Abstract
Studies using the Morris water maze to assess hippocampal function in animals, in which adult hippocampal neurogenesis had been suppressed, have yielded seemingly contradictory results. Cyclin D2 knockout (Ccnd2−/−) mice, for example, have constitutively suppressed adult hippocampal neurogenesis but had no overt phenotype in the water maze. In other paradigms, however, ablation of adult neurogenesis was associated with specific deficits in the water maze. Therefore, we hypothesized that the neurogenesis-related phenotype might also become detectable in Ccnd2−/− mice, if we used the exact setup and protocol that in our previous study had revealed deficits in mice with suppressed adult neurogenesis. Ccnd2−/− mice indeed learned the task and developed a normal preference for the goal quadrant, but were significantly less precise for the exact goal position and were slower in acquiring efficient and spatially more precise search strategies. Upon goal reversal (when the hidden platform was moved to a new position) Ccnd2−/− mice showed increased perseverance at the former platform location, implying that they were less flexible in updating the previously learned information. Both with respect to adult neurogenesis and behavioral performance, Ccnd2+/− mice ranged between wild types and knockouts. Importantly, hippocampus-dependent learning was not generally impaired by the mutation, but specifically functional aspects relying on precise and flexible encoding were affected. Whether ablation of adult neurogenesis causes a specific behavioral phenotype thus also depends on the actual task demands. The test parameters appear to be important variables influencing whether a task can pick up a contribution of adult neurogenesis to test performance.
Keywords: Adult neurogenesis, cognitive flexibility, dentate gyrus, Morris water maze, pattern separation, plasticity, proliferation, spatial learning, stem cells, strategies
In line with theoretical considerations suggesting different roles for adult neurogenesis in improving hippocampal function (Aimone et al. 2006, 2009; Appleby et al. 2011; Becker 2005; Wiskott et al. 2006), studies testing mice with substantially reduced numbers of new neurons in spatial learning tasks did seem to reveal a consistent behavioral phenotype. The phenotypes reported so far related to memory acquisition, memory retention and reversal learning (Dupret et al. 2008; Garthe et al. 2009; Jessberger et al. 2009; Martinez-Canabal et al. 2013; Snyder et al. 2005; Winocur et al. 2006; Zhang et al. 2008).
The observed discrepancies were often perceived as contradictions but in fact might primarily reflect a differential sensitivity of the tests and the test protocols for the different contributions of the new neurons to ‘function’. For the water maze task that has been used in many of these experiments, we have proposed that differences in the setup and protocols might be responsible for the seemingly contradictory results (Garthe et al. 2009). We have argued that the contribution of the new neurons might only become relevant and thus detectable when the actual task demands sufficiently match the functional implication of adult neurogenesis.
A model proposed by Wiskott et al. (2006) had suggested that adult neurogenesis improves the storage of multiple, highly similar and thus potentially overlapping memories as an example of ‘pattern separation’, a key functionality of the dentate gyrus. The model predicted that a lack of new neurons would result in catastrophic interference of highly similar information stored in CA3 and thus would impair spatial learning. We could confirm that prediction experimentally in the water maze paradigm by including a goal reversal, where a new target position has to be learned within the same general spatial context (Garthe et al. 2009). The relevance of adult neurogenesis for ‘pattern separation’ has also been confirmed using behavioral paradigms based on models by Aimone et al. (2006, 2009) that explicitly ask for an efficient discrimination of similar visual contexts (Clelland et al. 2009). We have also seen that in a panel of related strains of mice adult neurogenesis levels correlated with task acquisition but not with probe trial performance (Kempermann & Gage 2002). Taken together, there is good theoretical and experimental evidence in support of a role of adult neurogenesis in task acquisition during spatial learning.
As another phenotype presumably acting on a longer time scale, several studies have revealed significantly impaired retention in spatial learning tasks after ablation of adult neurogenesis by assessing spatial performance 3–4 weeks after acquisition. Although impaired retention appears to be the most robust and consistently reproducible neurogenesis-related phenotype in the water maze task (Fan et al. 2007; Raber et al. 2004; Snyder et al. 2005), it is still not clear whether retention relies on adult neurogenesis directly or whether it is rather indirectly facilitated by the increased integration of episodes or an optimized orthogonalization of memories in CA3 due to the new neurons.
Nevertheless, although the observed phenotypes were backed by theoretical models, the results of many studies addressing the functional relevance of adult neurogenesis not always appeared to be consistent (Deng et al. 2010). Specifically, the acquisition and reversal phenotypes were found only in some studies, whereas others failed to show a significant impairment in this respect (Garthe & Kempermann 2013).
An important caveat has been the question in how far the manipulations that were used to suppress or ablate adult neurogenesis were selective (Dupret et al. 2007; Garthe et al. 2009; Saxe et al. 2006; Snyder et al. 2005). But among the numerous models of suppressed adult neurogenesis, the cyclin D2 knockout (Ccnd2−/−) mice have stood out because of their unusual specificity in the absence of complex genetic constructs. The reason for this specificity lies in the fact that the cyclins as key regulatory factors of the cell cycle are usually redundant in precursor cells. At least in mice, however, this does not seem to be the case in the dentate gyrus (Kowalczyk et al. 2004). Albeit adult neurogenesis is essentially absent in Ccnd2−/− mice, a very low amount of residual neurogenesis persists in the mutants, indicating that the regulatory elements and microenvironmental conditions for neurogenesis are still in place and functional (Jaholkowski et al. 2009; Jedynak et al. 2012). Surprisingly, however, in a wide range of spatial and other learning tasks, no consistent reduction in learning performance was seen in the Ccnd2−/− mice. The mutants showed changes in some hippocampus-dependent behaviors but apparently normal learning (Jaholkowski et al. 2009; Jedynak et al. 2012; Urbach et al. 2013). Overall activity and exploratory behavior were increased but water maze performance, as the key example of a classical hippocampus-dependent learning task, seemed unimpaired (Jaholkowski et al. 2009). Interestingly, species-specific behaviors such as marble burying and nest building were on the other hand impaired in Ccnd2−/− mice (Jedynak et al. 2012). In another study, however, Ccnd2−/− mice did also show impaired retention in the presence of normal task acquisition and it was thus suggested that this phenotype could represent a common denominator for acute or genetically reduced adult neurogenesis (Ben Abdallah et al. 2013). According to our own hypothesis and previously obtained results, adult neurogenesis should in addition be instrumental for reversal learning, the flexible integration of novel information into previously learned contexts.
We therefore asked whether we could detect the apparently less robust reversal phenotype in the Ccnd2−/− mice if we applied the specific setup, protocol and analysis employed before (Garthe et al. 2009). We assessed the extent to which the mice relied on spatially precise and thus more efficient search strategies to locate the hidden platform.
Materials and methods
Animals
Experiments were done on Ccnd2−/− mice (Sicinski et al. 1996) after rederivation by embryo transfer and backcrossing into the C57BL/6J background for more than six generations. Embryos were taken from the Ccnd2 colony at the Nencki Institute, Warsaw. Rederived mice were group housed at the animal facility of the Medical Faculty of Technische Universität Dresden, Germany, under a natural light/dark cycle of 12 h each. All applicable local and federal regulations of animal welfare in research were followed. The experiments were approved by the responsible authority, Landesdirektion Dresden.
For all experiments, 6- to 8-week-old female mice with 10 animals per group (total of 30) were used. Wild-type littermates were used as controls. During the experiments, the experimenter was blinded for the genotypes.
We used female mice to remain consistent with our many previous studies based on the environmental enrichment and exercise paradigms that had to be carried out in females in order to avoid confounding stressful consequences of territorial behavior that is more prominent in male mice than in females (Kempermann & Gage 1999; Kempermann et al. 1997, 2002).
Tissue preparation
On three consecutive days before perfusion, animals received daily single injections of 5-bromo-2′-deoxyuridine (BrdU; 50 mg/kg body weight in sterile 0.9% saline; Sigma-Aldrich, Hamburg, Germany). Under deep anesthesia with ketamine (50 mg/kg body weight; Sigma), animals were perfused transcardially with 4% paraformaldehyde (PFA) in 0.1 m phosphate buffer, pH 7.4. After having been removed from the skulls, postfixed in 4% PFA overnight and transferred into a 30% sucrose solution in 0.1 m phosphate buffer (pH 7.4), brains were cut into 40 µm-thick coronal sections on a dry-ice-cooled copper block on a sliding microtome (SM 200R; Leica, Bensheim, Germany) and cryoprotected.
Immunohistochemistry
To detect dividing cells, the proliferation marker, BrdU, visualized with the diaminobenzidine tetrahydrochloride (DAB)-peroxidase method (ABC, Vectastain Elite; Vector Laboratories, Burlingame, CA, USA) was used. Pretreatment for BrdU staining was done with 2 n hydrochloric acid for 30 min in order to denature DNA. Monoclonal rat anti-BrdU (1:500; Biozol, Eching, Germany) was used as primary antibody and biotinylated donkey anti-rat (1:500; Dianova, Hamburg, Germany) as secondary antibody. For further details of the BrdU labeling and detection procedure see (Steiner et al. 2008). Sections were mounted on slides and coverslipped with Neomount.
Quantification
Our quantification protocol of cells labeled with the DAB-peroxidase method, e.g. BrdU-positive cells in this study, has been described elsewhere (Kempermann et al. 2003) and has been used in numerous studies thereafter. Sections of 40-µm thickness were counted in one-in-six series through a light microscope (× 40 objective; Leica) and the numbers multiplied by 6 to assess the total number of cells per dentate gyrus (subgranular zone and granule cell layer).
Morris water maze task
As described elsewhere (Garthe et al. 2009), mice were trained in the reference memory version of the Morris water maze task (Morris 1984) to locate a hidden escape platform in a circular pool (200 cm in diameter). Testing was done during the light-on phase. The water was made opaque with non-toxic white pigment and kept at a temperature of 19–20°C. Each mouse was given six trials per day for five consecutive days with an intertrial interval (ITI) of 30 min. Mice were released from one of four possible starting points and allowed to search up to 120 seconds for the platform. During each day the starting position remained constant. Irrespective of trial performance mice were guided to the platform and allowed to remain there for at least 15 seconds. The platform position was changed after day 3 (Fig. 2a). A probe trial lasting 60 seconds without a platform was performed before the first relearning trial on day 4. Swim paths were recorded using Ethovision, Version XT 7 (Noldus, Wageningen, the Netherlands).
Figure 2.
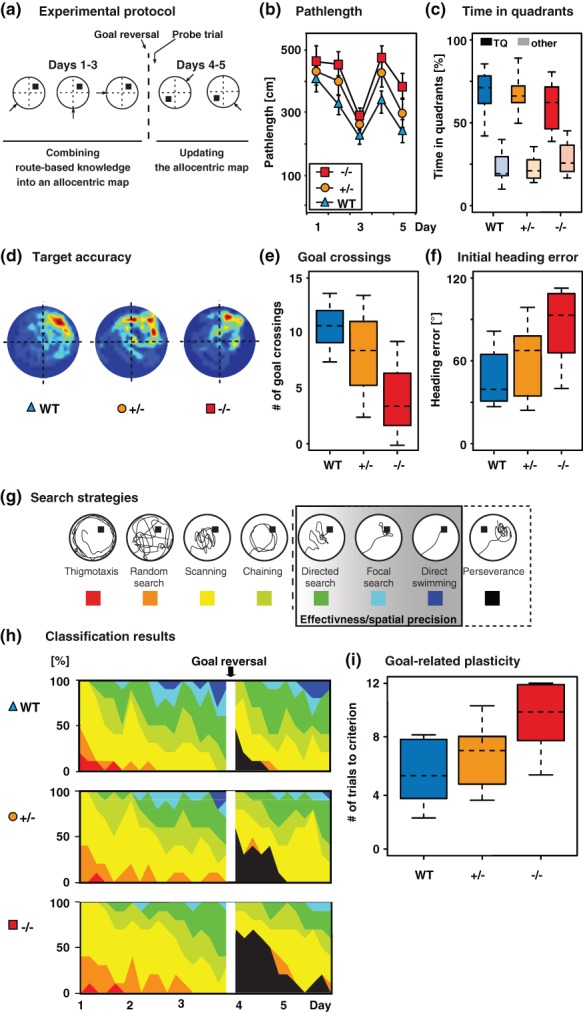
Differences in spatial learning abilities of Ccnd2−/−, Ccnd2+/− and wild-type mice in the water maze. (a) Experimental protocol including a first acquisition followed by another acquisition after moving the platform to the opposite quadrant. (b) Path length to reach the hidden platform. Shown are means ± standard deviations. (c) Probe trial performance indicated by time spent in the target quadrant (TQ, intense colors) vs. other quadrants combined (faint colors). (d) Target accuracy in probe trial indicated by heat maps. Dark red zones represent a sixfold presence probability. (e) Precise encoding of goal position indicated by number of crossings of former goal positions. (f) Precision of initial goal approaches indicated by the average initial heading error. (g) Examples of search strategies used for algorithm-based strategy classification. (h) Contribution of respective search strategies to group performance. Color code as indicated in (g). (i) Reversal learning performance indicated by trials needed to regain the average path length reached before goal reversal.
Path lengths, time spent in quadrants and number of goal crossings were analyzed using the in-built features of the Ethovision software. Further analyses were done based on the raw time-tagged xy-coordinates using Matlab, Version 2012b (The Mathworks, Ismaning, Germany). To obtain the initial heading error (Woolley et al. 2010), we calculated the average directional aberration until the cumulative distance covered had equaled the length of a direct connection between starting and goal positions.
Although decreasing latencies and path lengths are reliable indicators of successful learning, spatial learning that does not ultimately lead to reaching the platform cannot be detected using these parameters. Earlier phases of acquisition are characterized by search patterns that show a clear directional preference and thus the formation of a cognitive map but do not necessarily allow an animal to find the goal within a short time.
Analyzing the search strategies is based on two numeric parameters for each strategy that reflect the unique abstract properties of a given search pattern. Search strategies were classified according to parameters and an algorithm described in our previous study (Garthe et al. 2009), originally based on Balschun et al. (2003). Strategies were defined by no more than two parameters that are not dependent on pool dimensions.
During acquisition, mice show a sequential use of different swim patterns ranging from initially almost undirected to spatially precise, efficient search strategies. In the first trials undirected swim patterns covering most of the pool surface predominate, whereas the search strategies become directed and spatially confined for later stages. Accordingly, classification of thigmotaxis, random swimming and scanning is done by assorting each data point to different zones that represent different regions of the pool and by calculating the surface coverage of each path. Common to these three strategies is that any directional preference toward the goal position is absent. ‘Chaining’ is proposed to be a successor of scanning. Here, the animals show a clear preference for the correct distance of the expected goal to the wall. It is still not clear whether chaining involves the hippocampus. Technically, chaining behavior is identified by calculating the amount of time spent in the annulus zone at the right distance from the wall.
That the animals develop a directional preference for the goal position from different starting points suggests a contribution of a cognitive map. This point is marked by the ‘directed search’ strategy where the search becomes directional restricted in a triangular fashion pointing from the starting position toward the actual goal. The algorithm calculates the number of data points lying outside the triangular goal corridor. Although path lengths swum may be initially not significantly shorter than for the scanning or chaining, directed search is usually the beginning of a subsequent refinement in the efficiency of the search patterns applied to reach the goal platform from any possible start position. Focal search is characterized by an agglomeration of data points close to the hidden goal position that is measured by the distance of the path's centroid to the goal and an overall short average distance of data points to that position. A classification as direct swimming expects a small directional aberration of the path between two subsequent data points from the actual optimal course to the goal averaged for the full path.
To assess goal-related plasticity, we counted the number of trials needed by an animal to regain a path length that was smaller or at least equal to the averaged path length reached by that animal on day 3 at the end of the first acquisition.
Statistics
For testing the effects of the respective genotypes on BrdU cell counts, number of goal crossings, initial heading errors and trials to criterion following reversal, we applied a one-way between-subjects analysis of variance (anova) with genotype as the main factor. Effects on path length and spatial strategy use were assessed using repeated measurements anova with day as a second main factor. Testing of main and interaction effects was done using F-statistics. Tukey's HSD was used for post hoc testing. Significance level was set to 0.05. Statistical analyses were done using SPSS, Version 21 (IBM Germany, Ehningen, Germany) and Matlab, Version 2012b (The Mathworks).
Results
Decreased precursor cell proliferation in Ccnd2−/− mice
We first confirmed that the Ccnd2−/− mice available to us had indeed the adult neurogenesis phenotype described by Jaholkowski et al. (2009). In addition, we also investigated the heterozygotes, which had not been included in the previous study.
Using anova, we found a significant effect of genotype on BrdU cell counts (F2,27 = 163.7, P < 0.001). Post hoc analyses revealed that both knockouts (M = 127.4, SD = 38.2) and heterozygous Ccnd2+/− (M = 492.7, SD = 137.1) mice showed significantly reduced levels of precursor cell proliferation in the dentate gyrus compared with wild-type controls (M = 987.62, SD = 492.12, Fig. 1). Thus, both Ccnd2−/− and Ccnd2+/− mice had strongly reduced but not absent adult hippocampal neurogenesis compared with wild types. As a new observation, heterozygotes ranged in between knockouts and wild types, suggesting a gene dosage effect.
Figure 1.
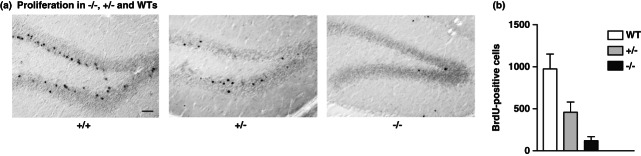
Quantitative assessment of proliferating cells (BrdU-labeled) in wild-type (Ccnd2+/+) mice, Ccnd2+/− and Ccnd2−/−animals. (a) The images show BrdU-stained hippocampal sections showing substantially decreased proliferation in the knockouts compared with wild-type controls, as well as between Ccnd2+/− and wild types. Scale bar is 100 µm. (b) Compared with wild types, BrdU-labeled cells were immensely decreased in both Ccnd2−/− (P < 0.001) and Ccnd2+/− mice (P = 0.022).
Ccnd2−/− mice successfully learned to find the hidden platform but showed deficits in specific aspects of the task in the water maze
Adult-generated hippocampal neurons have been implicated to be essential for forming an allocentric cognitive map of the test environment, resulting in increased adaptability and effective spatial navigation in the Morris water maze task (Dupret et al. 2007; Kempermann 2012). To address spatial learning in Ccnd2−/− and Ccnd2+/− mice along these lines of argument, we trained our mice on the hidden platform version of the water maze task and used a protocol consisting of 3 days of intensive training with six trials per day, each lasting a maximum of 120 seconds, unless the animal had found the hidden escape platform before. The platform location remained constant for these 3 days (in the northeastern quadrant), whereas the starting position was changed every day. On day 4, a probe trial was performed and the platform was then moved to the opposite southwestern quadrant (‘reversal’), where it also remained on day 5.
For the first acquisition period (days 1–3) the genotype had no significant effect on swim path lengths (repeated measurements anova: F2,18 = 0.738, P = 0.121, Fig. 2b), whereas the factor day did so (F2,18 = 12.47, P < 0.001). Post hoc comparisons revealed consistently shorter path lengths on day 3 compared with day 1, indicating a successful general task acquisition in all three groups. There was no interaction between genotype and day.
Consistently, in the probe trial all groups showed a clear preference for the correct goal quadrant and there was no significant effect of genotype on time spent in that quadrant (anova: F2,27 = 0.884, P = 0.316, Fig. 2c,d). Thus, Ccnd2−/− and Ccnd2+/− mice did acquire a spatial preference that allowed them to locate the hidden platform effectively.
While searching in the right direction of the hidden platform can already improve water maze performance, a cognitive map allows locating and approaching the goal location specifically and from arbitrary positions. As indication of the presence of such a map we measured the number of exact goal crossings during the probe trial and found a significant effect of the genotype (F2,27 = 9.27, P = 0.003, Fig. 2e). Post hoc analyses revealed that compared with wild-type mice (M = 10.83, SD = 6.34), knockouts showed a significantly lower number of goal crossings (M = 3.16, SD = 10.87). In turn, heterozygous Ccnd2+/− mice did not differ from controls in the number of goal.
After having been introduced to the pool at the start of each trial, the mice have to orient themselves in the environment and recognize their own position within that environment. Based on their previously acquired knowledge (and presumably the existence of the cognitive map that has been acquired) they have to decide how to reach the hidden goal in the most direct way. Therefore, the animal's heading direction and the straightness of the swim path approaching the goal the first time in a given trial are considered to be indicative of whether a cognitive map is available and is used. In contrast to an average heading direction (a commonly assessed parameter in the water maze), the initial heading direction (or initial heading error) considers the swim path only until the cumulative distance had equaled the direct start–goal connection (Woolley et al. 2010). Good learners possessing a full and specific cognitive map show initial goal approaches that bring the animals close to the hidden platform.
We found a significant effect of the genotype on the initial heading error (F2,27 = 7.78, P = 0.014, Fig. 2f). Compared with wild types (M = 39.24, SD = 33.19), the initial goal approaches of Ccnd2−/− mice had a significantly larger aberration from the straight start–goal connection (M = 94.56, SD = 70.97), whereas the heterozygotes did not show differences from wild types.
Mice can locate the hidden platform using different behavioral strategies that are indicative of the underlying cognitive processes and brain structures involved (Balschun et al. 2003; Garthe et al. 2009; Ruediger et al. 2012). Because the availability of a cognitive map determines the behavioral strategies that can be applied, we also analyzed the search patterns shown by the mice to locate the hidden goal during task acquisition (days 1–3). Specifically, we compared the ratio of spatially directed, effective search patterns to less directed patterns and thus less effective strategies shown by the Ccnd2−/− and Ccnd2+/− animals on each day of the first acquisition period with the ratios shown by control mice (Fig. 2g).
Repeated measurements anova revealed a significant effect of the genotype on the use of spatially directed search strategies (F2,18 = 2.48, P = 0.032, Fig. 2h). Specifically, while control mice efficiently proceeded toward a predominant use of search patterns clearly directed toward the hidden goal and thus suggesting the use of an underlying spatial representation (M = 1.3, SD = 0.67), Ccnd2−/− mice relied more on less effective strategies without a clear directional preference (M = 0.82, SD =0.65). Heterozygous Ccnd2+/− mice showed no difference regarding the use of directed and efficient search strategies.
We also found an effect of day on the use of directed search strategies (F2,18 = 15.73, P < 0.001). As for path length, post hoc comparisons revealed consistently higher directed-to-less directed ratios on day 3 compared with day 1. Importantly, while knockout mice successfully proceeded toward using the more efficient strategies and thus did not generally fail to show directed search patterns, this progression appeared to be delayed in those mice. In contrast to controls, the most direct and spatially precise swim paths, however, were not found with the knockouts at all.
Our analyses also revealed a significant interaction effect of genotype and day on the use of directed search strategies (F4,36 = 4.92, P = 0.014), indicating that the mutation affects only specific aspects of water maze acquisition.
Because previous studies had reported that mice with suppressed adult neurogenesis were impaired in relearning a new goal position after platform reversal (Garthe et al. 2009; Zhang et al. 2008), we measured how many trials it took the mice after goal reversal to regain an average path length that was equal or shorter than the one reached during the first acquisition period.
Again, there was a significant effect of the genotype (F2,18 = 3.04, P = 0.022, Fig. 2i). Compared with controls (M = 5.42, SD = 5.84) Ccnd2−/− mice needed significantly longer times to regain their initial acquisition performance (M = 9.75, SD = 6.06), indicating an impaired updating of a previously formed cognitive map and thus reduced goal-related plasticity. No difference was found for Ccnd2+/− mice compared with wild types.
Taken together, Ccnd2−/− mice learned to find the hidden goal and developed a clear preference for the correct quadrant. Apparent deficits were found in a lower number of goal crossings, a less frequent use of search strategies with a clear directional and spatially specific preference for the hidden platform and an impaired goal-related plasticity after platform reversal. Ccnd2+/− mice tended to show phenotypes between wild types and Ccnd2−/− mice, although conventional statistical significance was not always reached.
Discussion
In this study, we had asked whether Ccnd2−/− mice would show learning deficits related to task acquisition and reversal performance in the water maze as we had found in our previous study using a different method to ablate adult neurogenesis. Consistent with those findings we here show that this was indeed the case.
Mice with suppressed adult neurogenesis owing to their lack of cyclin D2 were not generally impaired in learning the water maze task. After first task acquisition, knockout mice reached normal performance levels as measured by path length and time spent in the correct goal quadrant during the probe trial (Fig. 2b,c). The lack of new neurons also did not prevent Ccnd2−/− mice from using directed and efficient search strategies that directly reflect successful spatial learning (Fig. 2h).
However, we did find a small impairment in aspects of task acquisition and clearly reduced reversal performance, the two neurogenesis-related phenotypes that we had observed in our previous study. Although Ccnd2−/− mice showed a clear learning curve during first acquisition and path lengths did not differ statistically, we saw a highly suggestive trend toward longer path lengths in the knockouts (P = 0.064). Mice lacking new neurons failed to cross the exact goal position as often as controls, and their initial goal approaches were less precise (Fig. 2e,f). Presumably, the ability to locate the hidden goal from an arbitrary starting position and with a direct swim path over a relatively long distance without major correction turns depends on the presence of a sufficiently precise cognitive map (Save & Poucet 2000). In line with this, Ccnd2−/− mice – like mice treated with chemically ablated adult neurogenesis – showed a delayed progression toward the use of directed and thus more efficient search strategies that – especially in a context of varying starting positions – rely on a cognitive map presumed to be located in the hippocampus (Fig. 2h; Garthe & Kempermann 2013). Quite generally, animals with hippocampal lesions will fail to proceed toward direct and efficient search strategies (Eichenbaum et al. 1990). After platform reversal, we observed a clearly reduced goal-related plasticity representing the second phenotype previously found to be related to a lack of new neurons (Garthe et al. 2009; Zhang et al. 2008). Heterozygous Ccnd2+/− mice consistently showed an intermediate phenotype.
Thus, learning the task per se does not seem to be affected by the mutation. Adult neurogenesis appears to be relevant only for specific aspects of water maze performance rather than being mandatory for spatial learning in general. This is also supported by results obtained in several studies by others (Dupret et al. 2007; Garthe et al. 2009; Martinez-Canabal et al. 2013; Zhang et al. 2008).
However, our new data still seem to be at odds with the earlier findings suggesting that the Ccnd2−/− mice would have no adult neurogenesis-related phenotype (Jaholkowski et al. 2009; Jedynak et al. 2012; Urbach et al. 2013). As mentioned above, we have proposed that differences in the setups and protocols used might be responsible for the divergent results (Garthe & Kempermann 2013). Because the new neurons specifically add to an already existing pathway or network within the hippocampus (rather than replacing that pathway or network), it can be assumed that an absence of adult neurogenesis should not disrupt hippocampal function in general. Adult neurogenesis appears to optimize hippocampal function rather than adding a qualitatively new mode of information processing. We thus argue that a lack of new granule cells in the dentate gyrus will only affect the effectiveness of information processing in the hippocampus and only in situations where the processing load is high and adult neurogenesis is especially relevant. In this view, ablating the new neurons likely will not always result in an overall behavioral phenotype, even though such phenotype might be apparent in some situations.
Compared with wild-type mice, the hippocampal volume of Ccnd2−/− mice was shown to be substantially smaller, possibly owing to developmental deficits caused by the enduring lack of neurogenesis (Kowalczyk et al. 2004). A rough estimate of the contribution of adult-generated neurons to granule cell numbers is 30%, based on a lineage tracing study (Ninkovic et al. 2007). Whether or not the change in neurogenesis alone can explain the gross morphological difference, however, is difficult to judge. In any case, however, the Ccnd2−/− mice did not show general impairments in hippocampal learning but exactly the phenotype that with some good reasons can be attributed to the new neurons. This does not generally exclude small additional other effects, which will vary between models. But in turn this is exactly the argument for studies like the present one that aim at mediating between the conclusions drawn in different experiments.
Yet another study by Jedynak et al. (2012), for example, has found that the lack of adult neurogenesis in Ccnd2−/− mice did not impair hippocampal learning in general and the knockouts also successfully learned the water maze and Barnes maze tasks (Jaholkowski et al. 2009; Urbach et al. 2013). These findings are nevertheless in line with our notion that adult neurogenesis does not contribute to hippocampal learning in general but only to highly specific aspects of learning.
Interestingly, Ccnd2−/− mice reportedly also showed deficits in species-specific behaviors such as nest construction, digging and marble burying (Jedynak et al. 2012). Common among such behaviors – at least under natural conditions – might be the need to differentiate a large number of places, where nesting material and food can be found, or where buried food can be retrieved. Again, albeit speculatively, this would highlight the important role of the specific task demands that are necessary to reveal a functional deficit due to a lack of new hippocampal neurons.
Relearning or updating the platform position in an already known general context after goal reversal represents a situation with the risk of catastrophic interference and requires pattern separation (Wiskott et al. 2006). Moving the goal to a new position calls for a subtle and specific update of the cognitive map in order to maintain efficient navigation. That Ccnd2−/− mice showed the same deficit in relearning the task as mice with pharmacologically suppressed adult neurogenesis presumably reflects the shared impaired ability to separate novel similar information from the previously learned memories.
A noteworthy detail of our study is the finding that the heterozygotes that ranged between controls and knockout mice with respect to the number of adult-generated neurons in the hippocampus also had an intermediate behavioral phenotype. This suggests not only a gene dosage effect but also a quantitative relationship between the number of new cells available and the test performance.
Our study provides evidence that the setups and protocols used to address the functional relevance of adult neurogenesis play an essential but as yet probably underestimated role for revealing the contribution of new neurons. Understanding the functional relevance of adult neurogenesis in complex spatial navigation tasks must challenge the conventional way spatial learning experiments are performed and analyzed. This common usage of the test, however, has always been far from uniform and standardized. Further studies have to identify the factors within a given setup that determine whether the efficient separation of contexts is a relevant issue to learn the task and to what degree adult neurogenesis can be beneficial in keeping hippocampal information processing efficient. Addressing the functional relevance of adult neurogenesis using different cue configurations and protocols will improve our understanding under which conditions and to what extent the new neurons gain specific importance.
Acknowledgments
This study was financed from Basic Institutional Funds and partial contribution from The Helmholtz Alliance on Systems Biology. The authors would like to thank Anne Karasinsky and Daniela Lasse for technical support and animal breeding, as well as Marcin Wawrzyniak from the Nencki Institute and Ronald Naumann from the Max Planck Institute for Cell Biology and Molecular Genetics, Dresden, for transfer of Ccnd2−/− embryos obtained from the Nencki Institute, Warsaw. The authors declare no conflict of interest.
References
- Aimone JB, Wiles J. Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J. Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby PA, Kempermann G. Wiskott L. The role of additive neurogenesis and synaptic plasticity in a hippocampal memory model with grid-cell like input. PLoS Comput Biol. 2011;7:e1001063. doi: 10.1371/journal.pcbi.1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G, Frey JU. Lipp HP. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;15:722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah NM, Filipkowski RK, Pruschy M, Jaholkowski P, Winkler J, Kaczmarek L. Lipp HP. Impaired long-term memory retention: common denominator for acutely or genetically reduced hippocampal neurogenesis in adult mice. Behav Brain Res. 2013;252:275–286. doi: 10.1016/j.bbr.2013.05.034. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH. Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB. Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV. Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN. Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Stewart C. Morris RG. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Liu Z, Weinstein PR, Fike JR. Liu J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25:38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- Garthe A. Kempermann G. An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front Neurosci. 2013;7:63. doi: 10.3389/fnins.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Behr J. Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaholkowski P, Kiryk A, Jedynak P, Ben Abdallah NM, Knapska E, Kowalczyk A, Piechal A, Blecharz-Klin K, Figiel I, Lioudyno V, Widy-Tyszkiewicz E, Wilczynski GM, Lipp HP, Kaczmarek L. Filipkowski RK. New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn Mem. 2009;16:439–451. doi: 10.1101/lm.1459709. [DOI] [PubMed] [Google Scholar]
- Jedynak P, Jaholkowski P, Wozniak G, Sandi C, Kaczmarek L. Filipkowski RK. Lack of cyclin D2 impairing adult brain neurogenesis alters hippocampal-dependent behavioral tasks without reducing learning ability. Behav Brain Res. 2012;227:159–166. doi: 10.1016/j.bbr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR. Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. New neurons for 'survival of the fittest'. Nat Rev Neurosci. 2012;13:727–736. doi: 10.1038/nrn3319. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Gage FH. Experience-dependent regulation of adult hippocampal neurogenesis: effects of long-term stimulation and stimulus withdrawal. Hippocampus. 1999;9:321–332. doi: 10.1002/(SICI)1098-1063(1999)9:3<321::AID-HIPO11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance in the water maze task. Eur J Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG. Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D. Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M. Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kowalczyk A, Filipkowski RK, Rylski M, Wilczynski GM, Konopacki FA, Jaworski J, Ciemerych MA, Sicinski P. Kaczmarek L. The critical role of cyclin D2 in adult neurogenesis. J Cell Biol. 2004;167:209–213. doi: 10.1083/jcb.200404181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Canabal A, Akers KG, Josselyn SA. Frankland PW. Age-dependent effects of hippocampal neurogenesis suppression on spatial learning. Hippocampus. 2013;23:66–74. doi: 10.1002/hipo.22054. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Mori T. Gotz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR. Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004;55:381–389. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- Ruediger S, Spirig D, Donato F. Caroni P. Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning. Nat Neurosci. 2012;15:1563–1571. doi: 10.1038/nn.3224. [DOI] [PubMed] [Google Scholar]
- Save E. Poucet B. Involvement of the hippocampus and associative parietal cortex in the use of proximal and distal landmarks for navigation. Behav Brain Res. 2000;109:195–206. doi: 10.1016/s0166-4328(99)00173-4. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R. Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ. Weinberg RA. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ. Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Steiner B, Zurborg S, Horster H, Fabel K. Kempermann G. Differential 24 h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience. 2008;154:521–529. doi: 10.1016/j.neuroscience.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Urbach A, Robakiewicz I, Baum E, Kaczmarek L, Witte OW. Filipkowski RK. Cyclin D2 knockout mice with depleted adult neurogenesis learn Barnes maze task. Behav Neurosci. 2013;127:1–8. doi: 10.1037/a0031222. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS. Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wiskott L, Rasch MJ. Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- Woolley DG, Vermaercke B, Op de Beeck H, Wagemans J, Gantois I, D'Hooge R, Swinnen SP. Wenderoth N. Sex differences in human virtual water maze performance: novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res. 2010;208:408–414. doi: 10.1016/j.bbr.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH. Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]


