Abstract
Although insufficient sleep is a well-recognized risk factor for overeating and weight gain, the neural mechanisms underlying increased caloric (particularly fat) intake after sleep deprivation remain unclear. Here we used resting-state functional magnetic resonance imaging and examined brain connectivity changes associated with macronutrient intake after one night of total sleep deprivation (TSD). Compared to the day following baseline sleep, healthy adults consumed a greater percentage of calories from fat and a lower percentage of calories from carbohydrates during the day following TSD. Subjects also exhibited increased brain connectivity in the salience network from the dorsal anterior cingulate cortex (dACC) to bilateral putamen and bilateral anterior insula (aINS) after TSD. Moreover, dACC-putamen and dACC-aINS connectivity correlated with increased fat and decreased carbohydrate intake during the day following TSD, but not during the day following baseline sleep. These findings provide a potential neural mechanism by which sleep loss leads to increased fat intake.
Population studies consistently find that short sleep duration (habitual sleep ≤ 6 h/night) is a significant risk factor for weight gain and obesity1,2,3,4,5,6,7. Recent laboratory studies demonstrate that sleep restriction leads to weight gain8,9, increased caloric intake8,9,10,11,12,13,14,15,16, increased portion sizes17, delayed meal timing8,9,10,11, increased fat consumption8,10,12, and greater impulsivity in response to food cues18. In order to investigate mechanisms underlying the effect of sleep loss on increased caloric intake, most previous research has primarily focused on changes in metabolic hormones, only a few recent studies have begun to examine how changes in brain activity may play a role19,20,21,22,23.
Compared to habitual sleep, subjects undergoing restricted sleep displayed greater overall neuronal activity in response to food stimuli, particularly in areas related to reward including the putamen, nucleus accumbens (NAC), thalamus, insula and prefrontal cortex19. When comparing activation in response to images of healthy versus unhealthy foods, subjects displayed greater activation in the insular cortex, orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex during sleep restriction but not during habitual sleep20. Compared with normal sleep, subjects undergoing total sleep deprivation showed increased activation in the right anterior cingulate cortex (ACC) in response to food images, which correlated with post-scan appetite ratings for pictures of high calorie foods21. Conversely, in another study, subjects undergoing total sleep deprivation displayed decreased activation in higher-order cortical evaluation regions (i.e., ACC, left lateral OFC, and anterior insula) and enhanced activation in the amygdala while rating desirability for various foods displayed in pictures22. Finally, self-reported daytime sleepiness correlated with reduced activation in the ventromedial prefrontal cortex (VMPFC) in response to high- versus low-calorie food images, and activation in this region predicted overeating in women23.
Notably, the brain regions showing altered activation in response to food stimuli following sleep loss are nodes in the “salience network”, which is thought to play a key role in conflict monitoring, interoceptive-autonomic processing, homeostatic regulation and reward-processing24,25. It has been posited that the salience network integrates processed sensory data with visceral, autonomic, and hedonic signals in order to guide behavior24,25. Relative to lean individuals, those who are obese exhibit abnormal activation in regions that are components of the salience network, including the ACC, insula and dorsal striatum, in response to food stimuli and when anticipating the consumption of high-calorie food26,27,28,29,30. Obese individuals also exhibit greater salience network functional connectivity at rest and in response to food cues31,32. Collectively, these studies suggest that sleep loss may alter neuronal activity within the salience network and that changes in salience network activation may relate to increases in caloric intake that have been previously observed during sleep loss.
The current study examined the effects of acute total sleep deprivation on salience network activity using resting-state functional connectivity (FC). Assessing brain function in a resting-state (when subjects are not performing a specific sensorimotor or cognitive task in the scanner) measures the effect of sleep loss without the interference of behavioral performance. FC also investigates the relationship between neural activity in anatomically distinct but functionally related brain regions, allowing for the identification and assessment of physiological coupling between brain regions within intrinsic networks33,34,35. The current study also assessed the relationship between salience network FC and actual caloric and macronutrient intake, rather than relying on self-reported ratings of hunger or food cravings that may not accurately reflect behavior. To our knowledge, only one previous experiment examined the relationship between changes in brain activity and actual caloric intake, in which changes in right insula activation in response to unhealthy food stimuli after sleep restriction was negatively correlated to the change in caloric intake and fat intake between habitual sleep and sleep restriction20.
We investigated the impact of total sleep deprivation on caloric intake, macronutrient intake and ACC-seed resting-state FC in a healthy and diverse sample of adult men and women, and in a control group who were not sleep deprived. We hypothesized that total sleep deprivation would increase caloric intake and fat intake as well as alter resting-state FC in the salience network. In addition, we hypothesized that, following total sleep deprivation, salience network FC would relate to caloric and macronutrient intake.
Results
Subject Characteristics
Sleep-deprived subjects (n = 34) did not differ from control subjects (n = 12) in age, BMI or in the percentage of African Americans or women (all p>0.50; Table 1). Sleep duration and timing during the week prior to the in-laboratory study were assessed using wrist actigraphy; there were no differences in pre-study sleep duration, onset, offset or midpoint between sleep-deprived and control subjects (all p>0.49).
Table 1. Mean ± SD Subject Characteristics.
| Age | BMI | % Women (n) | % African American (n) | Pre-Study Actigraphic Sleep Duration (h) | Pre-Study Actigraphic Sleep Midpoint (time ± minutes) | |
|---|---|---|---|---|---|---|
| Sleep-deprived subjects (n = 34) | 34.7 ± 7.9 | 24.8 ± 2.8 | 47.1 (16) | 64.7 (22) | 8.0 ± 0.5 | 0338h ± 50 minutes |
| Control subjects (n = 12) | 33.5 ± 9.3 | 24.4 ± 3.6 | 58.3 (7) | 75.0 (9) | 7.9 ± 0.4 | 0348h ± 48 minutes |
Caloric Intake
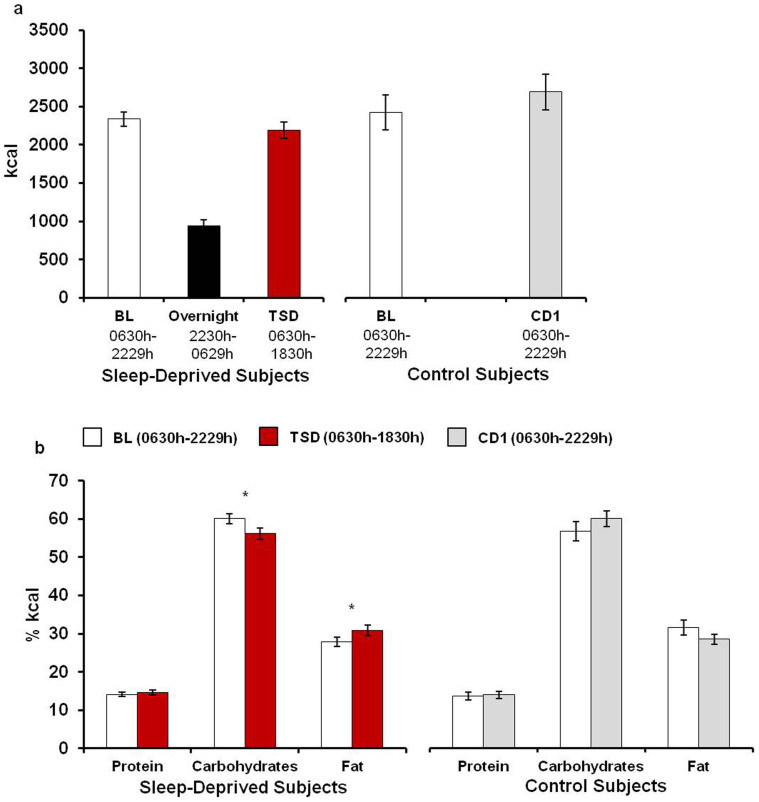
Subjects consumed 939.0 ± 81.7 (M ± SD) kcal during the overnight period of wakefulness and then consumed a similar amount of calories during the day following total sleep deprivation compared to the day following baseline sleep (BL: 2336.2 ± 560.7 kcal, TSD: 2188.8 ± 636.0 kcal; Figure 1a, p = 0.12). However, sleep-deprived subjects consumed a larger percentage of calories from fat (t(33) = −2.93, p = 0.006) and a smaller percentage of calories from carbohydrates (t(33) = 3.15, p = 0.004) during the day following total sleep deprivation compared to the day following baseline sleep (Figure 1b). Protein intake was not significantly different between baseline and total sleep deprivation days (p = 0.41). There were no differences between caloric intake and the percentage of calories derived from each macronutrient during corresponding days in the control condition (Figure 1, all p>0.09).
Figure 1. Caloric and macronutrient intake.
Caloric intake was ad libitum during the study. (a) Despite consuming nearly 1000 kcal during the overnight period of wakefulness, sleep-deprived subjects consumed a similar amount of calories during the day following total sleep deprivation (TSD) as during the day following baseline sleep (BL). Subjects randomized to the control condition consumed a similar amount of calories during corresponding days (BL and CD1). (b) Sleep-deprived subjects consumed a larger percentage of calories from fat and a smaller percentage of calories from carbohydrates during the day following TSD compared to the day following BL sleep. Macronutrient intake did not differ between corresponding days in control subjects (BL and CD1). Data presented as Mean ± SEM; *p<0.05.
Resting-State Functional Connectivity
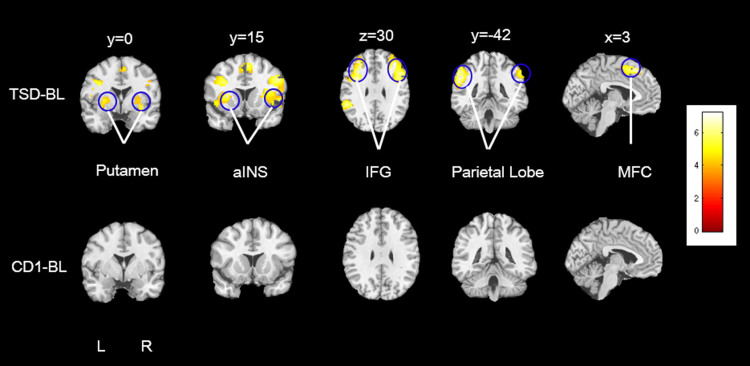
For resting-state functional connectivity analyses, we used ACC as the seed region to determine the salience network24,32. We used two types of ACC seeds, the structurally defined ACC and a literature defined dorsal ACC (dACC)31; Figure 2 depicts dACC connectivity changes. After total sleep deprivation, increased dACC connectivity was observed in bilateral putamen, bilateral anterior insula (aINS), bilateral parietal lobe, bilateral inferior frontal gyrus (IFG) and medial frontal cortex (MFC) (the same regions were observed using the structural ACC seed). All of these activation clusters survived whole brain cluster-level FWE corrected p<0.005 (Table 2). However, there were no significant changes in structural ACC or dACC connectivity between corresponding days in the control condition (Figure 2).
Figure 2. dACC-seed functional connectivity.
Compared to the scan following baseline sleep, resting-state FC between dorsal anterior cingulate cortex (dACC) and the following regions was increased during the scan following total sleep deprivation (TSD-BL): bilateral putamen, bilateral anterior insula (aINS), bilateral inferior frontal gyrus (IFG), bilateral parietal lobe, and medial frontal cortex (MFC). All of these areas survived whole brain cluster-level FWE corrected p<0.005. There were no significant changes in dACC connectivity between corresponding days in the control condition (CD1-BL).
Table 2. Difference in dACC functional connectivity between BL and TSD.
| TSD > BL | ||||||
|---|---|---|---|---|---|---|
| Peak MNI Coordinates | ||||||
| x | y | z | T Score | P value (cluster-level FWE correction) | Cluster Size | |
| R_aINS | 30 | 15 | 9 | 5.12 | <0.001 | 1,150 |
| L_aINS | −30 | 15 | 9 | 5.87 | <0.001 | 981 |
| R_putamen | 24 | 0 | 9 | 4.12 | <0.001 | |
| L_putamen | −24 | 0 | 6 | 5.44 | <0.001 | |
| R_IFG | 45 | 9 | 30 | 5.81 | <0.001 | 292 |
| L_IFG | −36 | 6 | 30 | 6.60 | <0.001 | 260 |
| R_parietal lobe | 57 | −36 | 42 | 6.32 | <0.001 | 128 |
| L_parietal lobe | −51 | −42 | 36 | 6.44 | <0.001 | 345 |
| MFC | 3 | 15 | 54 | 5.87 | <0.001 | 105 |
*The threshold was set as the whole brain cluster-level FWE corrected p<0.005 and cluster size>100 voxels.
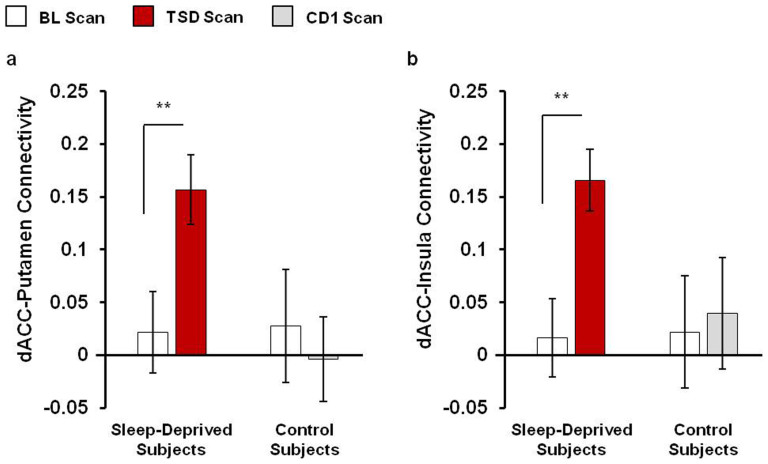
In the current study, we focused on regions of the salience network; therefore, we confirmed the whole brain results of altered dACC-bilateral putamen and dACC-bilateral insula resting-state FC using anatomical region of interests (ROI) analyses. Compared to the scan following baseline sleep, the strength of resting-state FC was significantly increased between the dACC and bilateral putamen (t(33) = −3.41, p = 0.002, Figure 3a) and between the dACC and bilateral insula (t(33) = -3.42, p = 0.002, Figure 3b) after total sleep deprivation. No changes in dACC-putamen or dACC-insula connectivity were observed between corresponding days in the control condition (all p>0.46, Figure 3). In addition, subjects randomized to TSD and control conditions did not differ in FC between dACC and putamen or insula during the baseline measurement (all p>0.90).
Figure 3. Salience network functional connectivity.
Region of interests (ROI) analyses confirmed altered dorsal anterior cingulate (dACC)-bilateral putamen and dACC-bilateral insula resting-state FC after total sleep deprivation (TSD). Anatomical bilateral putamen and bilateral insula were defined as the ROIs using wfupickatlas tool box. The correlation coefficients between these regions and the dACC were then extracted. Compared to the scan following baseline sleep (BL), resting-state FC was significantly increased between the dACC and bilateral putamen (a) and between the dACC and bilateral insula (b) after TSD. No changes in dACC connectivity with bilateral putamen or bilateral insula were observed between corresponding days in the control condition (BL and CD1). Data presented as Mean ± SEM; **p<0.005.
Relationship between Functional Connectivity and Macronutrient Intake
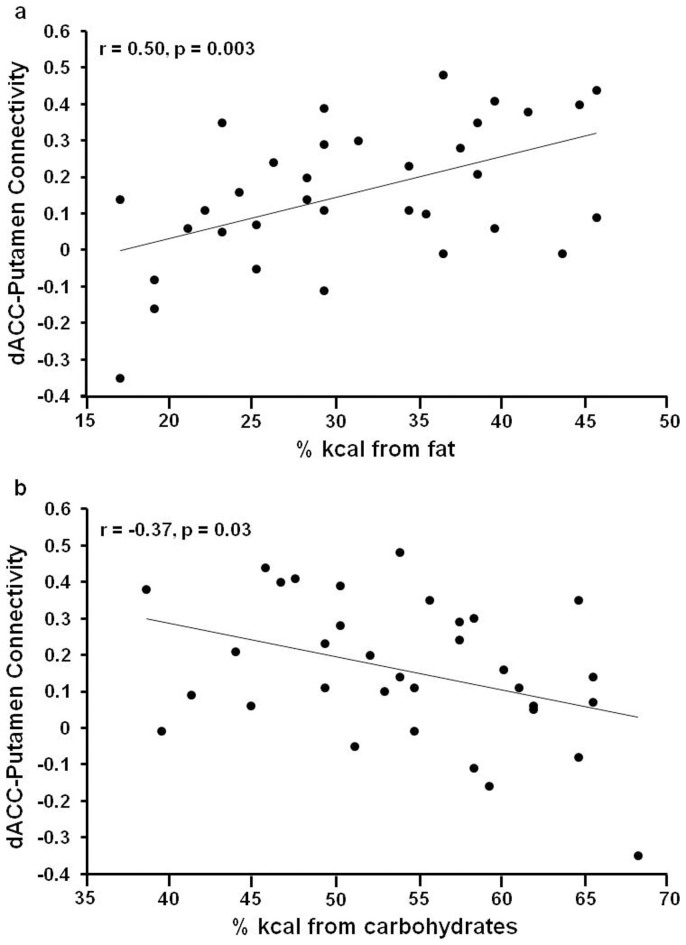
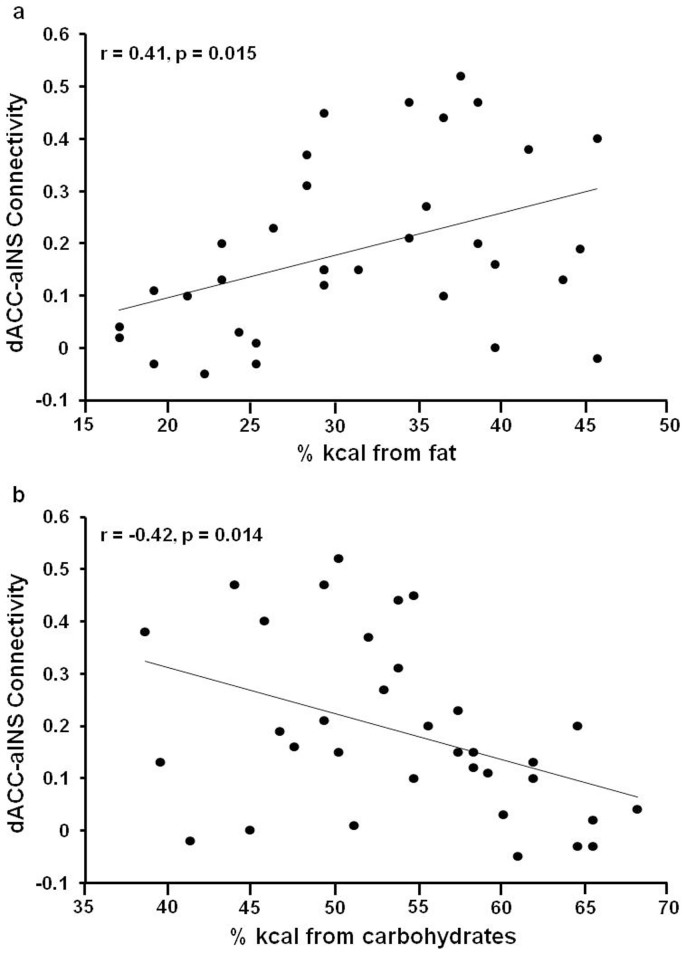
To investigate the relationship between the resting-state FC of the salience network and macronutrient intake, bilateral putamen and bilateral aINS ROIs were built based on the whole brain results summarized in the methods section using Marsbar toolbox (p<0.0001, cluster size>100 voxels). In sleep-deprived subjects, dACC-putamen connectivity during the scan following baseline sleep did not relate to macronutrient intake during the day following baseline sleep (all p>0.05). During the day following total sleep deprivation, dACC-bilateral putamen connectivity was positively correlated with fat intake (% kcal; Figure 4a, r = 0.50, p = 0.003) and negatively correlated with carbohydrate intake (%kcal; Figure 4b, r = −0.37, p = 0.03) but was not related to protein intake (p = 0.55). In sleep-deprived subjects, dACC-bilateral aINS connectivity during the scan following baseline sleep did not relate to macronutrient intake during the day following baseline sleep (all p>0.38). During the day following total sleep deprivation, ACC-bilateral aINS connectivity was positively correlated with fat intake (% kcal; Figure 5a, r = 0.41, p = 0.015) and negatively correlated with carbohydrate intake (%kcal; Figure 5b, r = −0.42, p = 0.014) but was not related to protein intake (p = 0.63). dACC connectivity with IFG, parietal lobes and MFC was not correlated with macronutrient intake during the day following either baseline sleep or total sleep deprivation (all p>0.30).
Figure 4. Correlation between dACC-Putamen connectivity and macronutrient intake after TSD.
During the day following total sleep deprivation (TSD), dorsal anterior cingulate cortex (dACC)-putamen connectivity was positively correlated with fat intake (a) and negatively correlated with carbohydrate intake (b) but was not significantly related to protein intake.
Figure 5. Correlation between dACC-aINS connectivity and macronutrient intake after TSD.
During the day following total sleep deprivation (TSD), dorsal anterior cingulate cortex (dACC)-bilateral anterior insula (aINS) connectivity was positively correlated with fat intake (a) and negatively correlated with carbohydrate intake (b) but was not significantly related to protein intake.
Discussion
Consistent with previous research examining the effect of chronic sleep restriction on caloric intake8,9,10,11,12,13,14,15,16,17,18, total sleep deprivation led to increased caloric intake; subjects remarkably consumed nearly 1000 calories during the overnight period of wakefulness. Despite consuming these additional calories overnight, subjects consumed a similar number of calories during the day following total sleep deprivation compared to the day following baseline sleep. In addition, subjects consumed a greater percentage of calories from fat and a smaller percentage of calories from carbohydrates during the day following total sleep deprivation compared to the day following baseline sleep. The observed increase in fat intake is consistent with previous studies showing increased fat intake during chronic sleep restriction8,10,12. At the neural level, one night of total sleep deprivation enhanced dACC functional connectivity with bilateral putamen and bilateral aINS, which are core regions of the salience network24,26. Moreover, dACC connectivity with these two areas positively correlated with the percentage of calories consumed from fat and negatively correlated with the percentage of calories consumed from carbohydrates after total sleep deprivation. Thus, total sleep deprivation altered salience network functional connectivity and the increased co-activation of dACC and aINS as well as dACC and putamen predicted subsequent macronutrient intake.
To our knowledge, this is the first study to examine the relationship between resting brain connectivity and actual macronutrient intake after baseline sleep and after total sleep deprivation. This approach assesses actual intake behavior rather than relying on self-reported hunger levels or food cravings, which are often inaccurate, and investigates the effect of sleep loss on brain function while avoiding the potentially confounding factors of task performance. When subjects are performing a task, such as viewing pictures of food stimuli or providing ratings for how much a food is desired in the scanner, it may be difficult to determine whether observed changes in brain activity reflect changes in task performance, effects of sleep loss, or an interaction of these two factors. In addition, we used FC analyses in order to examine changes in the functional coupling of a brain network rather than isolated brain regions.
The ACC, insula and putamen are all core regions of the salience network, which guides behavior by identifying important homeostatically relevant internal/external stimuli36. Increased ACC activation has been associated with homeostatically incongruous physical states (i.e., thirst, hunger, overheating) and encoding the pleasantness/averseness of stimuli in all sensory modalities37. The ACC becomes activated during challenging physical conditions that disturb homeostasis and, in conjunction with the insular cortex via autonomic information-processing24,25, guides behavior by altering the perception of salient stimuli and influencing emotional and cognitive responses37. Total sleep deprivation represents a physical homeostatic challenge38 that has been shown to influence ACC activation19,21 and ACC activation has been associated with oral fat administration specifically. ACC activation increases in response to oral fat administration and positively correlates with increasing fat concentrations39. Ventral ACC activation increases in response to oral fat and sucrose administration40 and ACC activation correlates with pleasantness ratings for oral fat administration41.
Co-activation of the ACC and insula has been associated with autonomic responses to salient stimuli24,25. It has been posited that the insula detects salient stimuli (detection) whereas the ACC modulates the response to salient stimuli via inputs to sensory, motor and association cortices (action selection); stimuli detected as salient by the insula receive preferential access to the brain's attentional resources42. The insular cortex plays a role in the sensory perception of food stimuli (i.e., sight, smell, and taste of food/drink) as well as the rewarding/hedonic aspects of food43,44. Healthy women in the fasted state, exhibited increased insula activation in response to high-calorie food stimuli and this activation positively correlated with acylated ghrelin levels (an orexigenic hormone secreted from the stomach)45. Of note, sleep loss increases acylated ghrelin46. Finally, like the ACC, oral fat administration increases insula activation39,47 and activation of the left insula in response to fattening food cues predicted greater caloric consumption of fat when subjects had ad libitum access to a buffet48.
Similar to the insula, the putamen nucleus plays a role in the detection of salient stimuli (particularly rewarding stimuli), including those related to food49,50,51 and may be particularly important during the anticipation of stimulus delivery52. Dopamine release in the dorsal striatum (putamen and caudate) relates to self-ratings of meal pleasantness, hunger, and food desire and is increased in response to food cues53,54. Garcia-Garcia and colleagues32, who found that obese individuals exhibited greater resting-state FC within the left putamen nucleus and between the putamen and insula, suggest that changes in the recruitment of the putamen nucleus within the salience network may reflect greater reliance on the reward response when visualizing or anticipating food stimuli. Sleep deprivation has been shown to increase the reactivity of brain reward regions (including the insular cortex and putamen) to positive experiences55.
Although we also observed increased FC between the ACC and the MFC, IFG and parietal lobe, the FC between these regions were not correlated with macronutrient intake. The MFC, IFG and parietal lobe all play a role in attention processing56,57 and previous studies have shown that total sleep deprivation leads to altered activation in these areas during cognitive performance58,59. It is possible that the effort to maintain wakefulness increased the co-activation of the ACC with each of these areas.
It is noteworthy that the sample consisted of healthy adults within a range of 21-50 years and a normal-to-overweight BMI (18-30). Therefore, our results may not generalize to children, adolescents or the elderly or those who are obese. Future studies are necessary to assess how sleep deprivation may affect salience network FC as well as macronutrient intake in obese adults. In addition, the sample size of this study was not large and we did not find any gender differences. However, given that men overeat to a greater degree than women during sleep loss10 and there are gender differences in the neural response to food stimuli60, future research is needed to compare gender differences in resting-state salience network FC. Furthermore, we permitted the use of oral contraceptives and did not track the menstrual cycle of female subjects, therefore we do not know whether this factor contributes to the observed changes in salience network connectivity. Finally, caloric intake was ad libitum throughout our entire study. Although this is a strength which allowed us to examine actual caloric and macronutrient intake (rather than relying on self-report assessments of hunger or food craving), the overnight intake was not controlled and varied between subjects.
In summary, we observed increased resting-state co-activation between the dorsal anterior cingulate cortex and bilateral putamen as well as between the dorsal anterior cingulate cortex and bilateral anterior insula after total sleep deprivation. Following total sleep deprivation, increased salience network functional connectivity predicted increased fat intake and decreased carbohydrate intake. When adults are forced to maintain wakefulness for an extended period of time due to work or lifestyle (e.g., truck-drivers, medical professionals, military personnel) changes in salience network activation may lead to high-fat, unhealthy food choices that could contribute to weight gain overtime.
Methods
Subjects
Forty-six healthy adults (right-handed, 23 male, age range 21–50 years, 18–30 BMI) were recruited in response to study advertisements. They reported habitual nightly sleep durations between 6.5 h–8.5 h, habitual bedtimes between 2200h–0000h, and habitual awakenings between 0600h–0900h; these reports were confirmed objectively using actigraphy. They had no evidence of habitual napping, no sleep disturbances and an absence of extreme morningness or extreme eveningness, assessed by questionnaire61. Subjects were free of acute or chronic medical and psychological conditions, as established by interviews, clinical history, questionnaires, physical examinations and blood (including a fasting blood glucose test) and urine tests. They were nonsmokers and did not participate in shift work, transmeridian travel, or irregular sleep-wake routines in the 60 days prior to the study. Enrolled subjects were monitored at home with actigraphy, sleep-wake diaries, and time-stamped call-ins to assess bedtime and waketime during the week before and after the in-laboratory phase. They were not permitted to use caffeine, alcohol, tobacco and medications (except oral contraceptives) in the week before the laboratory study, as verified by urine screenings. Sleep disorders were excluded by a night of laboratory polysomnography and oximetry measurements.
The study was approved by the Institutional Review Board of the University of Pennsylvania. All subjects provided written informed consent before enrollment, which was in accordance with the Declaration of Helsinki. Subjects were compensated for participating in the study.
Experimental Design
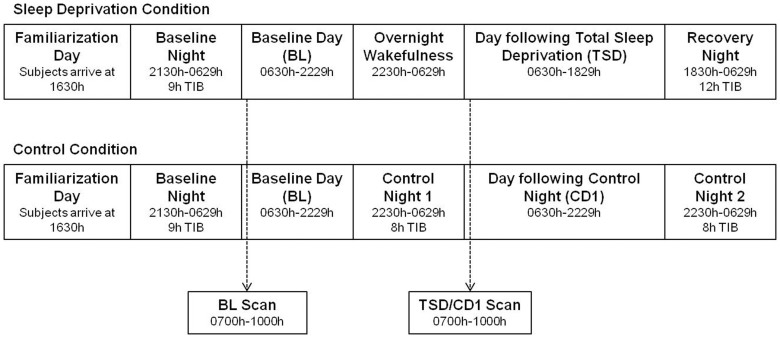
Subjects were screened twice prior to participating in the study; training in a mock MRI scanner occurred during the second screening session. During the study, subjects remained in the laboratory at the Clinical Translational Research Center at the Hospital of the University of Pennsylvania for 5 consecutive days (4 consecutive nights). Subjects arrived at the laboratory in the afternoon and were provided 9 h time-in-bed (TIB) for their baseline sleep night (Figure 6). The first functional magnetic resonance imaging (fMRI) scan session took place the next morning (Baseline [BL] day) from 0700h–1000h. Subjects were then randomized to either a total sleep deprivation (TSD) or control condition. During the second night of the study, sleep-deprived subjects were kept awake and control subjects were allowed 8 h TIB to sleep (Figure 6). The second fMRI scan session took place the next morning (total sleep deprivation [TSD] day or control day 1 [CD1]). Sleep-deprived subjects were then allowed 12 TIB for recovery sleep and control subjects were allowed 8 h TIB to sleep (Figure 6). Each subject was scanned at the same time for each scan to avoid potential time-of-day differences between scans.
Figure 6. Protocol summary.
Subjects arrived at the laboratory in the afternoon and were provided 9h time-in-bed (TIB) for their baseline sleep night. The first functional magnetic resonance imaging (fMRI) scan session took place the next morning (Baseline [BL] day) from 0700h-1000h. Subjects were then randomized to either a total sleep deprivation (TSD) or control condition. During the second night of the study, sleep-deprived subjects were kept awake and control subjects were allowed 8 h TIB to sleep. The second fMRI scan session took place the next morning from 0700h-1000h (total sleep deprivation [TSD] day or control day 1 [CD1]). Sleep-deprived subjects were then allowed 12 TIB for recovery sleep and control subjects were allowed 8 h TIB to sleep. Each subject was scanned at the same time for each scan to avoid potential time-of-day differences between scans. Subjects had ad libitum to food/drink during the study. Subjects remained in the laboratory for the duration of the study and were monitored by trained staff at all times to ensure adherence to the protocol.
Subjects were behaviorally monitored by trained staff continuously to ensure adherence and were not permitted to leave the laboratory during the study. Subjects were ambulatory and were allowed to watch television, read, play video or board games, and perform other sedentary activities between test bouts (which were completed while sitting at the computer) but they were not allowed to exercise. Subjects wore a wrist actigraph throughout the study and wore ambulatory electroencephalography and electrocardiography recording equipment for 24-h intervals. The light levels were held constant at <50 lux during scheduled wakefulness and <1 lux during scheduled sleep periods. Ambient temperature was maintained between 22°–24°C. Food/drink was ad libitum throughout the protocol (caffeine was prohibited).
Measures
Subjects selected their meals/snacks by choosing from various menu options, and by making requests to the monitors and study coordinator. In order to ensure that subjects were provided sufficient time to eat each day, three 30- to 45-min opportunities were specified in the protocol during the daytime and one additional 30-min opportunity to eat was specified in the protocol during the overnight period when subjects were kept awake. In addition to these specified meal times, subjects were also allowed to consume food/drink at any time during the protocol other than when they were completing neurobehavioral tests. All food was weighed and recorded prior to being provided to subjects. To enhance the measurement accuracy of each food's weight, food was provided in individual containers (for example, a dinner consisting of chicken, peas, and rice was provided in three separate containers). Each day, a detailed description of the items and the amount consumed and intake time was recorded by trained monitors. Additionally, any food/drink that was leftover after each meal was weighed and recorded. The intake data were entered into The Food Processor SQL program (ESHA Research, Salem, OR), a validated62 professional nutrition analysis software and database program that provides components of food/drink intake including calories and macronutrients.
Magnetic resonance imaging was conducted in a 3T whole-body scanner (Siemens Medical Systems, Erlangen, Germany), using a standard array coil. A standard EPI sequence was used for resting-state BOLD fMRI data acquisition with the following parameters: TR = 2s, TE = 24 ms, FOV = 220 × 220 mm, matrix = 64 × 64 × 36, slices thickness = 4 mm, inter-slice gap = 4 mm. A total of 210 images were acquired for each subject. Subjects were instructed to remain still in the scanner at rest and to keep their eyes open. An eye-tracker outsider the scanner was used to monitor subjects' eyes and ensure that they did not fall asleep during the scans. After the functional scans, high-resolution (1 × 1 × 1 mm3) T1-weighted anatomic images were obtained using a standard 3D MPRAGE sequence.
Image data processing and analyses were carried out using the Statistical Parametric Mapping software (SPM8, Wellcome Department of Cognitive Neurology, UK) and the REST 2.0 toolbox (http://resting-fmri.sourceforge.net/) implemented in Matlab 14 (Math Works, Natick, MA). After head motion correction and co-registration, functional images were smoothed using an isotropic Gaussian kernel with a full-width at half-maximum (FWHM) of 8 mm, and then normalized to the standard Montreal Neurological Institute (MNI) space. Linear trends were also removed. Finally, all functional volumes were band pass filtered (0.01 Hz < f < 0.08 Hz) in order to reduce low-frequency drift and physiological high-frequency respiratory and cardiac noise. Nuisance covariates including six head motion parameters, global mean signal, white matter signal and CSF signal were regressed out before the seed based functional connectivity (FC) analysis63.
The ACC seed was defined using an automated anatomical labeling region of interest library through the WFU_Pickatlas toolbox (http://fmri.wfubmc.edu/software/PickAtlas). The dACC seed was defined using MNI coordinates from reference 31 (x: ±6 y: 45 z: 9, radius: 6 mm) using Marsbar toolbox64. For each subject, the mean BOLD fMRI signal time series were extracted from the seed and used as the regressor in the FC analysis. The correlation coefficients between the time series of seed region and other brain areas were grouped into an individual FC map and transformed into z-score through a Fisher's r-to-z transformation to improve the normality of the correlation coefficients. These z-transformed individual FC maps were then entered into the second level group analysis using paired t-tests to examine connectivity differences between the scans following baseline sleep and total sleep deprivation. Threshold was defined as uncorrected p < 0.001 at voxel level and family-wise error (FWE) corrected p < 0.005 at cluster level. For ROI analyses, anatomical bilateral putamen and bilateral insula were also defined using an automated anatomical labeling region of interest library through the WFU_Pickatlas toolbox.
Statistical Analyses
Paired-samples t-tests were used to compare caloric intake and macronutrient intake during the day following baseline sleep and the day following either total sleep deprivation (sleep-deprived subjects) or the day following a night of sleep (control subjects). FC values of the dACC-Putamen and dACC-aINS were extracted and correlated with macronutrient intake on each day using Pearson's r (IBM SPSS Statistics for Windows, Version 19.0, Chicago, IL). False discovery rate correction was used to correct for multiple comparisons (protein, carbohydrate and fat) within each region for each day.
Author Contributions
H.R., N.G., J.A.D. and D.F.D. designed the study and supervised the research. H.R., Z.F., A.M.S., N.M., S.Z., and S.H. carried out the research, collected the data, and contributed to data analyses. Z.F., A.M.S., and H.R. prepared the figures and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Acknowledgments
This research was supported in part by NIH grants R01 HL102119, R01 NR004281, R21 DA032022, R03 DA027098, P30 NS045839, CTRC UL1RR024134, Department of the Navy, Office of Naval Research (Award No. N00014-11-1-0361), and a pilot grant from the Institute for Translational Medicine and Therapeutics (ITMAT) of the University of Pennsylvania.
References
- Ford E. S. et al. Sleep duration and body mass index and waist circumference among U.S. adults. Obesity 22, 598–607 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes W. et al. Association between body mass index and sleep duration assessed by objective methods in a representative sample of the adult population. Sleep Med. 14, 312–318 (2013). [DOI] [PubMed] [Google Scholar]
- Kobayashi D., Takahashi O., Deshpande G. A., Shimbo T. & Fukui T. Association between weight gain, obesity, and sleep duration: a large-scale 3-year cohort study. Sleep Breath. 16, 829–833 (2012). [DOI] [PubMed] [Google Scholar]
- Singh M., Drake C. L., Roehrs T., Hudgel D. W. & Roth T. The association between obesity and short sleep duration: a population-based study. J. Clin. Sleep Med. 1, 357–363 (2005). [PubMed] [Google Scholar]
- Di Milia L., Vandelanotte C. & Duncan M. J. The association between short sleep and obesity after controlling for demographic, lifestyle, work and health related factors. Sleep Med. 14, 319–323 (2013). [DOI] [PubMed] [Google Scholar]
- Hasler G. et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep 27, 661–666 (2004). [DOI] [PubMed] [Google Scholar]
- Chaput J. P., Després J. P., Bouchard C. & Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 31, 517–523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth A. M., Dinges D. F. & Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep 36, 981–990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwald R. R. et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. U S A 110, 5695–5700 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth A. M., Dinges D. F. & Goel N. Gender and race differences in caloric intake during sleep restriction in healthy adults. Am. J. Clin. Nutr. 100, 559–566 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeltcheva A. V. et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am. J. Clin. Nutr. 89, 126–133 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge M. P. et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am. J. Clin. Nutr. 94, 410–416 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosy-Westphal A. et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes. Facts 1, 266–273 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondel L., Romer M. A., Nougues P. M., Touyarou P. & Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am. J. Clin. Nutr. 91,1550–1559 (2010). [DOI] [PubMed] [Google Scholar]
- Calvin A. D. et al. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest 144, 79–86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman C. D. et al. Acute sleep deprivation increases food purchasing in men. Obesity 21, E555–60 (2013). [DOI] [PubMed] [Google Scholar]
- Hogenkamp P. S. et al. Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinology 38, 1668–1674 (2013). [DOI] [PubMed] [Google Scholar]
- Cedernaes J. et al. Increased impulsivity in response to food cues after sleep loss in healthy young men. Obesity 22, 1786–1791 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge M. P. et al. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am. J. Clin. Nutr. 95, 818–824 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge M. P., Wolfe S., Sy M., Shechter A. & Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int. J. Obes. (Lond). 38, 411–416 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C. et al. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI study. J. Clin. Endocrinol. Metab. 97, E443–447 (2012). [DOI] [PubMed] [Google Scholar]
- Greer S. M., Goldstein A. N. & Walker M. P. The impact of sleep deprivation on food desire in the human brain. Nat. Commun. 4, 2259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W. D. et al. Daytime sleepiness affects prefrontal regulation of food intake. Neuroimage 71, 216–223 (2013). [DOI] [PubMed] [Google Scholar]
- Seeley W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. S. Seminowicz D. A. & Davis K. D. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 30, 2731–2745 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Spoor S., Bohon C., Veldhuizen M. G. & Small D. M. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J. Abnorm. Psychol. 117, 924–935. (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. E. et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 18, 254–260 (2010). [DOI] [PubMed] [Google Scholar]
- García-García I. et al. Neural responses to visual food cues: insights from functional magnetic resonance imaging. Eur. Eat. Disord. Rev. 21, 89–98 (2013). [DOI] [PubMed] [Google Scholar]
- Stoeckel L. E. Weller R. E. Cook E. W. Twieg D. B. Knowlton R. C. & Cox J. E. Widespread reward-system activation in obese women in response to pictures of high- calorie foods. Neuroimage. 41, 636–647 (2008). [DOI] [PubMed] [Google Scholar]
- Rothemund Y. et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 37, 410–421 (2007). [DOI] [PubMed] [Google Scholar]
- Kullmann S. et al. Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cereb Cortex. 23, 1247–1256 (2013). [DOI] [PubMed] [Google Scholar]
- Garcia García-García I. et al. Alterations of the salience netw ork in obesity: a resting- state fMRI study. Hum. Brain. Mapp. 34, 2786–2797 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F. Z., Haughton V. M. & Hyde J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 (1995). [DOI] [PubMed] [Google Scholar]
- Cordes D. et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. Am. J. Neuroradiol. 22, 1326–1333 (2001). [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M. P. & Hulshoff Pol H. E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 20, 519–534 (2010). [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15, 483–506 (2011). [DOI] [PubMed] [Google Scholar]
- Gasquoine P. G. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci Biobehav Rev. 37, 340–348 (2013). [DOI] [PubMed] [Google Scholar]
- Goel N., Basner M., Rao H. & Dinges D. F. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 119, 155–190 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldeghaidy S. et al. The cortical response to the oral perception of fat emulsions and the effect of taster status. J Neurophysiol. 105, 2572–2581 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Araujo I. E. & Rolls E. T. Representation in the human brain of food texture and oral fat. J Neurosci. 24, 3086–3093 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E. T., Parris B. A. & d'Souza A. A. How the brain represents the reward value of fat in the mouth. Cereb Cortex. 20, 1082–1091 (2010). [DOI] [PubMed] [Google Scholar]
- Menon V. & Uddin L. Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 214, 655–667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta C. I. Sarkar P. R., Duong T. Q., Laird A. R. & Fox P. T. Neural bases of food perception: coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity (Silver Spring). 22, 1439–1446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S., Kullmann S. & Veit R. Food related processes in the insular cortex. Front Hum Neurosci. 7, 499–505 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen L. M., Lawson E. A., Christensen K., Klibanski A. & Goldstein J. M. Abnormal relationships between the neural response to high- and low-calorie foods and endogenous acylated ghrelin in women with active and weight-recovered anorexia nervosa. Psychiatry Res. 223, 94–103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K., Tasali E., Leproult R., Scherberg N. & Van Cauter E. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 96, 486–493 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger K. S. & Stice E. Elevated energy intake is correlated with hyperresponsivity in attentional, gustatory, and reward brain regions while anticipating palatable food receipt. Am J Clin Nutr. 97, 1188–1194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S. et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 96, 989–999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep N., Roefs A., Roebroeck A., Havermans R., Bonte M. L. & Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 198, 49–158 (2009). [DOI] [PubMed] [Google Scholar]
- Malik S., McGlone F. & Dagher A. State of expectancy modulates the neural response to visual food stimuli in humans. Appetite. 56, 302–309 (2011). [DOI] [PubMed] [Google Scholar]
- Schur E. A., Kleinhans N. M., Goldberg J., Buchwald D., Schwartz M. W. & Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond). 33, 653–661 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J. & Baler R. D. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 15, 37–46 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. M., Jones-Gotman M. & Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 19, 1709–1715 (2003). [DOI] [PubMed] [Google Scholar]
- Volkow N. D. et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 44, 175–180 (2002). [DOI] [PubMed] [Google Scholar]
- Gujar N., Yoo S. S., Hu P. & Walker M. P. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 31, 4466–4474 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E. & Posner I. M. The Attention System of the Human Brain: 20 Years After. Annu Rev Neurosci. 35, 73–89 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. & Li C. S. Functional clustering of the human inferior parietal lobule by whole- brain connectivity mapping of resting-state functional magnetic resonance imaging signals. Brain Connectivity. 4, 53–69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond S. P., Gillin J. C. & Brown G. G. Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res. 10, 85–92 (2001). [DOI] [PubMed] [Google Scholar]
- Poudel G. R., Innes C. R. & Jones R. D. Distinct neural correlates of time-on-task and transient errors during a visuomotor tracking task after sleep restriction. Neuroimage. 77, 105–113 (2013). [DOI] [PubMed] [Google Scholar]
- Frank S. et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res. 1350, 159–166 (2010). [DOI] [PubMed] [Google Scholar]
- Smith C. S., Reilly C. & Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 74, 728–738 (1989). [DOI] [PubMed] [Google Scholar]
- Hise M. E., Sullivan D. K., Jacobsen D. J., Johnson S. L. & Donnelly J. E. Validation of energy intake measurements determined from observer-recorded food records and recall methods compared with the doubly labeled water method in overweight and obese individuals. Am J Clin Nutr. 75, 263–267 (2002). [DOI] [PubMed] [Google Scholar]
- Fair D. A. et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 105, 4028–4032 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J., Valabregue R. & Poline J. Region of interest analysis using an SPM toolbox. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan: NeuroImage, 16 (2002, June 2–6).