Abstract
Estrogen receptor (ER)-positive breast cancer patients may turn ER-negative and develop acquired drug resistance, which compromises the efficacy of endocrine therapy. By investigating the phenomenon that gefitinib can re-sensitise tamoxifen (TAM)-resistant MCF-7 breast cancer cells (MCF-7/TAM) to TAM, the present study verified that gefitinib could reverse the acquired drug resistance in endocrine therapy and further explored the underlying mechanism.ERα-negative MCF-7/TAM cells were established. Upon treating the cells with gefitinib, the mRNA and protein levels of ERα and ERβ, as well as the expression of molecules involved in the MAPK pathway, were examined using the RT-PCR and immunocytochemistry. The RT-PCR results showed that the mRNA levels of ERα and ERβ in MCF-7/TAM cells were up-regulated following gefitinib treatment; specifically, ERα was re-expressed, and ERβ expression was up-regulated. The expression of molecules involved in the MAPK pathway, including RAS, MEK1/2, and p-ERK1/2, in MCF-7/TAM cells was significantly up-regulated, compared with MCF-7 cells. After the gefitinib treatment, the expression levels of MEK1/2 and p-ERK1/2 were significantly down-regulated. ERα loss is the primary cause for TAM resistance. Gefitinib reverses TAM resistance primarily by up-regulating the ERα mRNA level and inducing the re-expression of ERα. The MAPK pathway plays a key role in ERα re-expression.
As the primary therapeutic regimen for treating hormone receptor-positive breast cancers, endocrine therapy demonstrates an efficacy of approximately 50–60%. In clinical practice, TAM is the most commonly used endocrine therapeutic drug and has been shown to reduce the relapse and mortality rates of ER-positive breast cancers by 40–50% and 30–35%, respectively1. However, primary or acquired drug resistance is the primary cause of the compromised efficacy of endocrine therapy. Approximately 30–50% of ER-positive metastatic breast cancer patients effectively respond to initial TAM treatment. However, almost all patients ultimately develop acquired drug resistance, leading to disease progression or death, which significantly compromises the efficacy of endocrine therapy2,3. Studies of the mechanism underlying endocrine resistance and the corresponding intervention approaches have attracted substantial attention recently. In clinical practice, replacing endocrine therapeutic drugs or switching to chemotherapy is the most common strategy adopted once endocrine resistance has been developed. However, the ER status becomes negative when endocrine resistance occurs; thus, the treatment efficacy cannot be completely restored to the level of the initial treatment by switching to a different endocrine therapeutic drug4,5. Studies have demonstrated that re-expression of the ER, which is the gold standard for selecting patients for endocrine therapy, can re-sensitise breast cancer cells to endocrine therapy6,7. Therefore, searching for approaches to re-express the ER in endocrine resistant cells, investigating the mechanisms underlying ER re-expression, and screening for effective drugs to reverse endocrine resistance are key strategies for enhancing the efficacy of endocrine therapy for breast cancer.
As a small-molecule tyrosine kinase inhibitor, Gefitinib is commonly used in molecularly targeted therapy for lung cancer8,9,10. Gefitinib has also recently been used in studies of combination endocrine therapy for breast cancer11,12. Endocrine resistance is co-regulated by the signalling networks of both the ER and epidermal growth factor receptor (EGFR), and up-regulation of the MAPK signal converts ER-positive cells into ER-negative cells13. Oh et al. found that the transient transfection of MCF-7 cells with active human epidermal growth factor receptor 2 (Her-2), MEK, Raf, or ligand-activated EGFR could down-regulate the mRNA and protein expression of the ER. Moreover, the application of MEK inhibitors was shown to stimulate ER re-expression in breast cancer cells, with these cells subsequently regaining their sensitivity to selective ER antagonists14. However, Bayliss et al. demonstrated that ER re-expression does not always result in effective responses to endocrine therapy15, as certain cancer cells fail to re-express ER upon inhibition of the MAPK pathway. Therefore, ER re-expression in ER-negative breast cancer cells for re-sensitisation to endocrine therapy and the mechanism underlying how ER re-expression relates to the MAPK pathway remain crucial questions in endocrine therapy for breast cancers. In addition, the physiological function of estrogen is mediated by both ERα and ERβ subtypes. ERα is used not only for drug screening but also for evaluating patient prognosis, and ERα is therefore the gold standard for determining the responsiveness to endocrine therapy for breast cancer16. By comparison, the role of ERβ in endocrine therapy remains unclear.
In the present study, ERα-negative TAM-resistant MCF-7 breast cancer cells (MCF-7/TAM) were treated with a low dose of Gefitinib to induce ERα re-expression and TAM re-sensitisation. The role of Gefitinib in the mechanism underlying the reversal of endocrine resistance in breast cancer cells was investigated. By exploring this novel clinical application of Gefitinib, the present study sheds new light on reversing endocrine resistance and provides a reference for clinical applications.
Results
Effects of Gefitinib on the proliferation of MCF-7 and MCF-7/TAM cells
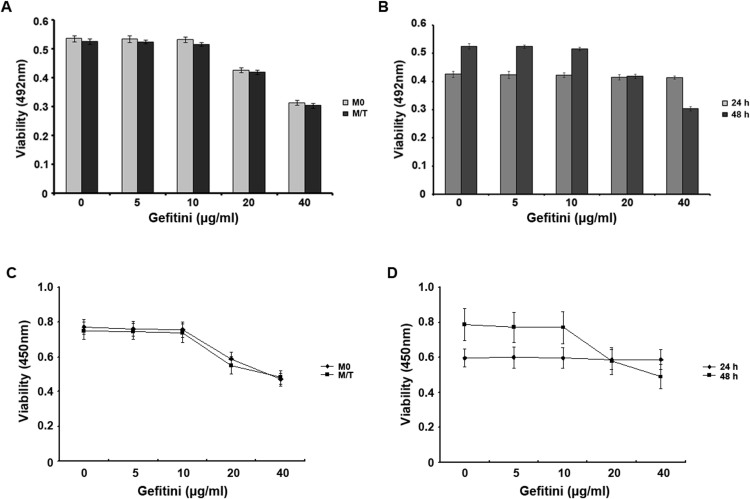
To determine the effects of Gefitinib on the proliferation of MCF-7 and MCF-7/TAM cells, we treated these cells with different doses of Gefitinib for different periods and then used the MTT assay to evaluate cell proliferation (Figure 1A, B). The results revealed that treatment with 10 μg/mL Gefitinib for 24 h or 48 h did not inhibit MCF-7/TAM cell proliferation (p > 0.05), whereas treatment with 20 μg/mL Gefitinib for 48 h significantly inhibited cell proliferation. Therefore, to optimise this experiment, a treatment time of 48 h was adopted for subsequent study. The results revealed that Gefitinib at 10 μg/mL did not inhibit MCF-7 and MCF-7/TAM cell proliferation (p > 0.05) and that Gefitinib at a dosage higher than 20 μg/mL inhibited MCF-7 and MCF-7/TAM cell proliferation (p < 0.05). Therefore, we treated cells with Gefitinib at 10 μg/ml for 48 h in the subsequent experiments.
Figure 1. Dose curve and time course of the effect of Gefitinib on the proliferation of MCF-7 and MCF-7/TAM cells.
(A): MCF-7 and MCF-7/TAM cells were treated with different doses of Gefitinib. The absorbance values determined at 492 nm in an ELISA analyser demonstrated that Gefitinib at different concentration gradients exhibited differing degrees of inhibition on cell proliferation. (B): The absorbance values of MCF-7/TAM cells treated with different concentrations of Gefitinib for 24 h or 48 h. Effects on MCF-7 and MCF-7/TAM cell proliferation in different time and different doses of Gefitinib for detection of CCK-8. (C): the effect of different doses of Gefitinib to inhibit the proliferation of M0 and M/T group, there showed inhibitory concentrations of Gefitinib in two kinds of cells for the proliferation. (D): different concentrations of Gefitinib changes in the range of 24 h, 48 h absorbance in group M/T cells. The experiments consisted of the following groups: the MCF-7 group (M0), the MCF-7/TAM group (M/T), and the MCF-7/TAM-Gefitinib (10 μg/ml) group (G10). All the experiments were repeated three times.
CCK-8 for cell proliferation rate (Figure 1C, D). As result of MTT, There showed no inhibitory effect on the proliferation of MCF-7/TAM cells in 24, 48 h with 10 μg/ml Gefitinib (p > 0.05). However There significantly inhibited cell proliferation in 48 h with 20 μg/ml Gefitinib. So 48 h was chosen as the processing time, we found the proliferation of MCF-7 and MCF-7/TAM cells had no inhibitory effect with Gefitinib in the dose of 10 μg/ml (p > 0.05), MCF-7 and MCF-7/TAM cells were inhibited of the proliferation in the above 20 μg/ml dose (p < 0.05). So, we finally determined the dose of 10 μg/ml Gefitinib, treatment time is 48 h to complete the following experiments.
Gefitinib enhanced the sensitivity of MCF-7/TAM cells to TAM
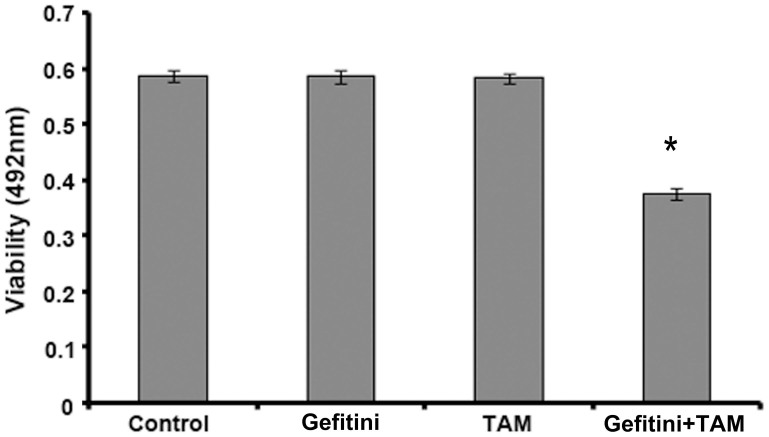
To investigate whether Gefitinib could enhance the sensitivity of MCF-7/TAM cells to TAM, we used 10 μg/mL Gefitinib to treat cells for 48 h and then assessed cell proliferation with the MTT assay (Figure 2). We found that treatment with 10 μg/mL Gefitinib for 48 h re-sensitised MCF-7/TAM cells to TAM, inhibited cell proliferation, and reversed drug resistance.
Figure 2. Gefitinib enhances the sensitivity of MCF-7/TAM cells to TAM.
The inhibitory effects of 10 μg/mL Gefitinib, 1.5 μmol/L TAM, or 10 μg/mL Gefitinib plus 1.5 μmol/L TAM on MCF-7/TAM cell proliferation were evaluated. The data revealed that treatment with Gefitinib re-sensitisedMCF-7/TAM cells to TAM and inhibited cell proliferation. All the experiments were repeated three times.* denotes p < 0.05.
Gefitinib up-regulated ERα mRNA and ERβ mRNA levels in MCF-7/TAM cells
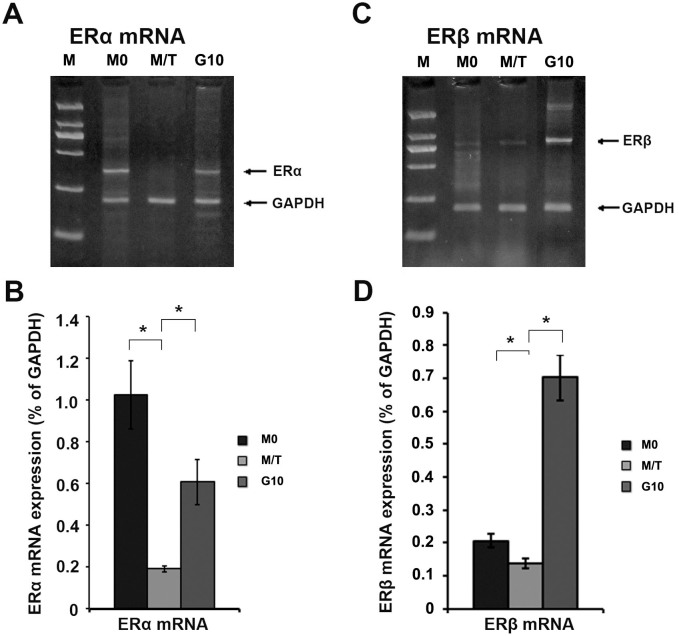
To investigate the mechanism underlying the Gefitinib-mediated reversal of drug resistance in MCF-7/TAM cells, we performed RT-PCR to examine the mRNA levels of ERα and ERβ (Figure 3). The results revealed that, compared with group M0, the mRNA levels of ERα and ERβ in the M/T group were down-regulated, with the down-regulation of the ERα mRNA level exhibiting a more significant difference (p < 0.05). Following Gefitinib treatment, the ERα mRNA level in the G10 group was significantly up-regulated (p < 0.05), and the ERβ mRNA level in the G10 group was also up-regulated (p < 0.05).
Figure 3. RT-PCR results for the ERα and ERβ mRNA levels in MCF-7/TAM cells.
(A): RT-PCR result for the ERα mRNA level. (B): Histogram of the ERα mRNA level. (C): RT-PCR result for the ERβ mRNA level. (D): Histogram of the ERβ mRNA level. All the experiments were repeated three times. * denotes p < 0.05.
Immunocytochemistry and Western blot to determine ERα and ERβ expression
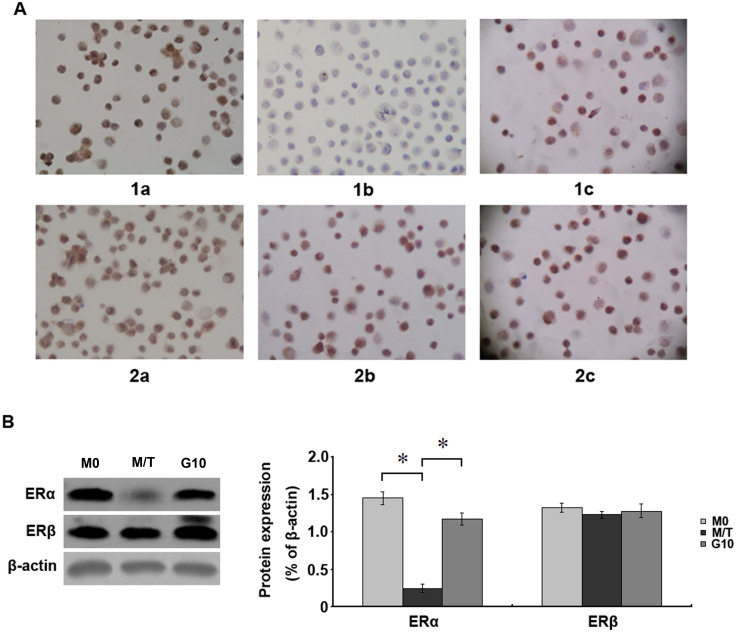
The expression levels of ERα in the M0, M/T, and G10 groups were 95.04 ± 1.81%, 2.10 ± 0.24%, and 75.04 ± 2.88%, respectively. The ERβ expression levels in each group were 96.13 ± 1.07%, 85.13 ± 2.17%, and 90.25 ± 2.15%, respectively. The results demonstrated that ERα expression in cells from the M/T group was significantly reduced (p < 0.05), and this expression was increased after Gefitinib treatment (p < 0.05). By contrast, the ERβ expression level in cells of the M/T group was only slightly decreased (p > 0.05), and Gefitinib treatment increased the ERβ expression level (Figure 4A). The above results suggest that the TAM resistance of MCF-7 cells is primarily associated with ERα loss, whereas the correlation between ERβ expression and TAM resistance is less clear.
Figure 4. Immunocytochemical staining for ERα and ERβ expression in cells.
(A): Sections 1a–1c represent ERα expression in the M0, M/T, and G10 groups, respectively. Sections 2a–2c represent ERβ expression in the M0, M/T, and G10 groups, respectively. ERα expression in cells of the M/T group was rarely observed. ERα was re-expressed in cells of the G10 group after treatment with 10 μg/mL Gefitinibfor 48 h. The ERβ expression in cells of the M/T group was also slightly down-regulated. These data were obtained from 3 independent experiments (DAB staining, ×400). (B): The expression of ERα in M/T group were significantly reduced than M0 group, with10 μg/ml Gefitini treatment for 48 h, the change of ERβ protein expression level is not obvious. All the experiments were repeated three times. *denotes p < 0.05.
Western blot analysis for the expression level of ERα and ERβ Western blot analysis showed that the expression of, ERα protein levels in M/T group were significantly lower than those of M0 group (p < 0.05). Those treated with 10 μg/ml of Gefitini for the treatment of ERα protein levels were significantly up-regulated after 48 h (p < 0.05). This shows that Gefitini can upregulate the expression of ERα levels, the resistant cells were sensitive to TAM. The chang of ERβ protein expression levels is not obvious between Gefitini treatment resistant cells and 10 g/ml Gefitini treatment (p > 0.05) (Figure 4B).
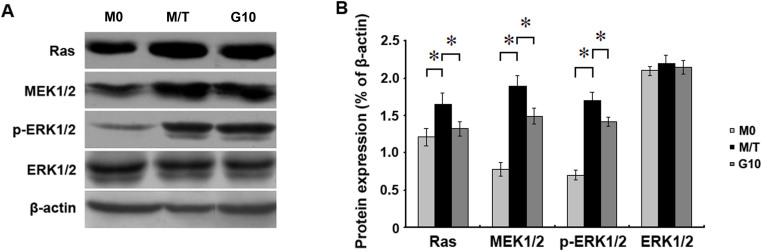
Gefitinib down-regulated the expression of signalling molecules in the MAPK pathway
Western blot results showed that the protein expression of molecules involved in the MAPK signalling pathway, including Ras, MEK1/2, and p-ERK1/2, was significantly enhanced in cells of the M/T group compared with the M0 group (p < 0.05). After treatment with 10 μg/mL Gefitinib for 48 h, the protein expression of Ras, MEK1/2, and p-ERK1/2 was reduced compared with the expression levels detected in the M/T group (p < 0.05). These results indicated that Gefitinib could reverse the drug resistance of MCF-7/TAM cells by altering the expression of molecules involved in the MAPK signalling pathway. These results also suggested that the MAPK pathway plays a key role in ERα re-expression (Figure 5).
Figure 5. Western blot results for the expression of molecules in the MAPK pathway.
(A): The expression of Ras, MEK1/2, and p-ERK1/2 in the M/T group was significantly enhanced compared with the expression levels detected in the M0 group. Treatment with 10 μg/mL Gefitinib for 48 h significantly reduced the expression of Ras, MEK1/2, and p-ERK1/2 compared with the expression levels detected in the M/T group. (B): Histograms of protein expression for Ras, MEK1/2, and p-ERK1/2. All the experiments were repeated three times.*denotes p < 0.05.
Discussion
It has been shown that combining Gefitinib with endocrine therapy can delay the development of drug resistance11,12. In addition, our previous study revealed that combining Gefitinib synergistically with TAM inhibits MCF-7 cell proliferation. Studies on the reversal of multi-drug resistance to chemotherapy have demonstrated that drugs with synergistic therapeutic effects play a critical role in the reversal of corresponding drug resistance in chemotherapy17,18. We previously found that elemene (ELE), a traditional Chinese medicine, could reverse the TAM resistance of breast cancer cells and that ERα loss was the primary cause for the development of TAM resistance in these cells. ELE stimulates ERα re-expression by increasing the ERα mRNA level to reverse drug resistance. Moreover, the MAPK pathway is known to be the key signalling pathway involved in ERα re-expression7. In the present study, we used established MCF-7/TAM cells to investigate whether Gefitinib could reverse TAM resistance and to explore the underlying mechanism.
A positive ER status is an important prerequisite for endocrine therapy in breast cancer patients. The ER regulates gene transcription through the MAPK pathway7,19, and ER-negative breast cancer cells and resistant breast cancer cells that become ER negative frequently exhibit active MAPK signal transduction pathways20,21,22. However, the mechanism underlying the reduced ER expression mediated by MAPK up-regulation remains unclear. Certain reports have proposed that both the MAPK pathway and NF-KB contribute to the loss of ER expression23,24. Gefitinib has been shown to down-regulate the expression of molecules involved in the MAPK pathway25,26, and our study demonstrated that Gefitinib at a low dose could reverse TAM resistance. We also examined the protein expression of MAPK pathway molecules, including Ras, MEK1/2, and P- ERK1/2ER, and found that MEK1/2 and P- ERK1/2ER expression levels in the M/T group were enhanced compared with the expression levels observed in the M0 group. Furthermore, treatment with Gefitinib significantly reduced the protein levels of these pathway components, suggesting that Gefitinib may reverse drug resistance in MCF-7/TAM cells by altering the expression of molecules involved in the MAPK pathway. Gefitinib was also shown to up-regulate the ERα mRNA level and induce ERα re-expression. Although this drug also increased the ERβ mRNA level, the association between ERβ expression and endocrine resistance remains unclear. Changes in the expression of molecules involved in the MAPK pathway affect both ERα and ERβ expression, and ERα re-expression mainly re-sensitises drug-resistant cells to TAM. Although the ERβ mRNA level was significantly increased in the G10 group, the change in ERβ protein expression was mild. Moreover, the ERβ expression in drug-resistant cells remained at a relatively high level. Treatment with Gefitinib increased both the mRNA and protein levels of ERα and ERβ in the G10 group compared with the M/T group, and the ERα/ERβ ratio in the G10 group was also higher than that observed in the M/T group. Consequently, the G10 group demonstrating a higher ratio of ERα/ERβ regained sensitivity to TAM. These results suggest that both ERα and ERβ are involved in the development of drug resistance and that their ratio may indicate sensitivity to endocrine therapy27.
In our previous study of ELE, we used the ERα mRNA/ERβ mRNA ratio as a criterion and found that ELE treatment yielded a ratio value greater than 1, suggesting that the effects on the ERα mRNA levels were significant. We also found that Gefitinib treatment yielded a ratio value less than 1, suggesting that the effects on ERβ mRNA levels were also significant7. Both ELE and Gefitinib treatments led to the reversal of drug resistance, indicating that the down-regulation of the ERα protein affects drug resistance, whereas the role of ERβ expression in drug resistance remains unclear. Indeed, ERβ may represent a new therapeutic target in endocrine-resistant cells, although further studies are required to investigate this potential. In addition, compared with our previous results for ELE, the changes in the ERβ mRNA level after Gefitinib treatment in the present study were significantly higher than those observed after ELE treatment. ERβ expression does not require a high mRNA level. Thus, it is possible that, in addition to affecting the MAPK pathway, Gefitinib is also involved in regulating transcription by other pathways; moreover, ERβ might play an important role in the proliferation of drug resistant cells. However, reports on the role of ERβ in endocrine resistance have been contradictory. Gruvberger et al.28 found that, in ERα-negative breast cancer patients, ERβ expression served as an independent prognostic factor, and such patients benefitted from TAM therapy with a significantly reduced relapse rate. Peach et al.29 reported that ERα activates transcription at the AP-1 site in an estradiol (E2)-dependent manner and that such activity was lost following treatment with an anti-estrogen. In contrast with ERα, the binding of ERβ to E2 does not activate transcription; instead, the binding to TAM stimulates transcription at the AP-1 site and induces cell proliferation. Hodges et al.30 proposed that TAM may block cell proliferation by inhibiting cyclin D1. Furthermore, the stimulation of transcription at AP-1 sites by the binding of ERβ and TAM is thought to provide a mechanism to explain how ERβ expression leads to TAM resistance. However, Strom et al.31 induced ERβ expression in T47D cells and found that neither E2 nor TAM could stimulate cell proliferation. According to other reports, a low level of ERβ may be a preliminary sign of TAM resistance32,33. Thus, these inconsistent results show that the role of ERβ in endocrine resistance remains inconclusive, and our study also failed to clarify the effects of ERβ on endocrine resistance.
Another mechanism underlying the conversion to ERα negative status is methylation of the CpG island in the promoter region of the ERα gene, in addition to histone deacetylation34,35,36. Overexpressed histone deacetylase (HDAC) was shown to convert ERα-positive MCF-7 cells into ERα-negative cells, whereas HDAC inhibitors restored these cells to an ERα-positive status37. In addition, Shen et al. found that DNA methylation inhibitors could induce ER-negative breast cancer cells to re-express active ER in nude mice38. In our study, MCF-7/TAM cells demonstrated low levels of ERα mRNA and the almost complete absence of ER protein expression. Whether ER gene silencing occurred in our experiments was not investigated. Therefore, in addition to altering the expression of molecules involved in the MAPK pathway to stimulate ER re-expression, whether Gefitinib inhibits DNA methylation or inhibits histone deacetylation requires further investigation.
The mechanisms underlying endocrine resistance are extremely complex, which provides many avenues for analysis. Indeed, endocrine therapy combined with Gefitinib, lapatinib, or everolimus is currently under investigation in clinical trials, and initial results have revealed that combination therapy may improve the progression and clinical benefit in treated patients6,39,40. Our investigation demonstrated that Gefitinib could reverse TAM resistance in breast cancer cells by inducing ERα re-expression. In terms of clinical applications, our results may allow patients who have already developed acquired drug resistance to benefit from re-exposure to endocrine therapy. Thus, we believe that our study will provide a novel therapeutic target and strategy for endocrine therapy.
Methods
Cell culture
MCF-7 cells (Shanghai, ChineseAcademy of Sciences Cell Repository) were cultured in DMEM/H-G media (USA, GIBCO) containing 10% fetal bovine serum (FBS) (GIBCO, USA). MCF-7/TAM cells (TRC, Canada) were cultured in complete DMEM/H-G containing 1.0 × 10−7 mol/L TAM for 6 months41. After drug resistance was established, the cells were cultured in DMEM/H-G media containing 10% FBS. The MCF-7/TAM cells were treated with 10 μg/mL or 20 μg/mL Gefitinib (AstraZeneca, USA) for 48 h. The experiments consisted of the following groups: the MCF-7 group (M0), the MCF-7/TAM group (M/T), and the MCF-7/TAM-Gefitinib (10 μg/ml) group (G10). Cell culture was performed at 37°C and 5% CO2, and the cells were passaged every 3–4 days.
MTT assay to determine viability
The cells were trypsinised with 0.25% trypsin (GIBCO, USA) and seeded into 96-well tissue culture plates at a density of 1 × 104 cells/mL and 100 μL per well. The cells were cultured at 37°C and 5% CO2 for 24 h. In total, 100 μL of culture medium containing a predetermined concentration gradient of Gefitinib or TAM was added, and the same type of medium was added to the control wells. Each treatment group contained 4 wells. After a 48-h incubation, 20 μL MTT (0.5 mg/mL) was added to each well. The cells were incubated at 37°C for 4 h, and then 200 μL DMSO was added. The plate was shaken for 15 min, and the absorbance values of each cell were measured at 492 nm using an enzyme-linked immunosorbent assay (ELISA) analyser42.
CCK8 cell viability assay
Cell viability was assessed by a Cell counting Kit-8 (CCK8) assay (KeyGEN BioTECH, China). Cells (1 × 104 cells/well) were plated into 96-well plates. After 24 h, the cells were treated with different concentrations of Gefitinib (0–40 μg/mL) or TAM (1.5 μmol/L) or DMSO (0.1%). After incubation with for 24 h, 48 h or 72 h, the CCK8 reagent was added to each well and cells were incubated for 2 h at 37°C. The absorbance (optical density) at 450 nm was measured.
Immunocytochemistry
The drop plate method was used. Cells were collected and trypsinised to produce a single-cell suspension. The cell density was adjusted to 1.0 × 106 cells/mL, and the cells were fixed with methanol for 20 min. Next, 10 μL of the cell suspension was placed onto poly-L-lysine-coated glass slides. The staining was performed according to the kit manual (the ERα and ERβ antibodies, ready-to-use SP kit, and concentrated DAB colour development kit were obtained from Beijing, Zhongshan Golden Bridge Biotechnology Co., Ltd.). The slides were treated with 0.5% Triton X-100 (in an ice-water bath) for 5 min, washed with PBS for 3 × 2 min, treated with 3% H2O2 (room temperature) for 15 min, washed again with PBS for 3 × 2 min, and blocked with the blocking serum provided in the kit at 37°C for 20 min. The slides were then incubated with the primary antibody (or with PBS for the control group) at 4°C overnight. The slides were kept at room temperature for 1 h, washed with PBS for 3 × 2 min, incubated with the secondary antibody at 37°C for 20 min, and washed with PBS for 3 × 2 min. The slides were then incubated with streptavidin-biotin complex (SABC) at 37°C in a moisturised chamber for 20 min and washed with PBS for 3 × 4 min. Next, the slides were stained with 3, 3′-diaminobenzidine (DAB)and were washed with tap water. Subsequently, the slides were stained with haematoxylin for 2–5 s, dehydrated, mounted, and then observed under a microscope.
Reverse transcription polymerase chain reaction (RT-PCR)
Cells were collected by centrifugation, and RNA was extracted using the Trizol method (TaKaRa, Dalian, China). The total RNA concentration was calculated. The following primers were designed according to previous reports7,43: ERα-forward: AATTCAGATAATCGACGCCAG, ERα-reverse: GTGTTTCAACATTCTCCCTCCTC; ERβ-forward: TAGTGGTCCATCGCCAGTTAT, ERβ-reverse: GGGAGCCAACACTTCACCAT; and GAPDH-forward: 5′-ACCACAGTCCATGCCATCAC-3, GAPDH-reverse: 5′-TCCACCACCCTGTTGCTGTA-3′. RT-PCR was performed according to the kit manual (TaKaRa, Dalian, China). The volume of each PCR reaction was 50 μL. The cycling conditions consisted of 94°C for 5 min, followed by 30 cycles of 94°C for 45 s for denaturing, 60°C for 45 s for annealling, and 72°C for 1 min for elongation. The PCR product was analysed using 1.5% agarose gel electrophoresis. A gel imaging system was used to scan the agarose gel.
Western blot
Cells were collected and washed twice with PBS, and 2 mL lysate buffer (50 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 100 mM sodium vanadate, 1 mM PMSF, 10 mg/mL aprotinin, 10 mg/mL leupeptin, and 1% NP-40; 5 mM cocktail; pH 7.4) was added to lyse the cells. The cell lysate was then centrifuged at 4°C and 25,000 rpm for 30 min, and the supernatant was collected. The protein concentration was determined using the bicinchoninic acid assay (BCA). The protein sample was dyed with bromophenol blue, and the same amount of protein was loaded into each well of a 10% SDS-PAGE gel. The separated protein on the gel was transferred to a polyvinylidenedifluoride (PVDF) membrane using the semi-dry transfer method. The membrane was blocked in 5% skim milk overnight. On the next day, the membrane was washed with tris-buffered saline and tween 20 (TBST) and then incubated with rabbit anti human monoclonal antibodies at 37°C for 1 h (anti-ERα and anti-ERβ:USA, Bioworld; anti-Ras: USA, Proteintech Group; anti-MEK1/2 and anti-p-ERK1/2: USA, Bioworld). The antibodies were diluted 1:500. The membrane was then washed with TBST and incubated with a horseradish peroxidase-conjugated goat anti-rabbit IgG secondary polyclonal antibody at 37°C for 1 h (Beijing, Zhongshan Golden Bridge Biotechnology Co., Ltd.). The signal was developed by adding chemiluminescence reagents, followed by exposure on X-ray film. The image was scanned and analysed by densitometry.
Statistical analysis
The data were analysed using the SPSS 16.0 statistical analysis software. Variance analysis was performed on 3 groups of data. The chi-square test was performed on the categorical data. Numeric data are presented as the mean ± standard deviation. p < 0.05 represents a statistically significant difference.
Author Contributions
X.Z. designed and carried out all the experiments and drafted the manuscript. Y.Z. participated in the research design and implementation of the study and interpreted data. L.J., J.W.L. and C.Z.L. constructed resistant cell lines and performed sensitivity analysis. Z.H.W. performed immunocytochemistry experiments and cell resistance analysis. W.D. cultured cell lines and performed RT-PCR. S.F. and M.M.L. performed western bolt and contributed to drafting the manuscript. B.S. helped writing of the manuscript. B.Z. and B.T. designed the study and interpreted the data and critically revised the manuscript. All authors read and approved the final manuscript for publication.
Supplementary Material
supplementary information
Acknowledgments
This work was supported by the Shandong Provincial Natural Science Foundation (Project NoZR2013HL048 and ZR2011HQ021), Natural Science Foundation of Shandong Academy of Medical Sciences(Project No 2013-35), Science Foundation of The First Affiliated Hospital of Dalian Medical University (2014QN004).
References
- Johnston S. R. Acquired tamoxifen resistance in human breast cancer-potential mechanisms and clinical implications. Anticancer Drugs 8, 911–930 (1997). [DOI] [PubMed] [Google Scholar]
- Smith R. A., Cokkinides V. & Brooks D. Cancer screening in the United States, 2011: A review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 61, 8–30 (2011). [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trailist Collaborative Group. Tamoxifen for early breast cancer:an overview of the randomized trials. Lancet 351, 1451–1467 (1998). [PubMed] [Google Scholar]
- Creighton C. J. et al. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. Cancer Res 8, 7493–7501 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. M., Clark G. M., Osborne C. K. & Allred D. C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J ClinOncol 17, 1474–1481 (1999). [DOI] [PubMed] [Google Scholar]
- Bayliss J., Hilger A., Vishnu P., Diehl K. & El-Ashry D. Reversal of the estrogen receptor negative phenotype in breast cancer and restoration of antiestrogen response. Clin Cancer Res 13, 7029–7036 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang B., Zhang X., Tang B., Zheng P. & Zhang Y. Investigation of elemene-induced reversal of tamoxifen resistance in MCF-7 cells through oestrogen receptor α (ERα) re-expression. Breast Cancer Res Treat 136, 399–406 (2012). [DOI] [PubMed] [Google Scholar]
- Janmaat M. L., Rodriguez J. A., Gallegos-Ruiz M., Kruyt F. A. & Giaccone G. Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. Int J Cancer 118, 209–214 (2006). [DOI] [PubMed] [Google Scholar]
- Huang Y. S., Huang B. & Wu Y. L. Manifestation of leukoencephalopathy in a patient with advanced non-small cell lung cancer following treatment with gefitinib. Chin Med J (Engl) 124, 3834–3837 (2011). [PubMed] [Google Scholar]
- Park H. S. et al. Gefitinib-induced pneumonitis in non-small cell lung cancer: radiological and clinical findings in five patients. Clin Imaging 31, 306–312 (2007). [DOI] [PubMed] [Google Scholar]
- Shou J. et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. Natl Cancer Inst 96, 926–935 (2004). [DOI] [PubMed] [Google Scholar]
- Osborne C. K. et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res 17, 1147–1159 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton C. J. et al. Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res 66, 3903–3911 (2006). [DOI] [PubMed] [Google Scholar]
- Oh A. S. et al. Hyperactivation of MAPK induces loss of ERalpha expression in breast cancer cells. MolEndocrinol 15, 1344–1359 (2011). [DOI] [PubMed] [Google Scholar]
- Bayliss J., Hilger A., Vishnu P., Diehl K. & El-Ashry D. Reversal of the estrogen receptor negative phenotype in breast cancer and restoration of antiestrogen response. Clin Cancer Res 13, 7029–7036 (2007). [DOI] [PubMed] [Google Scholar]
- Mirza S. et al. Clinical significance of Stratifin, ERalpha and PR promoter methylation in tumor and serumdNA in Indian breast cancer patients. ClinBiochem 43, 380–386 (2010). [DOI] [PubMed] [Google Scholar]
- Fu Z. Y., Han J. X. & Zhang H. Y. Effect s of emodin on gene expression profile in small cell lung cancer NCI-H446 cell. Chin Med J 120, 1710–1715 (2007). [PubMed] [Google Scholar]
- Clark A. S., West K., Streicher S. & Dennis P. A. Constitutive and inducible Akt activity promotes resistance to chemotherapy,trastuzumab,or tamoxifen in breast cancer cells. Mol Cancer Ther 1, 707–717 (2002). [PubMed] [Google Scholar]
- Migliaccio A. et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J 15, 1292–1300 (1996). [PMC free article] [PubMed] [Google Scholar]
- Krueger J. S., Keshamouni V. G., Atanaskova N. & Reddy K. B. Temporal and quantitative regulation of mitogen-activated protein kinase (MAPK) modulates cell motility and invasion. Oncogene 20, 4209–4218 (2001). [DOI] [PubMed] [Google Scholar]
- Jelovac D. et al. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res 65, 5380–5389 (2005). [DOI] [PubMed] [Google Scholar]
- Martin L. A., Farmer I., Johnston S. R., Ali S. & Dowsett M. Elevated ERK1/ERK2/estrogen receptor cross-talk enhances estrogen-mediated signaling during long-term estrogen deprivation. EndocrRelat Cancer 12, S75–84 (2005). [DOI] [PubMed] [Google Scholar]
- Allred D. C., Mohsin S. K. & Fuqua S. A. Histological and biological evolution of human premalignant breast disease. EndocrRelat Cancer 81, 47–61 (2001). [DOI] [PubMed] [Google Scholar]
- Buzdar A. U. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the international letrozole breast cancer group. J ClinOncol 22, 3199–3200 (2004). [DOI] [PubMed] [Google Scholar]
- Normanno N. et al. The MEK/MAPK pathway is involved in the resistance of breast cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J Cell Physiol 207, 420–427 (2006). [DOI] [PubMed] [Google Scholar]
- Matsuo M., Sakurai H., Ueno Y., Ohtani O. & Saiki I. Activation of MEK/ERK and PI3K/Akt pathways by fibronectin requires integrin alphav-mediated ADAM activity in hepatocellular carcinoma: a novel functional target for gefitinib. Cancer Sci 97, 155–162 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao K. et al. Quantitative analysis of estrogen receptor-alpha and -beta messenger RNA expression in breast carcinoma by real-time polymerase chain reaction. Cancer 89, 1732–1738 (2000). [DOI] [PubMed] [Google Scholar]
- Gruvberger-Saal S. K. et al. Estrogen Receptor {beta} Expression Is Associated with Tamoxifen Response in ER{alpha}-Negative Breast Carcinoma. Clin Cancer Res 13, 1987–1994 (2007). [DOI] [PubMed] [Google Scholar]
- Paech K. et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277, 1508–1510 (1997). [DOI] [PubMed] [Google Scholar]
- Hodges L. C. et al. Tamoxifen functions as a molecularagonist inducing cell cycle-associated genes in breast cancer cells. Mol Cancer Res 1, 300–311 (2003). [PubMed] [Google Scholar]
- Ström A. et al. Estrogen receptor β inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. ProcNatlAcadSci 101, 1566–1571 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgquist S. et al. Oestrogen receptors alpha and beta show different associations to clinicopathological parameters and their co-expression might predict a better response to endocrine treatment in breast cancer. J ClinPathol 61, 197–203 (2008). [DOI] [PubMed] [Google Scholar]
- Hopp T. A. et al. Low levels of estrogen receptor beta protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res 10, 7490–7499 (2004). [DOI] [PubMed] [Google Scholar]
- Lapidus R. G. et al. Mapping of ER gene CpG island methylation-specific polymerase chain reaction. Cancer Res 58, 2515–2519 (1998). [PubMed] [Google Scholar]
- Ottaviano Y. L. et al. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res 54, 2552–2555 (1994). [PubMed] [Google Scholar]
- Zhao L. et al. Silencing of estrogen receptor alpha(ERalpha) gene by promoter hypermethylation is a frequent event in Chinese women with sporadic breast cancer. Breast Cancer Res Treat 117, 253−259 (2009). [DOI] [PubMed] [Google Scholar]
- Kawai H., Li H., Avraham S., Jiang S. & Avraham H. K. Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor alpha. Int J Cancer 107, 353–358 (2003). [DOI] [PubMed] [Google Scholar]
- Fan J. et al. ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res ClinOncol 134, 883–890 (2008). [DOI] [PubMed] [Google Scholar]
- Villanueva C. et al. Phase II study assessing lapatinib added to letrozole in patients with progressive disease under aromatase inhibitor in metastatic breast cancer-Study BES 06. Target Oncol 8, 137–143 (2013). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Everolimus in combination with letrozole inhibit human breast cancer MCF-7/Aro stem cells via PI3K/mTOR pathway: an experimental study. Tumour Biol 35, 1275–1286 (2014). [DOI] [PubMed] [Google Scholar]
- Santen R. J., Song R. X., Zhang Z., Yue W. & Kumar R. Adaptive hypersensitivity to estrogen: mechanism for sequential responses to hormonal therapy in breast cancer. Clin Cancer Res 10, 337–345 (2004). [DOI] [PubMed] [Google Scholar]
- Pan G. D. et al. Reversal of multi-drug resistance by pSUPER-shRNA-mdr1 in vivo and in vitro. World J Gastroenterol 5, 431–440 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. P. et al. Expression of estrogen receptors alfa and beta mRNA and alkaline phosphatase in the differentiation of osteoblasts from elderly postmenopausal women: comparison with osteoblasts from osteosarcoma cell lines. Taiwan J ObstetGynecol 45, 307–312 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary information