Abstract
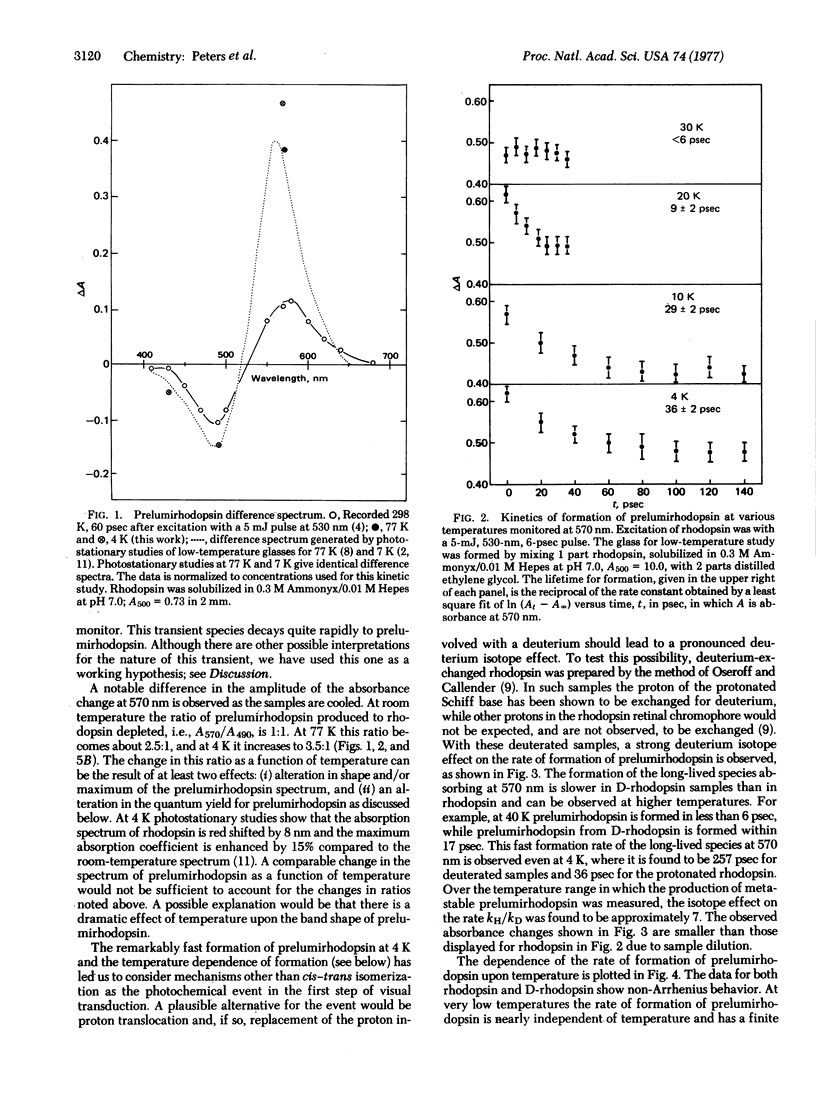
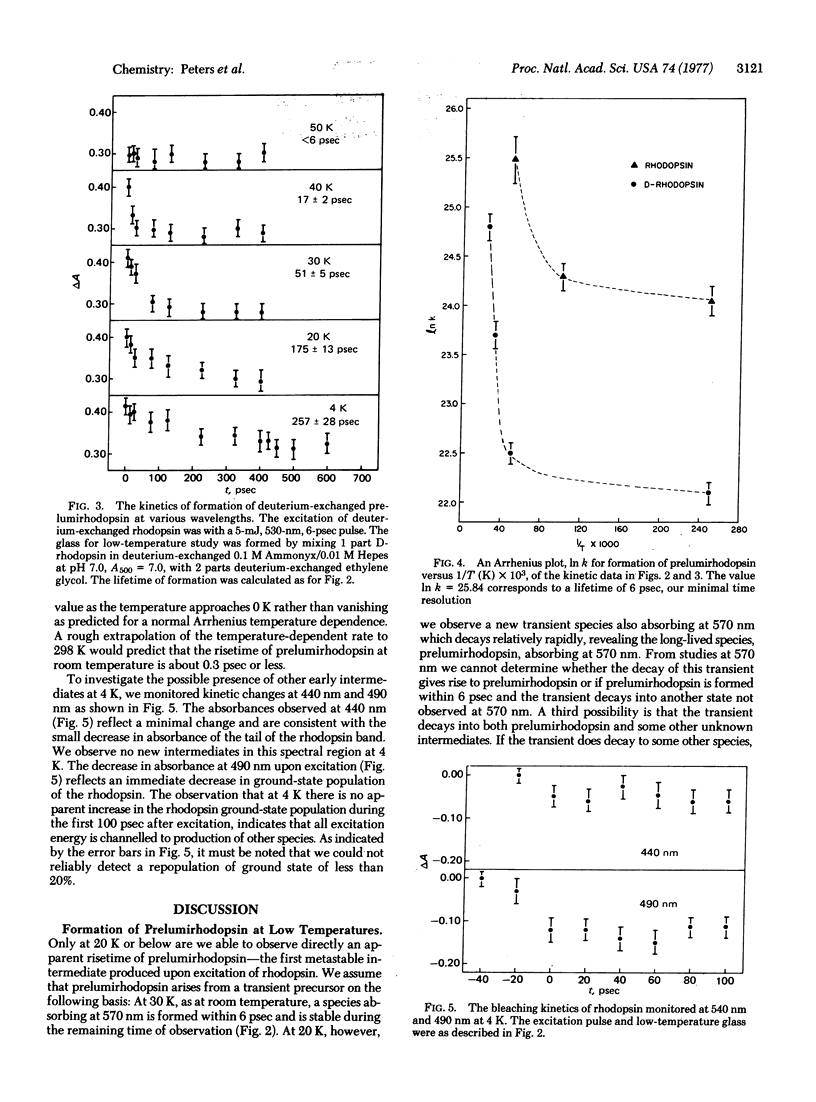
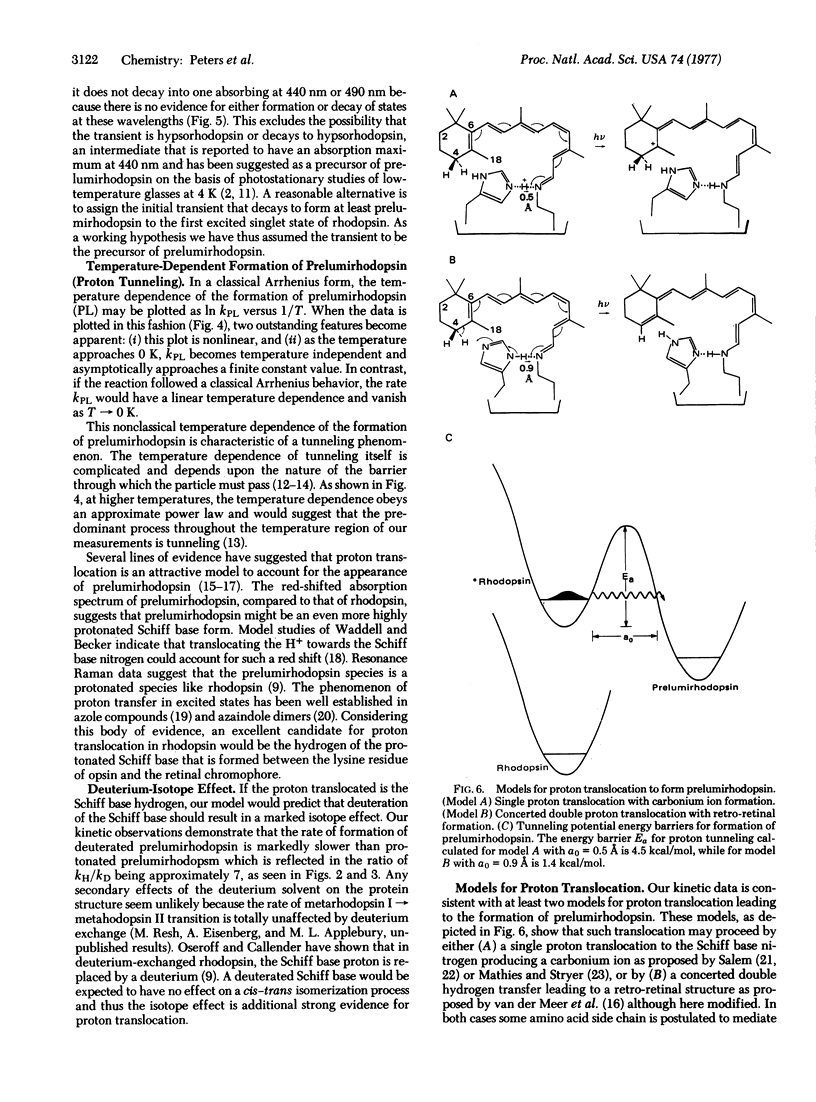
Picosecond studies of rhodopsin in low-temperature glasses have been carried out in order to observe directly the risetime of prelumirhodopsin, the first intermediate in the visual pathway. Only at 20 K or below can the risetime of this intermediate be resolved and even at 4 K it is astoundingly rapid, about 36 psec. An examination of the Arrhenius dependence on temperature of the rate of formation of prelumirhodopsin shows a strong deviation from linearity at low temperatures, i.e., non-Arrhenius behavior. This marked non-linear behavior is characteristic of a quantum mechanical tunneling event such as the translocation of hydrogen. An excellent candidate for the tunnelling process is the hydrogen of the protonated Schiff base formed between opsin and its retinal chromophore. Deuterium-exchanged rhodopsin, in which the Schiff base hydrogen is replaced by a deuterium, also shows a marked non-Arrhenius temperature dependence at low temperatures, consistent with tunneling. The rate of formation of prelumirhodopsin in deuterium-exchanged samples is much slower and a deuterium isotope effect kH/kD approximately or equal to 7 is observed. The data support a model in which the formation of prelumirhodopsin involves translocation of a proton toward the Schiff base nitrogen of the retinal chromophore.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Zuckerman D. M., Lamola A. A., Jovin T. M. Rhodopsin. Purification and recombination with phospholipids assayed by the metarhodopsin I leads to metarhodopsin II transition. Biochemistry. 1974 Aug 13;13(17):3448–3458. doi: 10.1021/bi00714a005. [DOI] [PubMed] [Google Scholar]
- Busch G. E., Applebury M. L., Lamola A. A., Rentzepis P. M. Formation and decay of prelumirhodopsin at room temperatures. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2802–2806. doi: 10.1073/pnas.69.10.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen M. R., Luyten W. C., Van Thuijl J., Jansen P. A., Van Breugel P. J., Daemen F. J. Structure of the chromophoric group in bathorhodopsin. Nature. 1976 Apr 22;260(5553):726–727. [PubMed] [Google Scholar]
- Kropf A. Is proton transfer the initial photochemical process in vision? Nature. 1976 Nov 4;264(5581):92–94. doi: 10.1038/264092a0. [DOI] [PubMed] [Google Scholar]
- Mathies R., Stryer L. Retinal has a highly dipolar vertically excited singlet state: implications for vision. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2169–2173. doi: 10.1073/pnas.73.7.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff A. R., Callender R. H. Resonance Raman spectroscopy of rhodopsin in retinal disk membranes. Biochemistry. 1974 Sep 24;13(20):4243–4248. doi: 10.1021/bi00717a027. [DOI] [PubMed] [Google Scholar]
- Salem L., Bruckmann P. Conversion of a photon to an electrical signal by sudden polarisation in the N-retinylidene visual chromophore. Nature. 1975 Dec 11;258(5535):526–528. doi: 10.1038/258526a0. [DOI] [PubMed] [Google Scholar]
- Salem L. Theory of photochemical reactions. Science. 1976 Feb 27;191(4229):822–830. doi: 10.1126/science.1251196. [DOI] [PubMed] [Google Scholar]
- Sundstrom V., Rentzepis P. M., Peters K., Applebury M. L. Kinetics of rhodopsin at room temperature measured by picosecond spectroscopy. Nature. 1977 Jun 16;267(5612):645–646. doi: 10.1038/267645a0. [DOI] [PubMed] [Google Scholar]
- Waddell W. H., Schaffer A. M., Becker R. S. Visual pigments. 3. Determination and interpretation of the fluorescence quantum yields of retinals, Schiff bases, and protonated Schiff bases. J Am Chem Soc. 1973 Dec 12;95(25):8223–8227. doi: 10.1021/ja00806a002. [DOI] [PubMed] [Google Scholar]
- Waddell W., Becker R. S. The hydrogen-bonded (protonated ) Schiff base of all-trans-retinal. J Am Chem Soc. 1971 Jul 28;93(15):3788–3789. doi: 10.1021/ja00744a050. [DOI] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- Warshel A. Bicycle-pedal model for the first step in the vision process. Nature. 1976 Apr 22;260(5553):679–683. doi: 10.1038/260679a0. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]
- van der Meer K., Mulder J. J., Lugtenburg J. A new facet in rhodopsin photochemistry. Photochem Photobiol. 1976 Oct;24(4):363–367. doi: 10.1111/j.1751-1097.1976.tb06837.x. [DOI] [PubMed] [Google Scholar]


