Abstract
Purpose
Male pattern baldness and prostate cancer appear to share common pathophysiologic mechanisms. However, results from previous studies that assess their relationship have been inconsistent. Therefore, we investigated the association of male pattern baldness at age 45 years with risks of overall and subtypes of prostate cancer in a large, prospective cohort—the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
Methods
We included 39,070 men from the usual care and screening arms of the trial cohort who had no cancer diagnosis (excluding nonmelanoma skin cancer) at the start of follow-up and recalled their hair-loss patterns at age 45 years. Hazard ratios (HRs) and 95% CIs were estimated by using Cox proportional hazards regression models with age as the time metric.
Results
During follow-up (median, 2.78 years), 1,138 incident prostate cancer cases were diagnosed, 571 of which were aggressive (biopsy Gleason score ≥ 7, and/or clinical stage III or greater, and/or fatal). Compared with no baldness, frontal plus moderate vertex baldness at age 45 years was not significantly associated with overall (HR, 1.19; 95% CI, 0.98 to 1.45) or nonaggressive (HR, 0.97; 95% CI, 0.72 to 1.30) prostate cancer risk but was significantly associated with increased risk of aggressive prostate cancer (HR, 1.39; 95% CI, 1.07 to 1.80). Adjustment for covariates did not substantially alter these estimates. Other classes of baldness were not significantly associated with overall or subtypes of prostate cancer.
Conclusion
Our analysis indicates that frontal plus moderate vertex baldness at age 45 years is associated with an increased risk of aggressive prostate cancer and supports the possibility of common pathophysiologic mechanisms.
INTRODUCTION
In US men, prostate cancer is the most frequently diagnosed noncutaneous cancer and the second leading cause of cancer death.1 Few risk factors have been established for prostate cancer except for advancing age, black race, family history of this malignancy,2–4 and certain genetic polymorphisms,5,6 which collectively explain only a fraction of the disease occurrence. Therefore, additional research to improve our understanding of the etiology of prostate cancer is needed. Male pattern baldness (or androgenic alopecia) seems to share pathologic mechanisms with prostate cancer in terms of advancing age, hereditability, and endogenous hormones.2,7 The fact that the age of observable hair loss coincides with the age of microscopic evidence for prostate cancer in autopsy studies,8,9 and that male pattern baldness represents cumulative exposures, as opposed to a single serum measurement, may help elucidate prostate cancer etiology.
Results from previous epidemiologic studies on the association between male pattern baldness and prostate cancer risk are inconclusive.10–22 Most studies were of cross-sectional or case-control design with small sample sizes and limited statistical power. Two cohort studies, as well as a meta-analysis of seven case-control studies, have suggested a positive association between male pattern baldness and prostate cancer risk,19,21,23 but subtype-specific analyses by prostate cancer aggressiveness were not presented. To overcome the noted shortfalls, we conducted an analysis of male pattern baldness at age 45 years in relation to risks of overall and subtypes of prostate cancer in the prospective Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
METHODS
The analytic cohort was drawn from the PLCO Cancer Screening Trial, the design of which has been described in detail previously.24,25 In brief, the trial was a multicenter, randomized, two-arm trial evaluating the effect of screening on disease-specific mortality end points. This study was approved by the institutional review boards of the National Cancer Institute and the 10 US screening centers.
Screening centers enrolled 76,683 men from 1993 to 2001. Eligible men were age 55 to 74 years at enrollment; had no history of prostate, lung, or colorectal cancer; had not undergone surgical removal of the entire prostate, a lung, or the entire colon; were not undergoing treatment for cancer (except nonmelanoma skin cancer [NMSC]); had not taken finasteride (Proscar) in the past 6 months; had no more than one prostate-specific antigen (PSA) test in the past 3 years from April 15, 1995 (date of trial protocol change). Eligible men were randomly assigned into the screening arm (ie, annual PSA test for the first 6 years, and annual digital rectal examination for the first 4 years), or the usual care arm. Men with suspected prostate cancer from an abnormal PSA test (> 4.0 ng/mL) and/or digital rectal examination result were referred for diagnostic work-up. Around the time of random assignment, each man was mailed a sex-specific baseline questionnaire–men (BQM). From 2006 to 2008, men who remained under active follow-up (Appendix Fig A1, online only) were mailed a supplemental questionnaire (SQX) to expand risk factor ascertainment.
Exposure Ascertainment
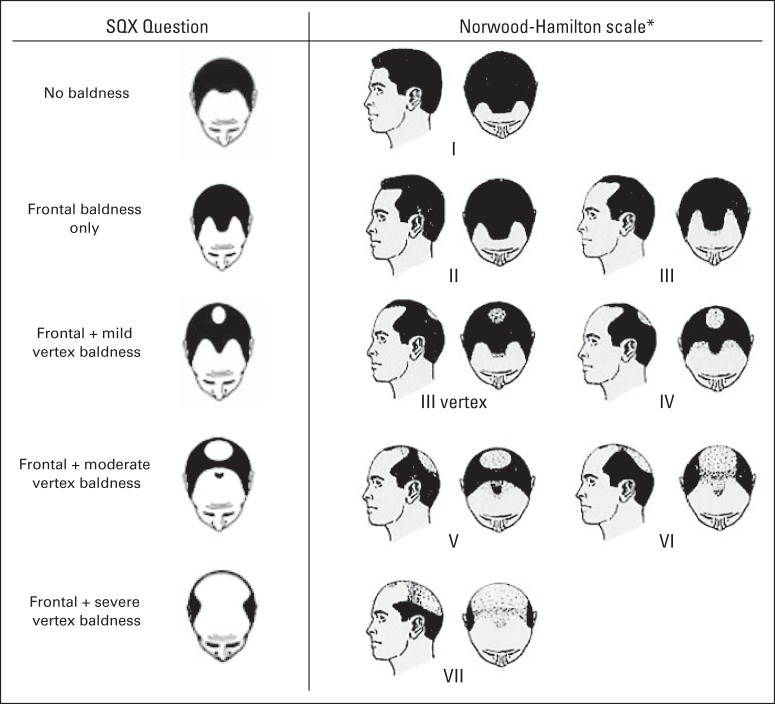
In the SQX, participants were asked to recall their hair-loss patterns at age 45 years from a modified Norwood-Hamilton scale (Fig 1): (1) no baldness, (2) frontal baldness only, (3) frontal plus mild vertex baldness, (4) frontal plus moderate vertex baldness, and (5) frontal plus severe vertex baldness.26
Fig 1.
Male pattern baldness at age 45 years as elicited in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Supplemental Questionnaire (SQX). (*) Scale image reproduced.26
Outcome Ascertainment
Diagnosed cancers and deaths were ascertained by active follow-up using annual mailed study update questionnaires, supplemented by linkage to the National Death Index. Cancer diagnoses were confirmed by medical record abstraction. Underlying causes of death were determined via a death review process that used information from death certificates as well as medical documents.27 In this analysis, incident prostate cancer was defined as having a first cancer diagnosis (excluding NMSC) of prostate cancer, or a second cancer diagnosis of prostate cancer within 30 days of the first cancer diagnosis because we considered that such close proximity of two primary cancer diagnoses indicated synchronous emergence.
Analytic Cohort
The analytic cohort comprised men from both trial arms who had no cancer diagnosis (excluding NMSC) at start of follow-up (ie, the time of the SQX) and who responded to the balding question. This resulted in a total of 39,070 men in the SQX cohort. Appendix Figure A1 describes exclusions that were used to form the analytic cohort.
Statistical Analyses
Pearson χ2 tests were used for categorical characteristics by case status and male pattern baldness. Cox proportional hazards regression models, with age in months as the time metric, were used to estimate hazards ratios (HRs) and 95% CIs of associations between male pattern baldness and prostate cancer risk. Follow-up started from age at SQX and continued until age at event (ie, incident prostate cancer defined above) or age at time of right-censoring (ie, study withdrawal, diagnosis with non–prostate cancer [excluding NMSC], death as a result of other causes, last date of follow-up), whichever occurred first.
The percentage of missing values was lower than 10% at BQM and SQX for all variables considered, except for family income (15%) and family history of prostate cancer (12%). For these missing values, we conducted multiple imputation using BQM and SQX data by a sequence of regression models28 via IVEware29 in SAS (SAS Institute, Cary, NC). Self-reported race was highly consistent across the two questionnaires. We thus used race reported at BQM if race at SQX was missing and imputed race if it was missing on both questionnaires (4%). Five imputed data sets were created and analyzed individually, and then HR estimates were combined by using PROC MIANALYZE in SAS. Proportional hazards assumptions were tested by using interaction terms and through visual inspection of log(-log) survival plots.
To explore the impact of possible confounders on the association between male pattern baldness and prostate cancer risk, we calculated HRs (95% CIs) by using unadjusted models and multivariable models adjusted for screening arm, screening center, race, education, marital status, cigarette smoking, body mass index (kg/m2) at age 50 years, regular aspirin use, history of diabetes, and history of myocardial infarction. Covariates chosen for the multivariable models had P values less than .15 in relation to both prostate cancer and male pattern baldness in univariable models (ie, χ2 tests) or were deemed a priori as potential confounders (screening arm, marital status, and diabetes). In addition, we adjusted for covariates that are potentially pathophysiologically related to prostate cancer (family history of prostate cancer in first-degree relative[s], and history of enlarged prostate).
We conducted subtype-specific analyses by prostate cancer aggressiveness, with aggressive defined as biopsy Gleason score ≥ 7, and/or clinical stage III or greater, and/or fatal prostate cancer (ie, as the underlying cause of death). For these analyses, men who experienced a prostate cancer subtype that was not of interest were censored at diagnosis. For example for aggressive prostate cancer, we censored men at the age at diagnosis of a nonaggressive tumor. To evaluate the robustness of associations, we also performed subtype-specific analyses by different definitions of aggressive prostate cancer: (1) biopsy Gleason score ≥ 8, and/or clinical stage III or greater, and/or fatal prostate cancer; (2) comprehensive Gleason score (from prostatectomy or biopsy) ≥ 7, and/or comprehensive stage (from pathologic or clinical TNMs) III or greater, and/or fatal prostate cancer; (3) comprehensive Gleason score ≥ 8, and/or comprehensive stage III or greater, and/or fatal prostate cancer; (4) modified D'Amico criteria30,31 as low risk (cT1 to cT2a, prediagnostic PSA within 6 months ≤ 10 ng/mL, and biopsy Gleason score ≤ 6); intermediate risk (cT2b, and/or prediagnostic PSA within 6 months > 10 ng/mL and ≤ 20 ng/mL, and/or biopsy Gleason score = 7); and high risk (≥ cT2c, and/or prediagnostic PSA within 6 months > 20 ng/mL, and/or biopsy Gleason score ≥ 8, and/or fatal prostate cancer). Other sensitivity analyses included combining classes of male pattern baldness (no/frontal only/frontal plus any vertex baldness; any/no baldness), adjusting for recall period from age 45 years to age at SQX, and restriction to men enrolled after trial protocol change. SAS v.9.3 was used for analyses. Two-sided P < .05 was considered statistically significant.
RESULTS
Of the 39,070 men in our analytic cohort, male pattern baldness at age 45 years was reported by 53.4%, of which 46.4% reported frontal baldness only, 23.5% frontal plus mild vertex baldness, 18.1% frontal plus moderate vertex baldness, and 12.0% frontal plus severe vertex baldness. During follow-up (median, 2.78 years), 1,138 incident prostate cancers were diagnosed, 571 of which were aggressive (biopsy Gleason score ≥ 7, and/or clinical stage III or greater, and/or fatal). The mean age at prostate cancer diagnosis was 72.2 years (range, 61.4 to 86.5 years).
Table 1 provides the characteristics of the analytic cohort by case status. The analytic cohort was predominantly white (89.3%). Compared with men without prostate cancer, men with aggressive prostate cancer were more likely to be in the usual care arm, married/cohabiting, have a history of enlarged prostate, and were less likely to ever smoke cigarettes; men with nonaggressive prostate cancer were more likely to be married/cohabiting, have a family history of prostate cancer, have a history of enlarged prostate, and were less likely to ever smoke cigarettes, and to have a history of diabetes or myocardial infarction.
Table 1.
Characteristics of Patients in the Analytic Cohort by Case Status
| Characteristic | Non–Prostate Cancer |
Incident Prostate Cancer |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 1,138) |
P† | Aggressive* (n = 571) |
P† | Nonaggressive* (n = 552) |
P† | ||||||
| No. | % | No. | % | No. | % | No. | % | ||||
| Randomly assigned group | .032 | .013 | .801 | ||||||||
| Usual care | 18,143 | 47.8 | 581 | 51.1 | 303 | 53.1 | 267 | 48.4 | |||
| Intervention | 19,789 | 52.2 | 557 | 48.9 | 268 | 46.9 | 285 | 51.6 | |||
| Age at SQX (years) | < .001 | .170 | < .001 | ||||||||
| < 65 | 5,378 | 14.2 | 161 | 14.1 | 70 | 12.3 | 88 | 15.9 | |||
| 65-69 | 10,985 | 29.0 | 388 | 34.1 | 166 | 29.1 | 216 | 39.1 | |||
| 70-74 | 9,896 | 26.1 | 319 | 28.0 | 170 | 29.8 | 147 | 26.6 | |||
| ≥ 75 | 11,673 | 30.8 | 270 | 23.7 | 165 | 28.9 | 101 | 18.3 | |||
| Median | 71 | 70 | 71 | 69 | |||||||
| IQR | 66-76 | 66-74 | 67-75 | 66-73 | |||||||
| Education | .021 | .061 | .407 | ||||||||
| Less than high school | 2,211 | 5.8 | 45 | 4.0 | 20 | 3.5 | 23 | 4.2 | |||
| High school graduate | 11,237 | 29.6 | 348 | 30.6 | 177 | 31.0 | 167 | 30.3 | |||
| Some college | 7,560 | 19.9 | 226 | 19.9 | 113 | 19.8 | 109 | 19.7 | |||
| College graduate | 7,661 | 20.2 | 212 | 18.6 | 104 | 18.2 | 107 | 19.4 | |||
| Postgraduate | 9,173 | 24.2 | 305 | 26.8 | 156 | 27.3 | 146 | 26.4 | |||
| Marital status | < .001 | .015 | < .001 | ||||||||
| Married or cohabiting | 30,888 | 81.4 | 978 | 85.9 | 488 | 85.5 | 478 | 86.6 | |||
| Single‡ | 6,721 | 17.7 | 151 | 13.3 | 79 | 13.8 | 69 | 12.5 | |||
| Race | .137 | .305 | .376 | ||||||||
| White | 33,871 | 89.3 | 1,023 | 89.9 | 511 | 89.5 | 498 | 90.2 | |||
| Black | 841 | 2.2 | 34 | 3.0 | 18 | 3.2 | 16 | 2.9 | |||
| Other§ | 1,684 | 4.4 | 43 | 3.8 | 23 | 4.0 | 20 | 3.6 | |||
| First-degree relative(s) had prostate cancer | < .001 | .222 | < .001 | ||||||||
| No | 30,129 | 79.4 | 853 | 75.0 | 438 | 76.7 | 402 | 72.8 | |||
| Yes | 3,297 | 8.7 | 138 | 12.1 | 57 | 10.0 | 80 | 14.5 | |||
| Diabetes | .037 | .279 | < .001 | ||||||||
| No | 27,581 | 72.7 | 867 | 76.2 | 410 | 71.8 | 444 | 80.4 | |||
| Yes | 6,207 | 16.4 | 163 | 14.3 | 104 | 18.2 | 59 | 10.7 | |||
| Myocardial infarction | .008 | .257 | .005 | ||||||||
| No | 28,137 | 74.2 | 875 | 76.9 | 427 | 74.8 | 438 | 79.3 | |||
| Yes | 5,343 | 14.1 | 129 | 11.3 | 70 | 12.3 | 56 | 10.1 | |||
| Enlarged prostate | < .001 | .008 | < .001 | ||||||||
| No | 20,710 | 54.6 | 510 | 44.8 | 280 | 49.0 | 220 | 39.9 | |||
| Yes | 16,889 | 44.5 | 619 | 54.4 | 286 | 50.1 | 328 | 59.4 | |||
| BMI at age 50 years (kg/m2) | .036 | .126 | .265 | ||||||||
| < 25.0 | 12,341 | 32.5 | 380 | 33.4 | 201 | 35.2 | 178 | 32.2 | |||
| 25.0-29.9 | 17,026 | 44.9 | 537 | 47.2 | 262 | 45.9 | 263 | 47.6 | |||
| ≥ 30 | 5,264 | 13.9 | 129 | 11.3 | 64 | 11.2 | 65 | 11.8 | |||
| Cigarette smoking | .001 | .022 | .007 | ||||||||
| Never | 12,999 | 34.3 | 442 | 38.8 | 220 | 38.5 | 220 | 39.9 | |||
| Ever | 23,771 | 62.7 | 659 | 57.9 | 329 | 57.6 | 317 | 57.4 | |||
| Aspirin use frequency | .097 | .242 | .442 | ||||||||
| None or < 1 time per month | 11,581 | 30.5 | 328 | 28.8 | 164 | 28.7 | 159 | 28.8 | |||
| ≤ 2 times per week | 3,668 | 9.7 | 113 | 9.9 | 56 | 9.8 | 56 | 10.1 | |||
| 3-6 times per week | 3,906 | 10.3 | 102 | 9.0 | 49 | 8.6 | 51 | 9.2 | |||
| ≥ 7 times per week | 17,903 | 47.2 | 581 | 51.1 | 293 | 51.3 | 281 | 50.9 | |||
| Male pattern baldness at age 45 years | .368 | .119 | .981 | ||||||||
| No baldness | 17,678 | 46.6 | 522 | 45.9 | 257 | 45.0 | 260 | 47.1 | |||
| Any baldness | 20,254 | 53.4 | 616 | 54.1 | .750‖ | 314 | 55.0 | .500‖ | 292 | 52.9 | .904‖ |
| Frontal baldness only | 9,411 | 24.8 | 279 | 24.5 | 138 | 24.2 | 139 | 25.2 | |||
| Frontal plus any vertex baldness | 9,411 | 24.8 | 279 | 24.5 | .625¶ | 138 | 24.2 | .448¶ | 139 | 25.2 | .816¶ |
| Frontal plus mild vertex baldness | 4,760 | 12.5 | 142 | 12.5 | 68 | 11.9 | 69 | 12.5 | |||
| Frontal plus moderate vertex baldness | 3,643 | 9.6 | 129 | 11.3 | 74 | 13.0 | 52 | 9.4 | |||
| Frontal plus severe vertex baldness | 2,440 | 6.4 | 66 | 5.8 | 34 | 6.0 | 32 | 5.8 | |||
NOTE. Column percent may not add up to 100% because of missing data.
Abbreviations: BMI, body mass index; IQR, interquartile range; SQX, supplemental questionnaire.
Aggressive prostate cancer was defined as biopsy Gleason score ≥ 7, and/or clinical stage III or greater, and/or prostate cancer as underlying cause of death. Nonaggressive prostate cancer was defined as otherwise. Fifteen prostate cancers lacked enough information to determine prostate cancer subtypes.
P values were calculated by Pearson χ2 tests by using nonmissing values with comparison to controls without cancer.
Single includes widowed, divorced, separated, and never married.
Other race includes Asian, Native Hawaiian or other Pacific Islander, American Indian, or Alaskan Native.
P value was calculated for any baldness and no baldness by Pearson χ2 tests compared with controls without cancer.
P value was calculated for frontal baldness only, frontal plus any vertex baldness, and no baldness by Pearson χ2 tests compared with controls without cancer.
Table 2 provides data showing that male pattern baldness at age 45 years was not significantly associated with overall prostate cancer risk, although frontal plus moderate vertex baldness showed a nonsignificant increased risk of approximately 19% compared with no baldness in the unadjusted model (HR, 1.19; 95% CI, 0.98 to 1.45). However, frontal plus moderate vertex baldness was significantly associated with an increased risk of aggressive prostate cancer compared with no baldness in the unadjusted model (HR, 1.39; 95% CI, 1.07 to 1.80). Conversely, classes of male pattern baldness were not significantly associated with nonaggressive prostate cancer. Further adjustment did not substantially alter the HR estimates.
Table 2.
Associations Between Male Pattern Baldness at Age 45 Years and Prostate Cancer Risk in the Analytic Cohort
| Male Pattern Baldness at Age 45 Years | Total Incident Prostate Cancer (N = 1,138) |
Aggressive Prostate Cancer* (n = 571) |
Nonaggressive Prostate Cancer* (n = 552) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted† |
Multivariable‡ |
Unadjusted† |
Multivariable‡ |
Unadjusted† |
Multivariable‡ |
|||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| No baldness | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Any baldness | 1.03 | 0.92 to 1.16 | 1.02 | 0.91 to 1.15 | 1.07 | 0.90 to 1.26 | 1.06 | 0.90 to 1.25 | 0.98 | 0.83 to 1.16 | 0.97 | 0.82 to 1.15 |
| Frontal baldness only | 1.00 | 0.87 to 1.16 | 1.00 | 0.86 to 1.16 | 1.00 | 0.82 to 1.23 | 1.00 | 0.81 to 1.23 | 1.01 | 0.82 to 1.24 | 1.00 | 0.82 to 1.23 |
| Frontal plus any vertex baldness | 1.05 | 0.92 to 1.21 | 1.04 | 0.91 to 1.20 | 1.12 | 0.92 to 1.36 | 1.11 | 0.92 to 1.35 | 0.96 | 0.78 to 1.17 | 0.95 | 0.77 to 1.16 |
| Frontal plus mild vertex baldness | 1.01 | 0.84 to 1.21 | 1.00 | 0.83 to 1.20 | 0.99 | 0.76 to 1.29 | 0.98 | 0.75 to 1.28 | 0.97 | 0.75 to 1.27 | 0.96 | 0.74 to 1.25 |
| Frontal plus moderate vertex baldness | 1.19 | 0.98 to 1.45 | 1.19 | 0.98 to 1.45 | 1.39 | 1.07 to 1.80 | 1.39 | 1.07 to 1.80 | 0.97 | 0.72 to 1.30 | 0.96 | 0.71 to 1.30 |
| Frontal plus severe vertex baldness | 0.93 | 0.72 to 1.20 | 0.92 | 0.71 to 1.19 | 0.97 | 0.68 to 1.39 | 0.97 | 0.68 to 1.39 | 0.90 | 0.63 to 1.30 | 0.89 | 0.61 to 1.28 |
NOTE. Missing values of covariates (excluding male pattern baldness at age 45 years) were imputed in sequential regression multiple imputation models via IVEware in SAS v.9.3.
Abbreviations: HR, hazard ratio; Ref., reference.
Aggressive prostate cancer was defined as biopsy Gleason score ≥ 7, and/or clinical stage III or greater, and/or prostate cancer as underlying cause of death. Nonaggressive prostate cancer was defined as otherwise. Fifteen prostate cancers lacked enough information to determine prostate cancer subtypes.
Unadjusted model: age (month) was used as the underlying time metric.
Multivariable model: adjusted for screening arm (usual care, screening), screening center (10 centers, not listed here), race (white, black, other), education (less than high school, high school graduate, some college, college graduate, postgraduate), married or cohabiting (yes, no), diabetes (yes, no), body mass index at age 50 years (< 18.5, 18.5-24.9, 25.0-29.9, ≥ 30 kg/m2), cigarette smoking (ever, never), aspirin use frequency (none or < 1 time per month, ≤ 2 times per week, 3-6 times per week, ≥ 7 times per week), and myocardial infarction (yes, no).
Sensitivity analyses by different definitions of aggressive prostate cancer showed stronger associations between frontal plus moderate vertex baldness and aggressive prostate cancer (Appendix Table A1, online only). Combined classes of male pattern baldness were not significantly associated with prostate cancer (Table 1). Additional adjustment for recall period, or covariates—which are potentially pathophysiologically related to prostate cancer—did not materially affect results (results not shown). Restriction to men enrolled after the date of trial protocol change (Appendix Table A2, online only) did not appreciably alter results.
DISCUSSION
In this large prospective study, frontal plus moderate vertex baldness at age 45 years was significantly associated with an increased risk of aggressive prostate cancer compared with no baldness. Other classes of baldness were not associated with aggressive prostate cancer, and no class of baldness was significantly associated with overall or nonaggressive prostate cancer.
Although the association between male pattern baldness and prostate cancer has been inconsistent, two recent cohort studies19,21 and a meta-analysis23 suggested a positive relationship. The National Health and Nutrition Examination Survey I (NHANES I) Epidemiologic Follow-up Study (NHEFS), which accrued 214 incident prostate cancer cases in 4,421 men during 20 years of follow-up, reported an HR of 1.50 (95% CI, 1.12 to 2.00) for prostate cancer in relation to any degree of baldness, as examined by dermatology residents at baseline.21 However, more than half the men were older than age 55 years at baseline. The other cohort study—the Melbourne Collaborative Cohort Study (MCCS)—comprised 9,448 men, 476 of whom were diagnosed with prostate cancer during 11 years of follow-up. This study predicted a 1.81 times (95% CI, 1.13 to 2.90 times) higher risk of prostate cancer by age 55 years among men with vertex baldness (Norwood-Hamilton scale III vertex to VII) at age 40 years than those without baldness, although the HR dropped to 1.00 by age 60 to 70 years.19However, male pattern baldness was recalled after the diagnosis of prostate cancer, and men with aggressive prostate cancer were more likely to have missing exposure because of loss to follow-up. Finally, a meta-analysis of seven case-control studies reported that men with any vertex baldness had an odds ratio (OR) of 1.25 (95% CI, 1.09 to 1.44) for prostate cancer compared with those without baldness, although no significant association was found for frontal recession only.23
We found no significant association of any classes of male pattern baldness at age 45 years with overall prostate cancer risk. This result is consistent with the MCCS null result for men age 60 to 70 years,19 a fair comparison to our study, given that the youngest age at the start of follow-up in our analytic cohort was 60 years. It is possible that, in relation to overall prostate cancer risk diagnosed in a population with high use of PSA screening, male pattern baldness is associated only with early-onset disease.
We found a significant and robust association between frontal plus moderate vertex baldness and increased risk of aggressive prostate cancer. This result is supported by similar associations observed in the NHEFS in which two thirds of follow-up occurred in the pre-PSA era, a period when a greater proportion of prostate cancer cases were identified symptomatically. In addition, akin to the MCCS, positive results in the NHEFS may also be partly attributable to an average younger cohort (median age, 55.1 years),21 if our prior hypothesis of an association between male pattern baldness and early-onset disease is true. Our result of the importance of frontal plus moderate vertex baldness in relation to aggressive prostate cancer may also be supported by a matched case-control study in Australian men, which reported a positive association (OR, 2.04; 95% CI, 1.35 to 3.08) of high-grade (Gleason scores 8 to 10) prostate cancer with vertex balding (Norwood-Hamilton stage III vertex and stage V) assessed by interviewers.12
We found no significant association between the highest class of male pattern baldness (frontal plus severe baldness) and prostate cancer risk. This observation may be explained by the failure to include younger men with prostate cancer or fatal/severe cardiovascular disease32,33 before start of follow-up (SQX), if this highest class of baldness is associated with such; measurement errors of recalled hair-loss via a modified Norwood-Hamilton scale; or different biologic mechanisms of balding patterns or extent. In addition, we did not find any significant association between the highest composite class of male pattern baldness (frontal with any vertex baldness) and prostate cancer risk; our observed association with frontal plus moderate vertex baldness and aggressive prostate cancer was masked by using this combined exposure.
Results from basic science and observational studies have suggested an association between male pattern baldness and prostate cancer with respect to aging, hereditability, and endogenous hormones. Advancing age is accompanied by increasing incidence and extent of baldness,34 as well as by increasing prostate cancer mortality.35 With regard to hereditability, it has been estimated that 42% of prostate cancer36 and 81% of male pattern baldness37 are attributed to heritable factors in twin studies. Moreover, two loci (Xq12 and 3q26) identified in genome-wide association studies of European men have separately been found to be associated with prostate cancer38 and male pattern baldness.39–42 Evidence for the importance of sex steroid hormones comes from the fact that hair follicles and the prostate gland are both androgen responsive. Men born with a congenital deficiency of 5-alpha reductase type II or who were prepubertally castrated do not develop prostate cancer and show complete retention of scalp hair.43 Baldness has also been associated with higher levels of dihydrotestosterone44 and increased expression of androgen receptor.45–47 Epidemiologic evidence for associations between circulating androgens and prostate cancer is inconclusive.48–50 Limitations of these studies include between-assay variations, lack of comparability of androgen levels, and use of a single blood measurement typically at middle age or later. Meanwhile, genetic polymorphisms in SRD5A2,51 CYP17,52 and HSD3B53 androgen metabolism genes as well as genomic regulatory elements binding to androgen receptor54,55 have been associated with prostate cancer, thus supporting the importance of sex steroid hormones.
Besides androgenic action, circulating insulin-like growth factors (IGFs) and hyperinsulinemia may also play roles in prostate carcinogenesis and baldness either directly or via interactions with androgens. A meta-analysis of 42 epidemiologic studies (14 cohort and 28 case-controls studies) reported that circulating IGF-I was associated with increased risk of prostate cancer.56 In addition, two case-control studies suggested that vertex balding was positively associated with increased circulating IGF-1 and inversely associated with plasma IGF binding protein-3.57,58 Moreover, hyperinsulinemia has been reported to be associated with accelerated growth of prostate cancer xenografts,59–61 as well as increased prostate cancer risk in two case-control studies.62,63 Insulin resistance can also lead to vasoconstriction and nutritional deficits in scalp follicles preceding hair loss,64,65 and minoxidil (an antihypertensive vasodilator) has been used to treat hair loss.
Several limitations of this study warrant discussion. First, the validity and reliability of our modified Norwood-Hamilton scale has not been assessed. However, previous validation studies for similar adapted scales showed that the validity (kappa = 0.47 to 0.60) of self-reported current hair patterns66,67 and reliability (kappa = 0.71 ± 0.07) of recalling hair patterns at age 45 years were moderate to good.66 Second, exposure was retrospectively recalled, which could have an impact on the accuracy of reporting. However, differential misclassification of exposure is not expected, given that exposure was recalled before prostate cancer diagnosis. Third, only a small number of black men were included in our cohort. In the NHEFS, black men with any degree of baldness had increased risk of prostate cancer (relative risk [RR], 2.10; 95% CI, 1.04 to 4.25).21 A case-control study in African American men showed that baldness at age 30 years recalled on a modified Norwood-Hamilton scale was significantly associated with prostate cancer risk (OR, 1.69; 95% CI, 1.05 to 2.74).20 Moreover, this case-control study demonstrated that frontal baldness was more strongly associated with high-stage (OR, 2.61; 95% CI, 1.10 to 6.18) and high-grade prostate cancer (OR, 2.20; 95% CI, 1.05 to 4.61).20 However, the paradox that black men are less likely to have male pattern baldness than their white counterparts68 (Appendix Table A3, online only) but have higher risk for prostate cancer requires further elucidation. Finally, we had only one single age point of male pattern baldness in this study, and a broader age range of male pattern baldness assessment may be more informative.
In summary, our analysis of the prospective PLCO Cancer Screening Trial has shown a positive association between frontal plus moderate vertex baldness at age 45 years and aggressive prostate cancer risk. Although the effect is moderate, it supports the possibility of overlapping pathogeneses between male pattern baldness and prostate cancer.
Supplementary Material
Acknowledgment
The authors thank Barry I. Graubard, PhD, and Amanda Black, PhD, from the Division of Cancer Epidemiology and Genetics, National Cancer Institute, for their consultation support.
Appendix
Table A1.
Associations Between Male Pattern Baldness at Age 45 Years and Prostate Cancer Risk in the Analytic Cohort by Four Different Definitions of Prostate Cancer Aggressiveness
| Male Pattern Baldness at Age 45 Years | Unadjusted* |
Multivariable† |
Unadjusted* |
Multivariable† |
Unadjusted* |
Multivariable† |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Definition 1‡ |
||||||||||||
| Aggressive (n = 169) |
Nonaggressive (n = 953) |
|||||||||||
| No baldness | Ref. | Ref. | Ref. | Ref. | ||||||||
| Any baldness | 1.27 | 0.93 to 1.72 | 1.26 | 0.93 to 1.71 | 0.98 | 0.87 to 1.12 | 0.98 | 0.86 to 1.11 | ||||
| Frontal baldness only | 1.21 | 0.83 to 1.77 | 1.21 | 0.83 to 1.76 | 0.97 | 0.83 to 1.14 | 0.96 | 0.82 to 1.13 | ||||
| Any frontal plus vertex baldness | 1.31 | 0.92 to 1.87 | 1.30 | 0.91 to 1.86 | 1.00 | 0.86 to 1.16 | 0.99 | 0.85 to 1.15 | ||||
| Frontal plus mild vertex baldness | 1.15 | 0.70 to 1.87 | 1.14 | 0.70 to 1.87 | 0.96 | 0.78 to 1.17 | 0.94 | 0.77 to 1.16 | ||||
| Frontal plus moderate vertex baldness | 2.03 | 1.32 to 3.13 | 2.02 | 1.31 to 3.12 | 1.05 | 0.84 to 1.30 | 1.04 | 0.84 to 1.30 | ||||
| Frontal plus severe vertex baldness | 0.53 | 0.22 to 1.32 | 0.53 | 0.21 to 1.30 | 1.00 | 0.76 to 1.30 | 0.99 | 0.76 to 1.30 | ||||
| Definition 2§ |
||||||||||||
| Aggressive (n = 628) |
Nonaggressive (n = 502) |
|||||||||||
| No baldness | Ref. | Ref. | Ref. | Ref. | ||||||||
| Any baldness | 1.09 | 0.93 to 1.28 | 1.08 | 0.93 to 1.27 | 0.96 | 0.81 to 1.15 | 0.95 | 0.80 to 1.14 | ||||
| Frontal baldness only | 1.07 | 0.88 to 1.30 | 1.06 | 0.87 to 1.29 | 0.95 | 0.76 to 1.18 | 0.94 | 0.76 to 1.17 | ||||
| Any frontal plus vertex baldness | 1.11 | 0.92 to 1.34 | 1.11 | 0.92 to 1.33 | 0.97 | 0.79 to 1.20 | 0.96 | 0.78 to 1.19 | ||||
| Frontal plus mild vertex baldness | 1.00 | 0.77 to 1.29 | 0.99 | 0.77 to 1.28 | 0.99 | 0.75 to 1.30 | 0.97 | 0.74 to 1.28 | ||||
| Frontal plus moderate vertex baldness | 1.42 | 1.11 to 1.81 | 1.42 | 1.11 to 1.81 | 0.93 | 0.68 to 1.28 | 0.93 | 0.68 to 1.27 | ||||
| Frontal plus severe vertex baldness | 0.87 | 0.60 to 1.24 | 0.87 | 0.60 to 1.24 | 1.01 | 0.70 to 1.46 | 0.99 | 0.69 to 1.43 | ||||
| Definition 3‖ |
||||||||||||
| Aggressive (n = 219) |
Nonaggressive (n = 911) |
|||||||||||
| No baldness | Ref. | Ref. | Ref. | Ref. | ||||||||
| Any baldness | 1.28 | 0.98 to 1.67 | 1.28 | 0.98 to 1.68 | 0.98 | 0.86 to 1.12 | 0.97 | 0.85 to 1.11 | ||||
| Frontal baldness only | 1.22 | 0.88 to 1.70 | 1.22 | 0.88 to 1.71 | 0.97 | 0.82 to 1.14 | 0.96 | 0.82 to 1.13 | ||||
| Any frontal plus vertex baldness | 1.33 | 0.97 to 1.81 | 1.33 | 0.97 to 1.82 | 0.99 | 0.85 to 1.16 | 0.98 | 0.84 to 1.14 | ||||
| Frontal plus mild vertex baldness | 1.17 | 0.77 to 1.80 | 1.18 | 0.77 to 1.80 | 0.96 | 0.78 to 1.18 | 0.94 | 0.76 to 1.16 | ||||
| Frontal plus moderate vertex baldness | 1.96 | 1.33 to 2.89 | 1.97 | 1.34 to 2.91 | 1.04 | 0.83 to 1.30 | 1.03 | 0.82 to 1.29 | ||||
| Frontal plus severe vertex baldness | 0.66 | 0.32 to 1.36 | 0.66 | 0.32 to 1.36 | 0.99 | 0.75 to 1.30 | 0.98 | 0.75 to 1.29 | ||||
| Definition 4¶ |
||||||||||||
| High Risk (n = 187) |
Intermediate Risk (n = 400) |
Low Risk (n = 415) |
||||||||||
| No baldness | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Any baldness | 1.17 | 0.87 to 1.56 | 1.16 | 0.87 to 1.55 | 1.06 | 0.87 to 1.30 | 1.07 | 0.88 to 1.30 | 0.98 | 0.81 to 1.18 | 0.97 | 0.80 to 1.18 |
| Frontal baldness only | 1.07 | 0.75 to 1.54 | 1.07 | 0.74 to 1.53 | 1.04 | 0.81 to 1.33 | 1.04 | 0.81 to 1.33 | 1.02 | 0.81 to 1.30 | 1.02 | 0.80 to 1.29 |
| Any frontal plus vertex baldness | 1.25 | 0.90 to 1.75 | 1.24 | 0.89 to 1.73 | 1.09 | 0.86 to 1.37 | 1.09 | 0.87 to 1.38 | 0.94 | 0.74 to 1.18 | 0.93 | 0.74 to 1.17 |
| Frontal plus mild vertex baldness | 1.08 | 0.68 to 1.72 | 1.07 | 0.67 to 1.71 | 1.03 | 0.75 to 1.41 | 1.03 | 0.75 to 1.41 | 0.93 | 0.68 to 1.27 | 0.92 | 0.67 to 1.25 |
| Frontal plus moderate vertex baldness | 1.93 | 1.28 to 2.91 | 1.92 | 1.27 to 2.90 | 1.12 | 0.80 to 1.57 | 1.13 | 0.81 to 1.59 | 0.96 | 0.68 to 1.36 | 0.97 | 0.68 to 1.36 |
| Frontal plus severe vertex baldness | 0.55 | 0.24 to 1.26 | 0.54 | 0.24 to 1.25 | 1.14 | 0.76 to 1.69 | 1.16 | 0.78 to 1.73 | 0.90 | 0.59 to 1.37 | 0.89 | 0.58 to 1.36 |
NOTE. Missing values of covariates (excluding male pattern baldness at age 45 years) were imputed in sequential regression multiple imputation models via IVEware in SAS v.9.3. Unadjusted model: age (month) was used as the underlying time metric. Multivariable model: adjusted for screening arm (usual care, screening), screening center (10 centers, not listed here), race (white, black, other), education (less than high school, high school graduate, some college, college graduate, postgraduate), married or cohabiting (yes, no), diabetes (yes, no), body mass index at age 50 years (< 18.5, 18.5-24.9, 25.0-29.9, ≥ 30 kg/m2), cigarette smoking (ever, never), aspirin use frequency (none or < 1 time per month, ≤ 2 times per week, 3-6 times per week, ≥ 7 times per week), and myocardial infarction (yes, no).
Abbreviations: HR, hazard ratio; Ref., reference.
Definition 1: aggressive prostate cancer was defined as biopsy Gleason score ≥ 8, and/or clinical stage III or greater, and/or fatal prostate cancer. There were 16 prostate cancers that lacked enough information to determine prostate cancer subtypes.
Definition 2: aggressive prostate cancer was defined as comprehensive Gleason score ≥ 7, and/or comprehensive stage III or greater, and/or fatal prostate cancer. There were eight prostate cancers that lacked enough information to determine prostate cancer subtypes.
Definition 3: aggressive prostate cancer was defined as comprehensive Gleason score ≥ 8, and/or comprehensive stage III or greater, and/or fatal prostate cancer. There were eight prostate cancers that lacked enough information to determine prostate cancer subtypes.
Definition 4: modified D'Amico criteria: low risk (cT1 to cT2a, prediagnostic prostate-specific antigen [PSA] within 6 months ≤ 10 ng/mL, and biopsy Gleason score ≤ 6); intermediate risk (cT2b, and/or prediagnostic PSA within 6 months > 10 ng/mL and ≤ 20 ng/mL, and/or biopsy Gleason score of 7); and high risk (≥ cT2c, and/or prediagnostic PSA within 6 months > 20 ng/mL, and/or biopsy Gleason score ≥ 8, and/or fatal prostate cancer). There were 136 prostate cancers that lacked enough information to determine prostate cancer subtypes.
Table A2.
Sensitivity Analysis: Associations Between Male Pattern Baldness at Age 45 Years and Prostate Cancer Risk in the Analytic Cohort Restricted to the Subcohort of Individuals Enrolled After the Date of Trial Protocol Change (N = 33,367)
| Male Pattern Baldness at Age 45 Years | Total Incident Prostate Cancer (N = 1,053) |
Aggressive Prostate Cancer* (n = 517) |
Nonaggressive Prostate Cancer* (n = 521) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted† |
Multivariable‡ |
Unadjusted† |
Multivariable‡ |
Unadjusted† |
Multivariable‡ |
|||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| No baldness | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Any baldness | 1.06 | 0.93 to 1.19 | 1.04 | 0.92 to 1.18 | 1.11 | 0.93 to 1.32 | 1.10 | 0.92 to 1.31 | 0.99 | 0.83 to 1.18 | 0.97 | 0.82 to 1.16 |
| Frontal baldness only | 1.04 | 0.90 to 1.21 | 1.03 | 0.89 to 1.20 | 1.05 | 0.85 to 1.31 | 1.04 | 0.84 to 1.30 | 1.04 | 0.84 to 1.28 | 1.02 | 0.83 to 1.26 |
| Any frontal plus vertex baldness | 1.07 | 0.93 to 1.23 | 1.05 | 0.91 to 1.22 | 1.16 | 0.95 to 1.42 | 1.15 | 0.94 to 1.41 | 0.95 | 0.77 to 1.17 | 0.93 | 0.76 to 1.15 |
| Frontal plus mild vertex baldness | 1.03 | 0.85 to 1.25 | 1.01 | 0.84 to 1.23 | 1.06 | 0.81 to 1.40 | 1.05 | 0.79 to 1.38 | 0.94 | 0.71 to 1.24 | 0.93 | 0.70 to 1.22 |
| Frontal plus moderate vertex baldness | 1.19 | 0.98 to 1.46 | 1.19 | 0.97 to 1.45 | 1.40 | 1.06 to 1.84 | 1.39 | 1.06 to 1.83 | 0.97 | 0.71 to 1.32 | 0.96 | 0.70 to 1.30 |
| Frontal plus severe vertex baldness | 0.95 | 0.73 to 1.23 | 0.93 | 0.72 to 1.22 | 0.99 | 0.68 to 1.44 | 0.99 | 0.68 to 1.44 | 0.93 | 0.64 to 1.35 | 0.90 | 0.62 to 1.31 |
NOTE. Missing values of covariates (excluding male pattern baldness at age 45 years) were imputed in sequential regression multiple imputation models via IVEware in SAS v.9.3.
Abbreviations: HR, hazard ratio; Ref., reference.
Aggressive prostate cancer was defined as biopsy Gleason score ≥ 7, and/or clinical stage III or greater, and/or prostate cancer as underlying cause of death. Nonaggressive prostate cancer was defined as otherwise. Fourteen prostate cancers lacked enough information to determine prostate cancer subtypes.
Unadjusted model: age (month) was used as the underlying time metric.
Multivariable model: adjusted for screening arm (usual care, screening), screening center (10 centers, not listed here), race (white, black, other), education (less than high school, high school graduate, some college, college graduate, postgraduate), married or cohabiting (yes, no), diabetes (yes, no), body mass index at age 50 years (< 18.5, 18.5-24.9, 25.0-29.9, ≥ 30 kg/m2), cigarette smoking (ever, never), aspirin use frequency (none or < 1 time per month, ≤ 2 times per week, 3-6 times per week, ≥ 7 times per week), and myocardial infarction (yes, no).
Table A3.
Characteristics of Participants in the Analytic Cohort by Male Pattern Baldness
| Characteristic | No Baldness |
Any Baldness |
P* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Baldness Only |
Frontal Plus Any Vertex Baldness |
||||||||||
| Frontal Plus Mild Vertex Baldness |
Frontal Plus Moderate Vertex Baldness |
Frontal Plus Severe Vertex Baldness |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Event status | .368 | ||||||||||
| Censored | 17,678 | 46.6 | 9,411 | 24.8 | 4,760 | 12.5 | 3,643 | 9.6 | 2,440 | 6.4 | |
| Event | 522 | 45.9 | 279 | 24.5 | 142 | 12.5 | 129 | 11.3 | 66 | 5.8 | |
| Randomly assigned group | .899 | ||||||||||
| Usual care | 8,750 | 46.7 | 4,659 | 24.9 | 2,332 | 12.5 | 1,785 | 9.5 | 1,198 | 6.4 | |
| Intervention | 9,450 | 46.4 | 5,031 | 24.7 | 2,570 | 12.6 | 1,987 | 9.8 | 1,308 | 6.4 | |
| Age at SQX, years | < .001 | ||||||||||
| < 65 | 2,569 | 46.4 | 1,301 | 23.5 | 796 | 14.4 | 517 | 9.3 | 356 | 6.4 | |
| 65-69 | 5,288 | 46.5 | 2,782 | 24.5 | 1,456 | 12.8 | 1,108 | 9.7 | 739 | 6.5 | |
| 70-74 | 4,829 | 47.3 | 2,487 | 24.3 | 1,286 | 12.6 | 971 | 9.5 | 642 | 6.3 | |
| ≥ 75 | 5,514 | 46.2 | 3,120 | 26.1 | 1,364 | 11.4 | 1,176 | 9.8 | 769 | 6.4 | |
| Education | .057 | ||||||||||
| Less than high school | 1,066 | 47.3 | 564 | 25.0 | 256 | 11.3 | 215 | 9.5 | 155 | 6.9 | |
| High school graduate | 5,402 | 46.6 | 2,932 | 25.3 | 1,400 | 12.1 | 1,095 | 9.5 | 756 | 6.5 | |
| Some college | 3,655 | 46.9 | 1,941 | 24.9 | 930 | 11.9 | 765 | 9.8 | 495 | 6.4 | |
| College graduate | 3,708 | 47.1 | 1,930 | 24.5 | 1,010 | 12.8 | 737 | 9.4 | 488 | 6.2 | |
| Postgraduate | 4,326 | 45.6 | 2,302 | 24.3 | 1,292 | 13.6 | 950 | 10.0 | 608 | 6.4 | |
| Marital status | .352 | ||||||||||
| Married or cohabiting | 14,867 | 46.7 | 7,931 | 24.9 | 3,967 | 12.4 | 3,048 | 9.6 | 2,053 | 6.4 | |
| Single† | 3,200 | 46.6 | 1,668 | 24.3 | 895 | 13.0 | 688 | 10.0 | 421 | 6.1 | |
| Race | < .001 | ||||||||||
| White | 15,874 | 45.5 | 8,816 | 25.3 | 4,440 | 12.7 | 3,448 | 9.9 | 2,316 | 6.6 | |
| Black | 483 | 55.2 | 172 | 19.7 | 116 | 13.3 | 61 | 7.0 | 43 | 4.9 | |
| Other‡ | 1,110 | 64.3 | 324 | 18.8 | 160 | 9.3 | 99 | 5.7 | 34 | 2.0 | |
| First-degree relative(s) had prostate cancer | .548 | ||||||||||
| No | 14,463 | 46.7 | 7,687 | 24.8 | 3,890 | 12.6 | 2,958 | 9.5 | 1,984 | 6.4 | |
| Yes | 1,560 | 45.4 | 863 | 25.1 | 440 | 12.8 | 353 | 10.3 | 219 | 6.4 | |
| Diabetes | .541 | ||||||||||
| No | 13,274 | 46.7 | 7,064 | 24.8 | 3,586 | 12.6 | 2,740 | 9.6 | 1,784 | 6.3 | |
| Yes | 2,947 | 46.3 | 1,566 | 24.6 | 788 | 12.4 | 643 | 10.1 | 426 | 6.7 | |
| Myocardial infarction | < .001 | ||||||||||
| No | 13,633 | 47.0 | 7,195 | 24.8 | 3,636 | 12.5 | 2,779 | 9.6 | 1,769 | 6.1 | |
| Yes | 2,438 | 44.6 | 1,373 | 25.1 | 678 | 12.4 | 570 | 10.4 | 413 | 7.5 | |
| Enlarged prostate | < .001 | ||||||||||
| No | 10,109 | 47.6 | 5,200 | 24.5 | 2,598 | 12.2 | 1,990 | 9.4 | 1,323 | 6.2 | |
| Yes | 7,946 | 45.4 | 4,410 | 25.2 | 2,255 | 12.9 | 1,746 | 10.0 | 1,151 | 6.6 | |
| BMI at age 50 years, kg/m2 | < .001 | ||||||||||
| < 25.0 | 6,023 | 47.3 | 3,275 | 25.7 | 1,534 | 12.1 | 1,191 | 9.4 | 698 | 5.5 | |
| 25.0-29.9 | 8,069 | 45.9 | 4,354 | 24.8 | 2,289 | 13.0 | 1,709 | 9.7 | 1,142 | 6.5 | |
| ≥ 30 | 2,506 | 46.5 | 1,245 | 23.1 | 682 | 12.6 | 550 | 10.2 | 410 | 7.6 | |
| Cigarette smoking | < .001 | ||||||||||
| Never | 6,027 | 44.8 | 3,290 | 24.5 | 1,780 | 13.2 | 1,378 | 10.3 | 966 | 7.2 | |
| Ever | 11,659 | 47.7 | 6,083 | 24.9 | 2,984 | 12.2 | 2,251 | 9.2 | 1,453 | 5.9 | |
| Aspirin use frequency | .001 | ||||||||||
| None or < 1 time per month | 5,719 | 48.0 | 2,967 | 24.9 | 1,434 | 12.0 | 1,083 | 9.1 | 706 | 5.9 | |
| 2 times per week or less | 1,756 | 46.4 | 924 | 24.4 | 492 | 13.0 | 383 | 10.1 | 226 | 6.0 | |
| 3-6 times per week | 1,820 | 45.4 | 1,050 | 26.2 | 502 | 12.5 | 372 | 9.3 | 264 | 6.6 | |
| ≥ 7 times per week | 8,492 | 45.9 | 4,530 | 24.5 | 2,363 | 12.8 | 1,860 | 10.1 | 1,239 | 6.7 | |
Abbreviations: BMI, body mass index; SQX, supplemental questionnaire.
P values were calculated from χ2 tests for nonmissing categorical variables with controls without prostate cancer as the comparison group.
Single includes widowed, divorced, separated, and never married.
Other race includes Asian, Native Hawaiian or other Pacific Islander, American Indian, or Alaskan Native.
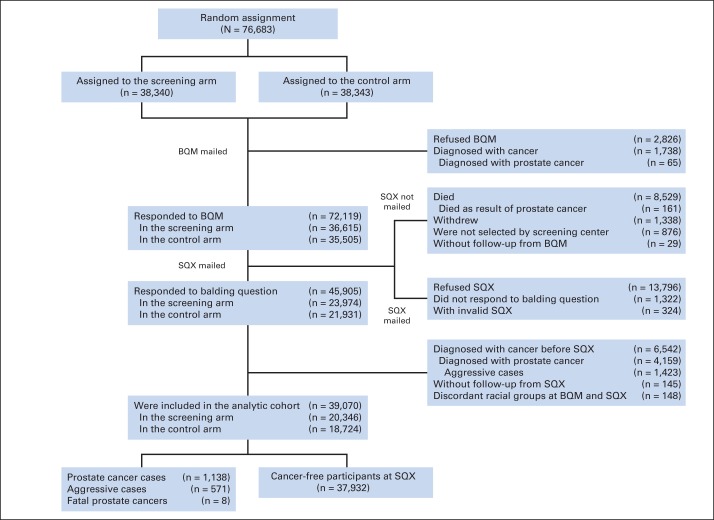
Fig A1.
Flow diagram showing how the analytic cohort was determined from participants in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. BQM, baseline questionnaire–men; SQX, supplemental questionnaire.
Footnotes
See accompanying editorial on page 386
Supported by the Intramural Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Cindy Ke Zhou, Sean D. Cleary, Paul H. Levine, Lisa W. Chu, Ann W. Hsing, Michael B. Cook
Collection and assembly of data: Cindy Ke Zhou, Ann W. Hsing
Data analysis and interpretation: Cindy Ke Zhou, Ruth M. Pfeiffer, Sean D. Cleary, Heather J. Hoffman, Paul H. Levine, Lisa W. Chu, Ann W. Hsing, Michael B. Cook
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society; [Google Scholar]
- 2.Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer. 2004;101:2371–2490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- 3.Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47:273–287. doi: 10.3322/canjclin.47.5.273. [DOI] [PubMed] [Google Scholar]
- 4.Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 5.Eeles RA, Kote-Jarai Z, Giles GG, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40:316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 6.Shui IM, Lindström S, Kibel AS, et al. Prostate cancer (PCa) risk variants and risk of fatal PCa in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Eur Urol. 2014;65:1069–1075. doi: 10.1016/j.eururo.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alsantali A, Shapiro J. Androgens and hair loss. Curr Opin Endocrinol Diabetes Obes. 2009;16:246–253. doi: 10.1097/med.0b013e32832b100a. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Chapado M, Olmedilla G, Cabeza M, et al. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: An autopsy study. Prostate. 2003;54:238–247. doi: 10.1002/pros.10177. [DOI] [PubMed] [Google Scholar]
- 9.Soos G, Tsakiris I, Szanto J, et al. The prevalence of prostate carcinoma and its precursor in Hungary: An autopsy study. Eur Urol. 2005;48:739–744. doi: 10.1016/j.eururo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Cremers RG, Aben KK, Vermeulen SH, et al. Androgenic alopecia is not useful as an indicator of men at high risk of prostate cancer. Eur J Cancer. 2010;46:3294–3299. doi: 10.1016/j.ejca.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Demark-Wahnefried W, Schildkraut JM, Thompson D, et al. Early onset baldness and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:325–328. [PubMed] [Google Scholar]
- 12.Giles GG, Severi G, Sinclair R, et al. Androgenetic alopecia and prostate cancer: Findings from an Australian case-control study. Cancer Epidemiol Biomarkers Prev. 2002;11:549–553. [PubMed] [Google Scholar]
- 13.Greenwald P, Damon A, Kirmss V, et al. Physical and demographic features of men before developing cancer of the prostate. J Natl Cancer Inst. 1974;53:341–346. doi: 10.1093/jnci/53.2.341. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh CC, Thanos A, Mitropoulos D, et al. Risk factors for prostate cancer: A case-control study in Greece. Int J Cancer. 1999;80:699–703. doi: 10.1002/(sici)1097-0215(19990301)80:5<699::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Oishi K, Okada K, Yoshida O, et al. Case-control study of prostatic cancer in Kyoto, Japan: Demographic and some lifestyle risk factors. Prostate. 1989;14:117–122. doi: 10.1002/pros.2990140205. [DOI] [PubMed] [Google Scholar]
- 16.Wright JL, Page ST, Lin DW, et al. Male pattern baldness and prostate cancer risk in a population-based case-control study. Cancer Epidemiol. 2010;34:131–135. doi: 10.1016/j.canep.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynder EL, Mabuchi K, Whitmore WF., Jr Epidemiology of cancer of the prostate. Cancer. 1971;28:344–360. doi: 10.1002/1097-0142(197108)28:2<344::aid-cncr2820280214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Yassa M, Saliou M, De Rycke Y, et al. Male pattern baldness and the risk of prostate cancer. Ann Oncol. 2011;22:1824–1827. doi: 10.1093/annonc/mdq695. [DOI] [PubMed] [Google Scholar]
- 19.Muller DC, Giles GG, Sinclair R, et al. Age-dependent associations between androgenetic alopecia and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2013;22:209–215. doi: 10.1158/1055-9965.EPI-12-0860. [DOI] [PubMed] [Google Scholar]
- 20.Zeigler-Johnson C, Morales KH, Spangler E, et al. Relationship of early-onset baldness to prostate cancer in African-American men. Cancer Epidemiol Biomarkers Prev. 2013;22:589–596. doi: 10.1158/1055-9965.EPI-12-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawk E, Breslow RA, Graubard BI. Male pattern baldness and clinical prostate cancer in the epidemiologic follow-up of the first National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 2000;9:523–527. [PubMed] [Google Scholar]
- 22.Thomas JA, Antonelli JA, Banez LL, et al. Androgenetic alopecia at various ages and prostate cancer risk in an equal-access multiethnic case-control series of veterans. Cancer Causes Control. 2013;24:1045–1052. doi: 10.1007/s10552-013-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amoretti A, Laydner H, Bergfeld W. Androgenetic alopecia and risk of prostate cancer: A systematic review and meta-analysis. J Am Acad Dermatol. 2013;68:937–943. doi: 10.1016/j.jaad.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 24.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 25.Gohagan JK, Prorok PC, Hayes RB, et al. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: History, organization, and status. Control Clin Trials. 2000;21:251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 26.Norwood OT. Hair Transplant Surgery. Springfield, IL: Charles C. Thomas; 1973. [Google Scholar]
- 27.Miller AB, Yurgalevitch S, Weissfeld JL, et al. Death review process in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:400S–406S. doi: 10.1016/s0197-2456(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 28.Raghunathan TE, Lepkowski JM, Van Hoewyk J, et al. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodol. 2001;27:85–95. [Google Scholar]
- 29.Raghunathan TE, Solenberger P, Van Hoewyk J. IVEware: Imputation and variance estimation software. Survey Methodology Program, Survey Research Center, Institute for Social Research, University of Michigan. 2002 [Google Scholar]
- 30.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 31.Cooke EW, Shrieve DC, Tward JD. Clinical versus pathologic staging for prostate adenocarcinoma: How do they correlate? Am J Clin Oncol. 2012;35:364–368. doi: 10.1097/COC.0b013e31821241fc. [DOI] [PubMed] [Google Scholar]
- 32.Yamada T, Hara K, Umematsu H, et al. Male pattern baldness and its association with coronary heart disease: A meta-analysis. BMJ Open. 2013:3. doi: 10.1136/bmjopen-2012-002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lotufo PA, Chae CU, Ajani UA, et al. Male pattern baldness and coronary heart disease: The Physicians' Health Study. Arch Intern Med. 2000;160:165–171. doi: 10.1001/archinte.160.2.165. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton JB. Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. 1951;53:708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 35.Hsing AW, Devesa SS. Trends and patterns of prostate cancer: What do they suggest? Epidemiol Rev. 2001;23:3–13. doi: 10.1093/oxfordjournals.epirev.a000792. [DOI] [PubMed] [Google Scholar]
- 36.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer: Analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 37.Nyholt DR, Gillespie NA, Heath AC, et al. Genetic basis of male pattern baldness. J Invest Dermatol. 2003;121:1561–1564. doi: 10.1111/j.1523-1747.2003.12615.x. [DOI] [PubMed] [Google Scholar]
- 38.Kote-Jarai Z, Al Olama AA, Giles GG, et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet. 2011;43:785–791. doi: 10.1038/ng.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellis JA, Scurrah KJ, Cobb JE, et al. Baldness and the androgen receptor: The AR polyglycine repeat polymorphism does not confer susceptibility to androgenetic alopecia. Hum Genet. 2007;121:451–457. doi: 10.1007/s00439-006-0317-8. [DOI] [PubMed] [Google Scholar]
- 40.Ellis JA, Stebbing M, Harrap SB. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J Invest Dermatol. 2001;116:452–455. doi: 10.1046/j.1523-1747.2001.01261.x. [DOI] [PubMed] [Google Scholar]
- 41.Hillmer AM, Flaquer A, Hanneken S, et al. Genome-wide scan and fine-mapping linkage study of androgenetic alopecia reveals a locus on chromosome 3q26. Am J Hum Genet. 2008;82:737–743. doi: 10.1016/j.ajhg.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hillmer AM, Hanneken S, Ritzmann S, et al. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Am J Hum Genet. 2005;77:140–148. doi: 10.1086/431425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marks LS. 5alpha-reductase: History and clinical importance. Rev Urol. 2004;6:S11–S21. [PMC free article] [PubMed] [Google Scholar]
- 44.Schweikert HU, Wilson JD. Regulation of human hair growth by steroid hormones: II. Androstenedione metabolism in isolated hairs. J Clin Endocrinol Metab. 1974;39:1012–1019. doi: 10.1210/jcem-39-6-1012. [DOI] [PubMed] [Google Scholar]
- 45.Price VH, Menefee E, Sanchez M, et al. Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): Three- and 4-year results. J Am Acad Dermatol. 2006;55:71–74. doi: 10.1016/j.jaad.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Randall VA, Thornton MJ, Messenger AG. Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g. beard) contain more androgen receptors than those from non-balding areas of scalp. J Endocrinol. 1992;133:141–147. doi: 10.1677/joe.0.1330141. [DOI] [PubMed] [Google Scholar]
- 47.Sawaya ME, Honig LS, Hsia SL. Increased androgen binding capacity in sebaceous glands in scalp of male-pattern baldness. J Invest Dermatol. 1989;92:91–95. doi: 10.1111/1523-1747.ep13071290. [DOI] [PubMed] [Google Scholar]
- 48.Endogenous Hormones and Prostate Cancer Collaborative Group. Roddam AW, Allen NE, et al. Endogenous sex hormones and prostate cancer: A collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss JM, Huang WY, Rinaldi S, et al. Endogenous sex hormones and the risk of prostate cancer: A prospective study. Int J Cancer. 2008;122:2345–2350. doi: 10.1002/ijc.23326. [DOI] [PubMed] [Google Scholar]
- 50.Gershman B, Shui IM, Stampfer M, et al. Prediagnostic circulating sex hormones are not associated with mortality for men with prostate cancer. Eur Urol. 2014;65:683–689. doi: 10.1016/j.eururo.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Huang Y, Fu X, et al. Meta-analysis of three polymorphisms in the steroid-5-alpha-reductase, alpha polypeptide 2 gene (SRD5A2) and risk of prostate cancer. Mutagenesis. 2011;26:371–383. doi: 10.1093/mutage/geq103. [DOI] [PubMed] [Google Scholar]
- 52.Wang F, Zou YF, Feng XL, et al. CYP17 gene polymorphisms and prostate cancer risk: A meta-analysis based on 38 independent studies. Prostate. 2011;71:1167–1177. doi: 10.1002/pros.21332. [DOI] [PubMed] [Google Scholar]
- 53.Chang BL, Zheng SL, Hawkins GA, et al. Joint effect of HSD3B1 and HSD3B2 genes is associated with hereditary and sporadic prostate cancer susceptibility. Cancer Res. 2002;62:1784–1789. [PubMed] [Google Scholar]
- 54.Jia L, Landan G, Pomerantz M, et al. Functional enhancers at the gene-poor 8q24 cancer-linked locus. Plos Genet. 2009;5:e1000597. doi: 10.1371/journal.pgen.1000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Y, Zhang Z, Yu H, et al. Functional annotation of risk loci identified through genome-wide association studies for prostate cancer. Prostate. 2011;71:955–963. doi: 10.1002/pros.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowlands MA, Gunnell D, Harris R, et al. Circulating insulin-like growth factor peptides and prostate cancer risk: A systematic review and meta-analysis. Int J Cancer. 2009;124:2416–2429. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Platz EA, Pollak MN, Willett WC, et al. Vertex balding, plasma insulin-like growth factor 1, and insulin-like growth factor binding protein 3. J Am Acad Dermatol. 2000;42:1003–1007. [PubMed] [Google Scholar]
- 58.Signorello LB, Wuu J, Hsieh CC, et al. Hormones and hair patterning in men: A role for insulin-like growth factor 1? J Am Acad Dermatol. 1999;40:200–203. doi: 10.1016/s0190-9622(99)70188-x. [DOI] [PubMed] [Google Scholar]
- 59.Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99:1793–1800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 60.Freedland SJ, Mavropoulos J, Wang A, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68:11–19. doi: 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ngo TH, Barnard RJ, Cohen P, et al. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003;9:2734–2743. [PubMed] [Google Scholar]
- 62.Albanes D, Weinstein SJ, Wright ME, et al. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst. 2009;101:1272–1279. doi: 10.1093/jnci/djp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsing AW, Gao YT, Chua S, Jr, et al. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95:67–71. doi: 10.1093/jnci/95.1.67. [DOI] [PubMed] [Google Scholar]
- 64.Klemp P, Peters K, Hansted B. Subcutaneous blood flow in early male pattern baldness. J Invest Dermatol. 1989;92:725–726. doi: 10.1111/1523-1747.ep12721603. [DOI] [PubMed] [Google Scholar]
- 65.Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, et al. Androgenetic alopecia and cardiovascular risk factors in men and women: A comparative study. J Am Acad Dermatol. 2010;63:420–429. doi: 10.1016/j.jaad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Littman AJ, White E. Reliability and validity of self-reported male balding patterns for use in epidemiologic studies. Ann Epidemiol. 2005;15:771–772. doi: 10.1016/j.annepidem.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Taylor R, Matassa J, Leavy JE, et al. Validity of self reported male balding patterns in epidemiological studies. BMC Public Health. 2004;4:60. doi: 10.1186/1471-2458-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Setty LR. Hair patterns of scalp of white and negro males. Am J Phys Anthropol. 1970;33:49–55. doi: 10.1002/ajpa.1330330108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.