist G. H., Hyde J. S., Vänngård T. Electron-Nuclear Double Resonance of a Protein That Contains Copper: Evidence for Nitrogen Coordination to Cu(II) in Stellacyanin. Proc Natl Acad Sci U S A. 1970 Sep;67(1):79–86. doi: 10.1073/pnas.67.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen P., Pecht I. Conformational equilibria accompanying the electron transfer between cytochrome c (P551) and azurin from Pseudomonas aeruginosa. Biochemistry. 1976 Feb 24;15(4):775–786. doi: 10.1021/bi00649a008. [DOI] [PubMed] [Google Scholar]
- Ryden L., Lundgren J. Homology relationships among the small blue proteins. Nature. 1976 May 27;261(5558):344–346. doi: 10.1038/261344a0. [DOI] [PubMed] [Google Scholar]
- Schugar H. J., Ou C., Thich J. A., Potenza J. A., Lalancette R. A., Furey W., Jr Letter: Molecular structure and copper(II)-mercaptide charge-transfer spectra of a novel Cu14(SC(CH3)2CH2NH2)12Cl cluster. J Am Chem Soc. 1976 May 12;98(10):3047–3048. doi: 10.1021/ja00426a078. [DOI] [PubMed] [Google Scholar]
- Siiman O., Young N. M., Carey P. R. Resonance raman spectra of "blue" copper proteins and the nature of their copper sites. J Am Chem Soc. 1976 Feb 4;98(3):744–748. doi: 10.1021/ja00419a017. [DOI] [PubMed] [Google Scholar]
- Solomon E. I., Clendening P. J., Gray H. B., Grunthaner F. J. Letter: Direct observation of sulfur coordination in bean plastocyanin by X-ray photoelectron spectroscopy. J Am Chem Soc. 1975 Jun 25;97(13):3878–3879. doi: 10.1021/ja00846a087. [DOI] [PubMed] [Google Scholar]
- Solomon E. I., Hare J. W., Gray H. B. Spectroscopic studies and a structural model for blue copper centers in proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1389–1393. doi: 10.1073/pnas.73.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon E. I., Rawlings J., McMillin D. R., Stephens P. J., Gray H. B. Infrared and visible circular dichroism and magnetic circular dichroism studies on cobalt (II)-substituted blue copper proteins. J Am Chem Soc. 1976 Dec 8;98(25):8046–8048. doi: 10.1021/ja00441a028. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Hirayama Y. Copper(II) and nickel(II) complexes of sulfhydryl and imidazole containing peptides: characterization and a model for "blue" copper sites. J Am Chem Soc. 1977 Mar 2;99(5):1581–1585. doi: 10.1021/ja00447a049. [DOI] [PubMed] [Google Scholar]
- Tang S. C., Koch S., Papaefthymiou G. C., Foner S., Frankel R. B., Ibers J. A., Holm R. H. Axial ligation modes in iron(III) Porphyrins. Models for the oxidized reaction states of cytochrome P-450 enzymes and the molecular structure of iron(III) protoporphyrin IX dimethyl ester p-nitrobenzenethiolate. J Am Chem Soc. 1976 Apr 28;98(9):2414–2434. doi: 10.1021/ja00425a008. [DOI] [PubMed] [Google Scholar]
- Wurzbach J. A., Grunthaner P. J., Dooley D. M., Gray H. B., Grunthaner F. J., Gay R. R., Solomon E. I. Sulfur 2p photoelectron spectrum of Limulus oxyhemocyanin. Reply to observations on the ESCA spectra of plastocyanins. J Am Chem Soc. 1977 Feb 16;99(4):1257–1258. doi: 10.1021/ja00446a043. [DOI] [PubMed] [Google Scholar]


