Abstract
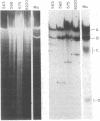
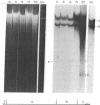
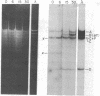
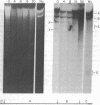
We have compared the process of prophage lambda induction with that of prophage Mu. According to the Campbell model, rescue of lambda DNA from the host DNA involves reversal of lambda integration such that the prophage DNA is excised from the host chromosome. We have monitored this event by locating the prophage DNA with a technique in which DNA of the lysogenic cells is cleaved with a restriction endonuclease and fractionated in agarose gels. The DNA fragments are denatured in gels, transferred to a nitrocellulose paper, and hybridized with 32P-labeled mature phage DNA. The fragments containing prophage DNA become visible after autoradiography. Upon prophage lambda induction, the phage-host junction fragments disappear and the fragment containing the lambda att site appears. No such excision is seen in prophage Mu. The Mu-host junction fragments remain intact well into the lytic cycle, when Mu DNA has undergone many rounds of replication and apparently many copies of Mu DNA have been integrated into the host DNA. Therefore, we postulate that Mu DNA replicates in situ and the replication generates a form of Mu DNA active in the integrative recombination between Mu DNA and host DNA. This type of mechanism may be common to many transposable elements.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Allet B. Plaque-forming lambda-Mu hybrids. Virology. 1975 Jan;63(1):30–39. doi: 10.1016/0042-6822(75)90367-0. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I. Bacteriophage mu as a transposition element. Annu Rev Genet. 1976;10:389–412. doi: 10.1146/annurev.ge.10.120176.002133. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Froshauer S., Botchan M. Ends of bacteriophage mu DNA. Nature. 1976 Dec 9;264(5586):580–583. doi: 10.1038/264580a0. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I. Reversal of mutator phage Mu integration. J Mol Biol. 1975 Jul 25;96(1):87–99. doi: 10.1016/0022-2836(75)90183-7. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Taylor A. L. Influence of insertions on packaging of host sequences covalently linked to bacteriophage Mu DNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4399–4403. doi: 10.1073/pnas.72.11.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. M. How viruses insert their DNA into the DNA of the host cell. Sci Am. 1976 Dec;235(6):102-9, 111-3. doi: 10.1038/scientificamerican1276-102. [DOI] [PubMed] [Google Scholar]
- Daniell E., Roberts R., Abelson J. Mutations in the lactose operon caused by bacteriophage Mu. J Mol Biol. 1972 Aug 14;69(1):1–8. doi: 10.1016/0022-2836(72)90019-8. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Kirschner I., Goldstein R., Baran N. Physical study of prophage excision and curing of lambda prophage from lysogenic Escherichia coli. J Mol Biol. 1973 Mar 15;74(4):703–720. doi: 10.1016/0022-2836(73)90058-2. [DOI] [PubMed] [Google Scholar]
- Howe M. M., Bade E. G. Molecular biology of bacteriophage mu. Science. 1975 Nov 14;190(4215):624–632. doi: 10.1126/science.1103291. [DOI] [PubMed] [Google Scholar]
- Pirrotta V. Two restriction endonucleases from Bacillus globiggi. Nucleic Acids Res. 1976 Jul;3(7):1747–1760. doi: 10.1093/nar/3.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaki T., Bukhari A. I. Events following prophage Mu induction. J Bacteriol. 1975 May;122(2):437–442. doi: 10.1128/jb.122.2.437-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wijffelman C., Gassler M., Stevens W. F., van de Putte P. On the control of transcription of bacteriophage Mu. Mol Gen Genet. 1974;131(2):85–96. doi: 10.1007/BF00266145. [DOI] [PubMed] [Google Scholar]