Abstract
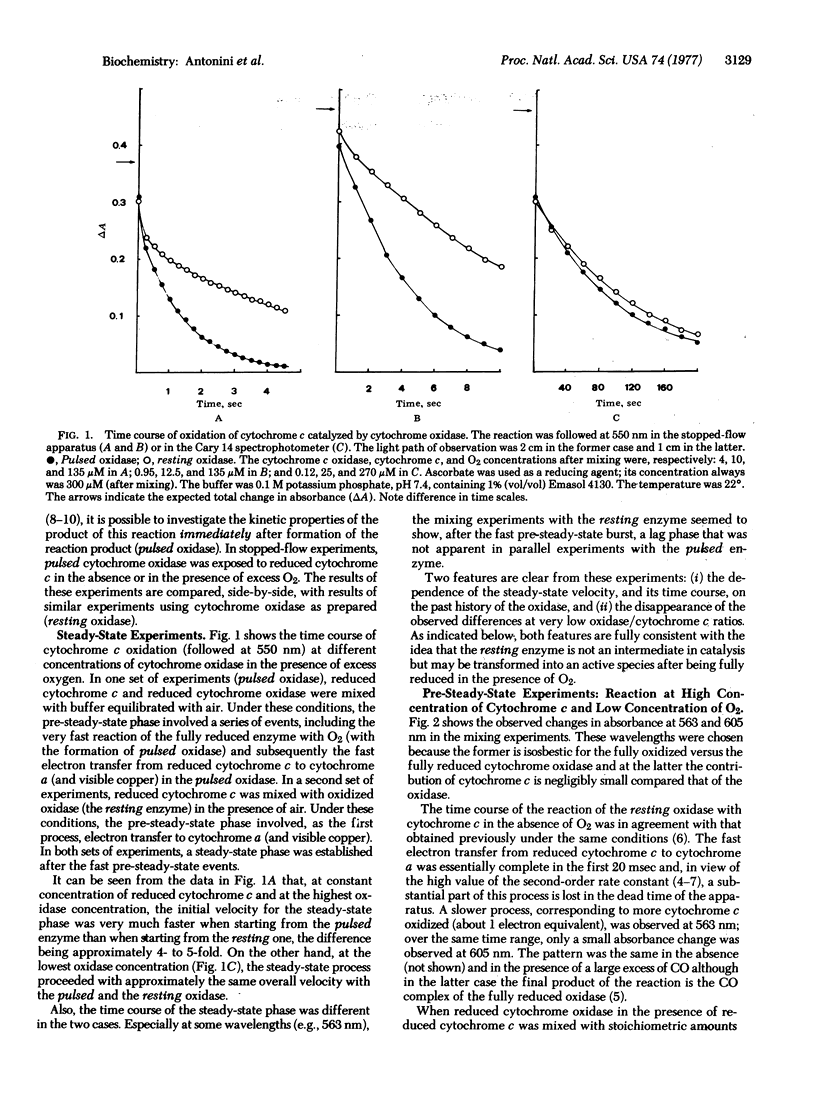
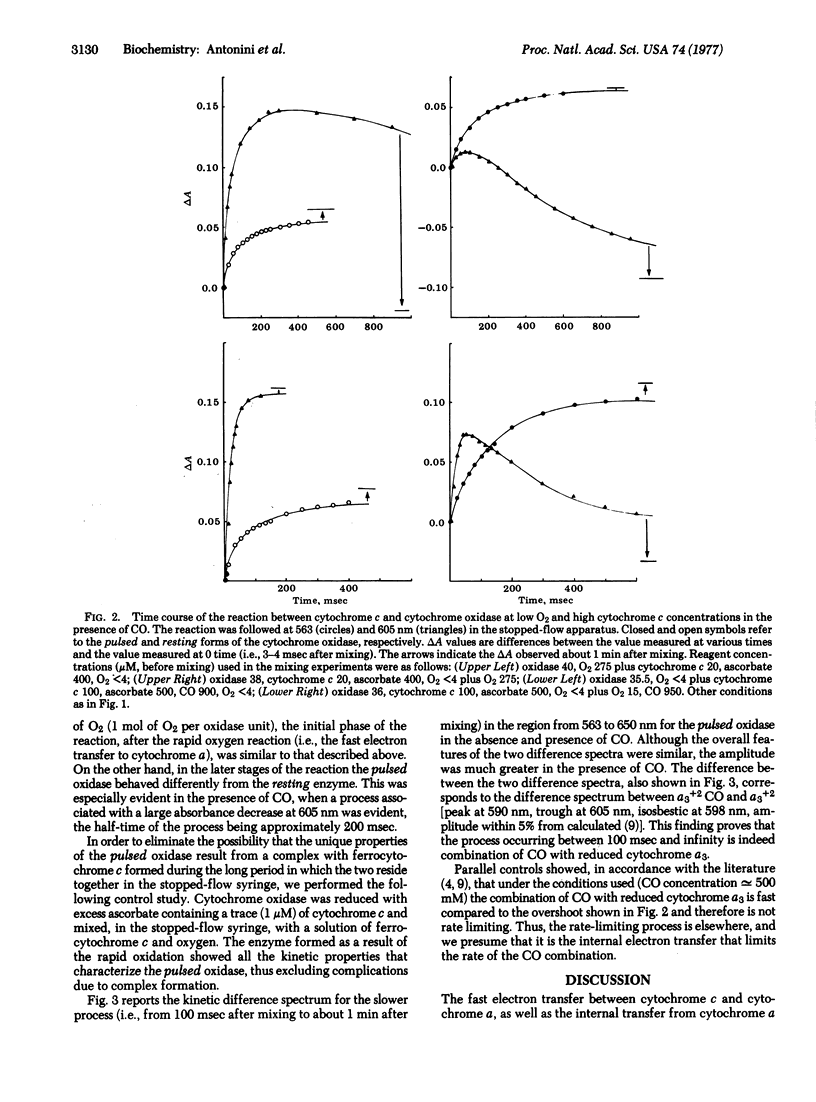
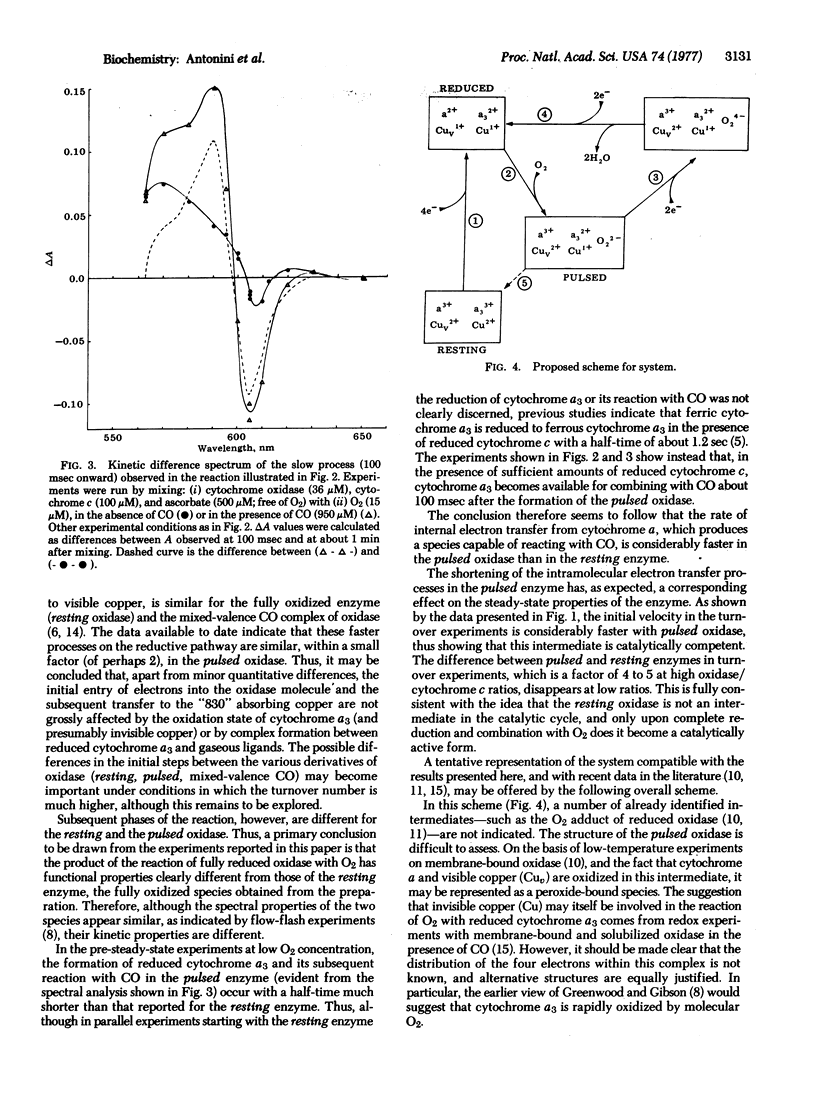
The kinetics of the reaction of cytochrome c with solubilized mammalian cytochrome c oxidase (ferrocytochrome c:oxygen oxidoreductase, EC 1.9.3.1) has been studied by a stopped-flow technique under two different experimental situations: (i) the completely oxidized enzyme (resting oxidase as obtained from the preparation) was mixed with reduced cytochrome c, and (ii) the completely reduced enzyme in the presence of reduced cytochrome c was exposed to a "pulse" of O2 (pulsed oxidase). Both sets of experiments were performed with either "limiting" or "excess" O2 (relative to oxidase), in the presence or absence of CO. Both the pre-steady-state events and the steady-state kinetics of cytochrome oxidase are found to be different in the two cases. This shows that the product of the reaction of fully reduced oxidase with O2 (pulsed oxidase) is functionally different from the oxidase as prepared (resting oxidase). These differences are interpreted with the assumption of a different rate of intramolecular electron transfer in the pulsed and resting oxidases. Implications of these experimental findings are discussed in the general framework of a tentative model for the catalytic cycle of the oxidase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Brunori M., Greenwood C., Malmström B. G. Catalytic mechanism of cytochrome oxidase. Nature. 1970 Dec 5;228(5275):936–937. doi: 10.1038/228936a0. [DOI] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in reaction of cytochrome oxidase with oxygen. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1635–1640. doi: 10.1073/pnas.72.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. Reactions of cytochrome oxidase with oxygen and carbon monoxide. Biochem J. 1963 Mar;86:541–554. doi: 10.1042/bj0860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. THE REACTION OF CYTOCHROME OXIDASE WITH CYTOCHROME C. J Biol Chem. 1965 Feb;240:888–894. [PubMed] [Google Scholar]
- Greenwood C., Brittain T. Studies on partially reduced mammalian cytochrome oxidase reactions with ferrocytochrome c. Biochem J. 1976 Sep 1;157(3):591–598. doi: 10.1042/bj1570591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood C., Gibson Q. H. The reaction of reduced cytochrome C oxidase with oxygen. J Biol Chem. 1967 Apr 25;242(8):1782–1787. [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J. G., Owen C. S., Wilson D. F. The invisible copper of cytochrome c oxidase. pH and ATP dependence of its midpoint potential and its role in the oxygen reaction. Arch Biochem Biophys. 1975 Aug;169(2):492–505. doi: 10.1016/0003-9861(75)90192-7. [DOI] [PubMed] [Google Scholar]
- Van Buuren J. H., Van Gelder B. F., Wilting J., Braams R. Biochemical and biophysical studies on cytochrome C oxidase. XIV. The reaction with cytochrome as studied by pulse radiolysis. Biochim Biophys Acta. 1974 Mar 26;333(3):421–429. doi: 10.1016/0005-2728(74)90126-1. [DOI] [PubMed] [Google Scholar]
- Wilson M. T., Greenwood C., Brunori M., Antonini E. Kinetic studies on the reaction between cytochrome c oxidase and ferrocytochrome c. Biochem J. 1975 Apr;147(1):145–153. doi: 10.1042/bj1470145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960 Mar;235:845–852. [PubMed] [Google Scholar]



