Abstract
Dysregulation of cell surface proteolysis has been strongly implicated in tumorigenicity and metastasis. In this study, we delineated the role of hepatocyte growth factor activator inhibitor-2 (HAI-2) in prostate cancer (PCa) cell migration, invasion, tumorigenicity and metastasis using a human PCa progression model (103E, N1, and N2 cells) and xenograft models. N1 and N2 cells were established through serial intraprostatic propagation of 103E human PCa cells and isolation of the metastatic cells from nearby lymph nodes. The invasion capability of these cells was revealed to gradually increase throughout the serial isolations (103E<N1<N2). In this series of cells, the expression of HAI-2 but not HAI-1 was significantly decreased throughout the progression and occurred in parallel with increased activation of matriptase. The expression level and activity of matriptase increased while the HAI-2 protein level decreased over the course of orthotopic tumor growth in mice, which was consistent with the immunohistochemical profiles of matriptase and HAI-2 in archival PCa specimens. Knockdown of matriptase reduced the PCa cell invasion induced by HAI-2 knockdown. HAI-2 overexpression or matriptase silencing in N2 cells down-regulated matriptase activity and significantly decreased tumorigenicity and metastatic capability in orthotopically xenografted mice. These results suggest that during the progression of human PCa, matriptase activity is primarily controlled by HAI-2 expression. The imbalance between HAI-2 and matriptase expression led to matriptase activation, and thereby increasing cell migration, invasion, tumorigenicity and metastasis.
Keywords: prostate cancer, hepatocyte growth factor activator inhibitor-2, cancer cell invasion, tumorigenicity and metastasis
Introduction
Cell invasion and metastasis are the life-threatening aspects of malignancies including prostate cancer (PCa) (1, 2). The acquisition of invasive capacity involves many alterations in the proteins that regulate cell-cell and cell-matrix interactions, including the extracellular matrix (ECM)-modulating proteases (3). Among these proteins, membrane-anchored proteases have received recent attention, owing to their aberrant proteolytic actions in ECM degradation and/or the activation of membrane-associated substrates, ligands, or receptors (4), which contribute to tumorigenesis and metastasis (5, 6).
Matriptase is a membrane-anchored serine protease, first identified in breast cancer cells (7, 8). This protease is mainly expressed in the epithelial components of tissues (9), and often co-expressed with a cognate inhibitor, hepatocyte growth factor activator inhibitor-1 (HAI-1) (10–12). In the clinical context, the ratio of matriptase to HAI-1 is known to be increased in many cancers such as breast (13) and prostate (14); an aberration that is often associated with poor prognosis. Moreover, matriptase has recently been shown to be involved in ErbB-2-promoted PCa cell invasion (15). Aberrant matriptase activity has been strongly implicated in carcinogenesis (16) and cancer progression (17, 18) and several of the matriptase-activated substrates identified, such as HGF, PAR2, MMP3, and uPA, are involved in promoting cell motility and invasion (19–21). Moreover, matriptase can also directly degrade ECM including laminin and fibronectin (22). Thus, dysregulation of matriptase may lead to enhanced malignant progression of tumors through enhanced invasive and metastatic potential.
HAI-2 was isolated from the conditioned medium of a human stomach carcinoma cell line, identified as a serine protease inhibitor, and named after its homolog HAI-1 (23). The protein was also independently identified as placental bikunin, a transmembrane glycoprotein with two Kunitz domains (23, 24). The HAI-2 transcript is detectable in a variety of human tissues, with relatively high levels of expression in the kidney, placenta, and prostate (23, 24). In addition to epithelial cells, HAI-2 expression is also found in non-epithelial cells in the brain and lymph nodes (25). Thus, HAI-2 has been proposed to have a physiological role in targeting a broad spectrum of serine proteases.
Altered levels of HAI-2 have been implicated in several human diseases. Syndromic congenital sodium diarrhea (CSD) has been shown to be caused by loss of HAI-2 function (26). Up-regulation of HAI-2 expression in cholangiopathies affects liver fibrosis and differentiation (27). In mice, HAI-2 is essential for placental development, neural tube closure and embryonic survival (28). The levels of HAI-2 gene expression are reported to be down-regulated in several cancers including breast cancer (29) and PCa (30), although in pancreatic cancer, HAI-2 expression has been shown to be up-regulated (31). The loss of HAI-2 expression in cancer appears to be mainly due to epigenetic silencing by DNA hypermethylation at CpG islands in the promoter region (32–34). HAI-2 expression has been proposed as a marker of favorable prognosis by virtue of its suppressive activity on cell growth, tumorigenesis, and cancer cell invasion (32, 33, 35). Through biochemical and genetic analysis, HAI-2 has been shown to be a potential inhibitor of hepsin and matriptase (25, 36). However, the role of HAI-2 in the PCa progression remains poorly understood. In this study, we established a human PCa metastatic progression model by repeated isolation of PCa cells that spontaneously metastasized from the prostate to regional lymph nodes in mice. Analysis of data generated with this model showed that HAI-2 was down-regulated and matriptase was up-regulated in metastatic PCa cells. This observation was further corroborated by results from the immunohistochemical analyses of a longitudinal series of orthotopic xenografted prostate tumors and human archival specimens. Increasing the level of matriptase in PCa cells resulted in enhanced cell migration, invasion, tumorigenicity and metastasis, all of which were specifically counteracted by a concomitant increase in the level of HAI-2. These data support the notion that down-regulation of HAI-2 in PCa can promote enhanced proteolytic activity and accelerate tumor progression.
Results
Propagation of a human prostate cancer cell line in the mouse prostate results in spontaneous metastasis
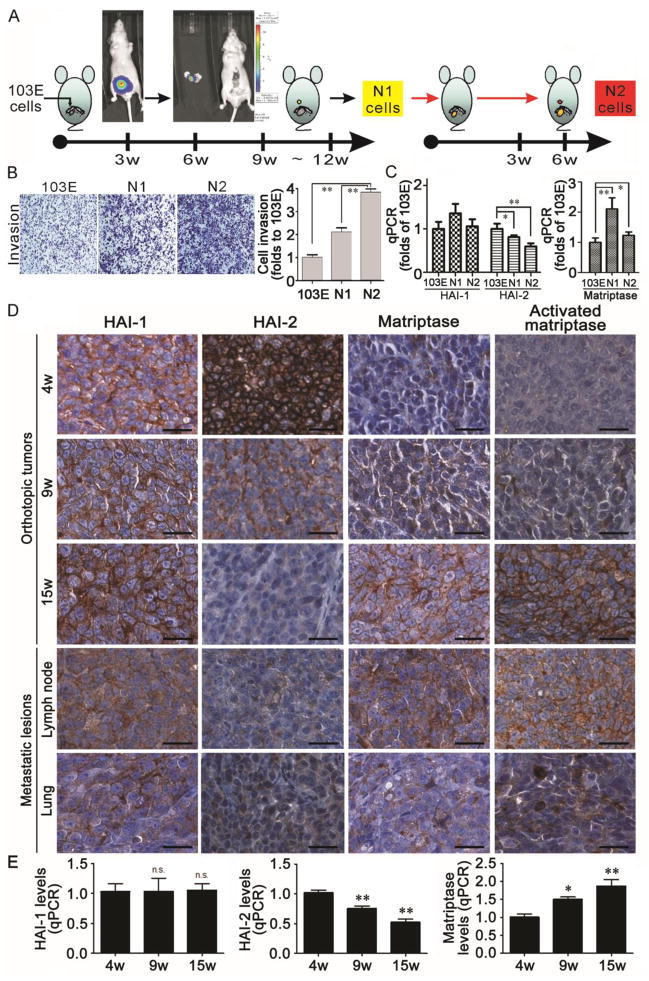
To try to mimic the progression of human PCa, we established a human PCa metastatic progression model by implanting human PCa cells into the mouse prostate, allowing them to grow, and isolating the metastatic cancer cells that escaped the confines of the prostate to reach the nearby lymph nodes (Figure 1A). In order to track the metastasis of PCa cells in the mouse body, we first transfected CWR22Rv1 cells with a luciferase reporter plasmid driven by the PSA promoter, and selected a stable clone with strong PSA promoter activity which we named 103E cells (37). Orthotopic xenografts were then initiated by implanting 103E cells into the anterior prostate glands of nude mice. After 9–12 weeks of tumor growth, metastatic cells that had colonized the lumbar lymph nodes nearby the prostate were isolated and named N1 cells (Figure 1A, middle panel). N1 cells were again engrafted into anterior prostates of mice and we observed that the metastasis of cells to the lymph node occurred in a shorter interval of 4–6 weeks after the implantation. Once again the metastatic cells were retrieved from the lumbar lymph nodes and called N2 cells (Figure 1A, right panel). Thus, 103E cells are parental cells with low metastatic capability and N2 cells derived from this animal model may have acquired a more aggressive phenotype.
Figure 1.
(A) Establishment of a human PCa progression model by orthotopic xenograft in mice. 103E cells derived from CWR22Rv1 cells by stable transfection with a luciferase reporter gene driven by the PSA promoter were implanted into the anterior prostate of mice. Nine to twelve weeks after implantation, the cells with luciferase activity were isolated from nearby lymph nodes and named N1 cells. The N1 cells were then also orthotopically injected into anterior prostate of mice. N1 cells metastasized to the proximate lymph nodes in a shorter period than before (4–6 weeks) and were isolated and named N2 cells. (B) Analysis of the invasive potential of 103E, N1 and N2 cells by transwell assays. Each subline was seeded at 3 × 105 cells per well in the upper well of a Matrigel-coated Boyden chamber in serum-free media. The lower chamber of each transwell was filled with RPMI1640 media containing 8% FBS as chemoattractant and the chambers were cultivated for 16 hr. Invasive cells were stained with crystal violet, photographed with a microscope, and quantified using ImageJ analysis software. Data represent mean ± SD of three independent experiments. (C) Analysis of matriptase (MTX), HAI-1 and HAI-2 mRNA levels by q-PCR in 103E, N1 and N2 cells. Total RNA was prepared from the indicated cells with Trizol reagent, reversely transcribed and subjected to qPCR analysis with a set of MTX, HAI-1 and HAI-2 primers and using GAPDH as an internal control. After qPCR, the gene expression levels were statistically calculated with ratios to 103E cells. (D) Immunohistochemical analysis of HAI-1, HAI-2, matriptase and activated matriptase in orthotopic xenograft tumors, and metastatic lesions in the lymph nodes and lung. 103E cells were orthotopically implanted into the anterior prostate of mice. After implantation, the local tumors were harvested from mice euthanized 4, 9 and 15 weeks after injection. Metastatic lesions were excised from the 15 mice from the lymph nodes and lung. Tumor tissues were embedded with paraffin, used for tissue array preparation and immunohistochemically stained with specific Abs as marked. The images were visualized with DAB and photographed under a light microscope. Scale bar = 25 μM. (E) The tumor tissues harvested from the mice 4, 9 and 15 weeks after injection were used for RNA extraction, reverse transcription and qPCR analysis for the expression levels of HAI-1, HAI-2 and matriptase as described previously. *, P< 0.05; **, P<0.01.
The invasion and metastatic potential of PCa cells is correlated with increased matriptase and decreased HAI-2 expression in vivo
To characterize the relative cell invasion potentials of 103E, N1 and N2 cells, Matrigel-coated Boyden chambers were used to examine the ability of the cells to penetrate through a layer of Matrigel – a standard assay of invasiveness. As expected, 103E cells were found to be less invasive than N1 and N2 cells, which exhibited progressively increased invasion activity up to approximately 4-fold that of the parental cells (Figure 1B). Thus, the gradual change in cancer cell invasion from 103E, to N1 then N2 cells may reflect the different metastatic potentials during PCa progression.
Decreased levels of matriptase inhibitors HAI-1 and HAI-2, and/or increased matriptase have been implicated in PCa progression (14, 30). Therefore, we analyzed the expression levels of HAI-1, HAI-2 and matriptase by qPCR in the cells of this cancer progression model. Relative to 103E cells, the level of HAI-2 expression but not that of HAI-1 was down-regulated approximately by 50% in N2 cells following progression whereas the matriptase expression level was significantly increased in N1 and N2 cells (Figure 1C). These observations suggest that during progression human PCa may alter pericellular proteolysis by up-regulating matriptase, down-regulating HAI-2 expression or both. The shift in the balance of matriptase and HAI-2 levels correlated with the increase in cellular invasion and metastatic potentials.
HAI-2 expression and matriptase activity are altered during tumorigenicity and metastasis of orthotopic PCa xenograft in mice
Next, to analyze the expression of HAI-1, HAI-2 and matriptase in vivo during the prostate tumor growth, orthotopic xenografts of 103E tumors in nude mice were resected 4, 9, and 15 weeks after the implantation of the cells, and the tumor specimens were stained with antibodies specific for HAI-1, HAI-2, total matriptase, and the activated form of matriptase. During the harvest of the tumors at the 15 week time-point, several metastatic nodules were observed in the lymph nodes and lungs of the animals, and so these tissues were also collected for further analysis. Immunohistochemistry (IHC) revealed that during the tumor growth, HAI-2 protein levels, but not those of HAI-1, fell progressively, whereas in contrast, the matriptase protein level increased (Figure 1D, left three columns). This altered expression was particularly marked between the 9- and 15-week time-points during which a dramatic decrease in HAI-2 and increase in matriptase occurred. This change agreed with the gene expression levels examined by qPCR (Figure 1E), and correlated with the time at which the N1 cells were isolated from the nearby lymph nodes (Figure 1A, middle panel). IHC showed that the level of HAI-2 in the metastatic nodules within the lymph nodes and lungs was similar to that in the 15-week orthotopic tumors (Figure 1D, lower panel). Although HAI-1 expression in the lymph node and pulmonary metastasis was marginally to moderately lower than in the other tumor specimens, alterations in HAI-1 level were not obvious during the growth of the primary tumors (Figure 1D & 1E, left panels). In addition, the IHC images may reveal that HAI-1 expression seemed to switch from predominantly membranous expression (up to 15 weeks) to a cytoplasmic (intra-cellular) pattern in the lymph node and lung metastasis. In contrast to the decrease in HAI-2 levels, matriptase expression levels and activation increased along with the tumorigenesis (Figure 1D, middle to right columns). These data suggest that loss of HAI-2 or gain-of-function of matriptase in PCa cells may be involved in the acquisition of an invasive phenotype, and the combination of decreased HAI-2 and increased matriptase levels may promote PCa metastasis to distant organs.
The relative levels of matriptase and HAI-2 expression are inversely correlated in clinical prostate cancer
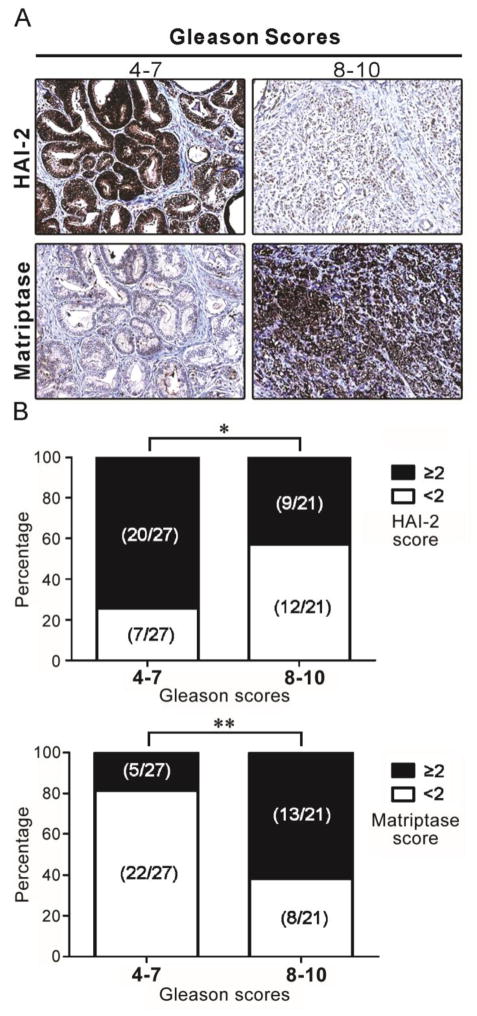
In order to explore whether these intriguing findings might be replicated in clinical specimens, we next investigated the HAI-2 and matriptase protein levels in archival specimens of human PCa by IHC. The antibodies used for the staining have all been well characterized previously (38, 39) and also as shown in Supplementary Figure 1. The results (Figure 2A) from two representative patients showed that HAI-2 expression was lower in the patient with Gleason score 8–10 than that in the patient with Gleason score 4–7, while matriptase expression was higher in the patient with the higher Gleason score. The staining was further evaluated visually using a three point scale (0: no staining; +1: weak staining; +2: moderate staining; +3: strong staining) to score a tumor more than 10% of cells with elevated protein levels of matriptase or HAI-2. As shown in Figure 2B, approximately 74% (20/27 cases) of HAI-2 immunostaining in Gleason score 4–7 PCa specimens had scores of equal to or greater than +2, whereas approximately 57% (12/21 cases) of the HAI-2 staining levels in Gleason score 8–10 cancer tissues were below 2. Matriptase immunostaining in Gleason score 4–7 cancer tissues had predominately lower intensity (22/27 cases (~81.5%) having scores of 0 or +1), while 13/21 cases (~61.9%) in the Gleason score 8–10 group had matriptase immunostaining scores of +2 or +3. Compared to Gleason score 4–7 tumor tissues, HAI-2 protein expression was significantly lower in advanced PCa (Gleason scores 8–10) (Fisher’s exact test P = 0.039), while the matriptase protein level was found to be significantly increased (Fisher’s exact test P = 0.0029). Among advanced PCa patients, five patients (5/21 cases) simultaneously exhibited a decrease in HAI-2 expression and an increase in matriptase expression. The gene expression levels of HAI-2 in the patients with Gleason score 6–7 and 8–9 were significantly reduced, compared to normal persons (Supplementary Figure 1C). The observation that low levels of HAI-2 or higher levels of matriptase staining were associated with tumor progression as assessed by Gleason score was consistent with the pattern of acquired changes in the levels of these proteins observed in the orthotopic xenograft model (Figure 1D), suggesting the potential involvement of HAI-2 and matriptase in the development of metastatic PCa.
Figure 2.
HAI-2 and matriptase expression in human archival specimens. Human PCa tissue microarrays (PR243a, US Biomax, USA) were used for immunohistochemical staining with anti-HAI-2 and anti-matriptase (S5) antibodies. (A) Representative examples of HAI-2 and matriptase staining in PCa tissues within two groups: Gleason scores 4–7 vs. 8–10. (B) Statistical analysis of HAI-2 and matriptase staining in the PCa tissues. Intensity scoring (0: no staining; +1: weak staining; +2: moderate staining; +3 strong staining) was conducted, Fisher’s exact test was used to evaluate the intensity differences (intensity score ≥ +2 vs. < +2) of immunohistochemistry signals of HAI-2 or matriptase between tissues with higher and lower Gleason scores. The P values of Fisher’s exact test for HAI-2 and matriptase staining between the two groups with higher and lower Gleason scores were 0.039 and 0.003, respectively. A two-sided P value of less than 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.005.
Matriptase activity in PCa cells is enhanced during lymph node metastasis
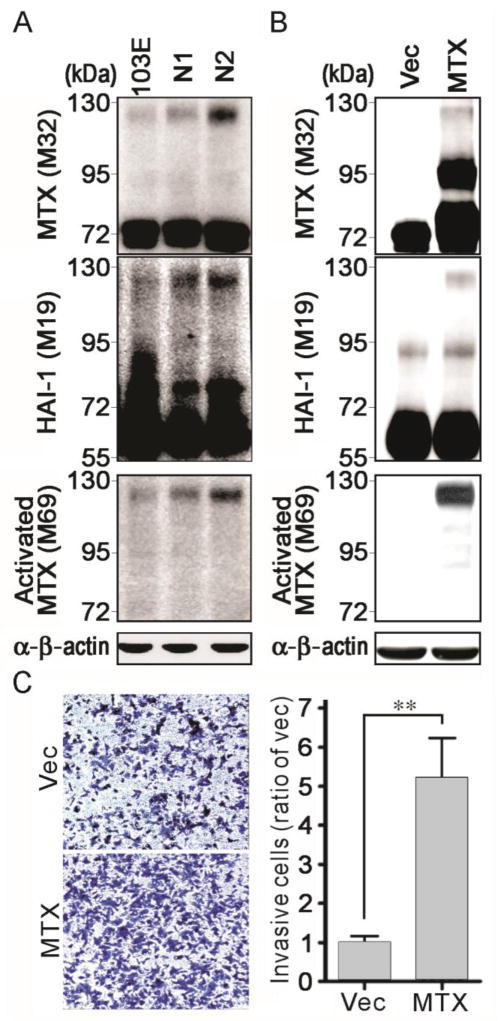
The activation of matriptase is tightly regulated in normal tissues, and increased activation of this enzyme has been associated with malignant progression (17, 40–42). Therefore, next we used antibodies for total matriptase, activated matriptase and HAI-1 (17, 43) to analyze matriptase activation levels in 103E, N1 and N2 cells. The results (Figure 3A) showed that the level of activated matriptase, detectable as a 120 kDa complex with HAI-1, was increased in N1 cells compared to 103E cells and was increased again comparing N2 with N1 cells – concomitant with their more aggressive properties. Moreover, M69 staining further revealed that the level of activated matriptase increased from 103E, to N1 and again to N2 cells (Figure 3A, bottom panel), again correlating with the invasive potential of the cells (Figure 1B), the up-regulation of matriptase, and the down-regulation of HAI-2 (Figure 1C). These results suggest that the imbalance between matriptase and HAI-2 in favor of proteolysis may also contribute to matriptase activation during PCa progression.
Figure 3.
Matriptase activation levels in 103E, N1 and N2 cells and the role of matriptase in PCa cell invasion. (A) Analysis of total matriptase, activated matriptase and HAI-1 in 103E, N1 and N2 cells. Cell lysates were prepared for immunoblot analysis with anti-total matriptase (M32), anti-HAI-1 (M19) and anti-activated matriptase (M69) under a non-boiling and non-reduced condition. Equal loading was assessed by immunoblotting with an anti-β-actin Ab. (B) Analysis of matriptase (MTX) and HAI-1 in matriptase-overexpressing 103E cells. 103E cells were transfected with matriptase plasmids (46) and stable pools of transfectants were selected by G418. Control cells were transfected with vector alone. Cell lysates were collected for immunoblotting analysis with anti-total matriptase (M32), anti-HAI-1 (M19) and anti-activated matriptase (M69) under non-boiling and non-reduced conditions. An anti-β-actin Ab was used as a loading control. (C) Effect of matriptase overexpression on PCa cell invasion. Matriptase-overexpressing 103E and control cells were seeded at a density of 3×105 cells per chamber and cultured for 20 h. Invasive cells were assayed as before. The results are statistically calculated and presented as the average ± S.D. of three independent experiments. **, P<0.01.
Matriptase enhances prostate cancer cell invasion
To further examine the role of matriptase in PCa cell invasion, we transfected the poorly invasive 103E cells with a matriptase expression vector and analyzed the effect of enhanced matriptase levels on cell invasion using the cell invasion assay. Overexpression of matriptase in 103E cells significantly increased the level of active enzyme (Figure 3B) and the cell invasion, by approximately 5-fold (Figure 3C). It appears, therefore, that PCa cells may enhance matriptase activation by increasing matriptase expression to promote their malignant behavior.
Expression of HAI-2 in 103E, N1 and N2 cells
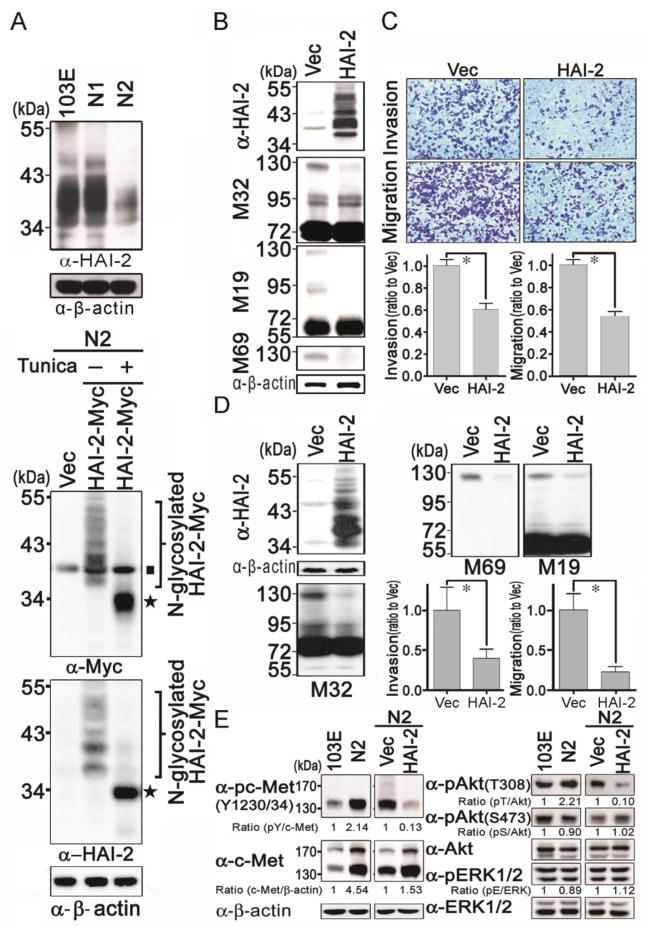
To further clarify the role of HAI-2 in human PCa progression, we examined HAI-2 protein expression levels in 103E, N1 and N2 cells by immunoblotting. The HAI-2 antibody detected a stack of multiple bands ranging from 34 to 50 kDa that were of somewhat reduced intensity in N1 cells compared to 103E cells, and were much less intense in N2 cells (Figure 4A, upper panel). HAI-2 is believed to be a highly glycosylated protein, and so we suspected that the multiple bands observed with the HAI-2 antibody were due to the presence of multiple glycosylated forms of the protein. To confirm the specificity of the HAI-2 antibody, and test this hypothesis, we constructed a HAI-2 mammalian expression plasmid that included a Myc epitope tag and used this construct to transfect N2 cells, in which HAI-2 levels are apparently quite low. N2 cells transfected with the empty vector or expressing the tagged HAI-2 construct were cultured in the presence or absence of the N-glycosylation inhibitor tunicamycin. The results (Figure 4A, lower panel) from western blot analysis with anti-Myc and anti-HAI-2 antibodies, showed that an array of bands similar to that seen with the HAI-2 antibody was detected in the lysates from the cells transfected with the tagged HAI-2 construct. Treatment with tunicamycin resulted in the detection of a single strong band of approximately 34 kDa, consistent with the unglycosylated size of HAI-2. These results suggest that HAI-2 has multiple N-glycosylation forms or biosynthetic intermediates, resulting in the molecular masses of HAI-2 in whole cell lysate reflecting the different extents of glycosylation. Together, the data indicated that the level of HAI-2 protein was inversely correlated with PCa cell invasion and metastatic capability.
Figure 4.
Analysis of HAI-2 protein expression in 103E, N1 and N2 cells, and its role in PCa cell invasion and migration. (A) Top panel, analysis of HAI-2 protein levels in 103E, N1 and N2 cells. Cell lysates were prepared for immunoblot analysis with an anti-HAI-2 Ab, or an anti-β-actin Ab as a loading control. Lower panel, HAI-2 was glycosylated and resulted in multiple bands. To examine whether multiple HAI-2 bands were caused by glycosylation, N2 cells were transfected with a myc-tagged HAI-2 expression construct and treated with or without tunicamycin (1 μg/ml) (Tunica) for 24 hr. Cell lysates were harvested and analyzed by SDS-PAGE and blotted with anti-Myc and anti-HAI-2 Abs. The ■ symbol marks endogenous Myc; and the ★ symbol indicates non-glycosylated HAI-2. (B) Effects of HAI-2 overexpression on matriptase in N2 cells. N2 cells were transfected with vector or HAI-2 expression plasmid, and selected by G418 as stable pools of transfectants. HAI-2 protein levels in HAI-2-overexpressing N2 cells were examined by immunoblotting analysis with an anti-HAI-2 Ab. β-actin levels were used for loading controls. Cell lysates were also prepared for immunoblotting analysis with anti-total matriptase (M32), anti-HAI-1 (M19) and anti-activated matriptase (M69) under non-boiling and non-reduced conditions. (C) Effect of HAI-2 overexpression on cell invasion and migration of N2 cells. For analyzing the invasion and migration of N2 stable transfectants, cells were seeded at a density of 3 × 105 per chamber and cultured for 16 h. Invasive and migratory cells were conducted as described in Figure 1B. The results were statistically calculated and represented as mean ± S.D. of three independent experiments. (D) Effect of HAI-2 overexpression on matriptase, PC3 cell invasion and migration. The effects of HAI-2 overexpression on matriptase, PCa invasion and migration were analyzed in PC3 cells using the same methods as in Figure 4B and 4C. *, P<0.05. (E) Analysis of phosphorylation levels of c-Met, Akt and Erk1/2 in 103E/N2 and HAI-2-overexpressing N2/Vec N2 cells using immunoblotting with anti-phospho-c-Met (Y1230/34), anti-c-Met, anti-phospho-Akt (T308), anti-phospho-Akt (S473), Anti-Akt, anti-phospho-ERK1/2 (Thr202/Tyr204 and Thr185/Tyr187 for ERK1 and ERK2, respectively), and anti-ERK Abs. The images were taken by X-ray films and their intensities were measured by a densitometer and statistically calculated with ratios to controls.
HAI-2 plays inhibitory roles in prostate cancer cell invasion and migration
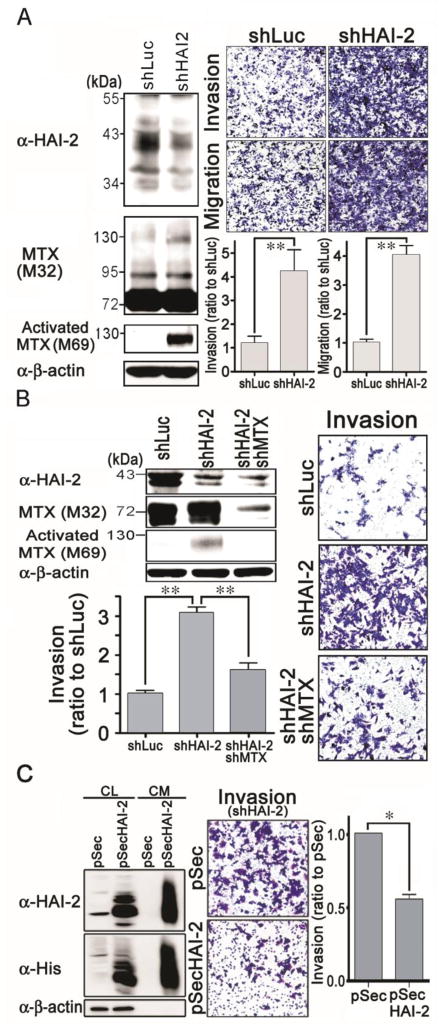
To further investigate whether HAI-2 expression might have any causal relationship with PCa cell invasion and migration, we overexpressed HAI-2 in N2 cells, and examined the effect of this intervention on cell migration and invasion. The ectopic expression of HAI-2 in the stable N2/HAI-2 cells was verified by western blot analyses (Figure 4B). As a result of HAI-2 overexpression in N2 cells, matriptase activation was drastically reduced resulting in the loss of the 120 kDa matriptase-HAI-1complex (Figure 4B, middle to lower panels). Using the MTT assay, ectopic expression of HAI-2 had no significant effect on the growth of N2 cells within 2 days (Supplementary Figure 2B). We then performed cell migration and invasion assays within 16 hours and found that enhanced HAI-2 expression caused a marked decrease (approximately 40 %) in both the invasion capability and chemoattractive migration, compared to control cells (Figure 4C).
To further confirm the inhibitory role of HAI-2 in matriptase activation, cell migration and invasion in PCa, we next transfected PC-3 PCa cells with the HAI-2 plasmid and established stable HAI-2 overexpression transfectants. When compared to control cells, the cells transfected to overexpress HAI-2 exhibited drastically decreased levels of activated matriptase, and concomitantly reduced cell migration and invasion (Figure 4D). Taken together, these data indicated that HAI-2 likely plays an inhibitory role for the activation of matriptase, and suppresses PCa cell migration and invasion. To dissect the molecular mechanism in which HAI-2 inhibits PCa cell migration and invasion, we investigated whether HAI-2 could inhibit c-Met signaling since matriptase has been reported to be a protease that can initiate c-Met-induced epithelial carcinogenesis (44). As shown in Figure 4E (left panels), the tyrosine phosphorylation levels of c-Met in N2 cells were increased approximately 2 folds compared to 103E cells, and HAI-2 overexpression in N2 cells dramatically reduced the activity of c-Met, as indicated by tyrosine phosphorylation. Moreover, HAI-2 overexpression also could decrease the phosphorylation levels of Akt (T308) but not the Erk1/2 (two main downstream molecules of c-Met) (Figure 4E, right panel). Taken together, the data indicate that HAI-2 can inhibit matriptase activity, leading to reduction of c-Met signaling, PCa cell migration and invasion.
To further confirm the link between HAI-2 levels and invasive behavior, we made use of a retroviral HAI-2 shRNA construct to suppress HAI-2 expression in CWR22Rv1 cells. HAI-2 protein levels were reduced in shHAI-2-infected CWR22Rv1 cells (Figure 5A, upper-left panel), and the level of activated matriptase was found to be increased in these cells (Figure 5A, lower-left panel), with no significant effect on the cell growth within 2 days (Supplementary Figure 2C). The suppression of HAI-2 in CWR22Rv1 cells significantly increased the cell migration and invasion (Figure 5A, right panel).
Figure 5.
Effect of HAI-2 knockdown on matriptase, PCa cell invasion and migration, as well as the effects of matriptase knockdown and recombinant HAI-2 proteins on HAI-2-silencing-induced cancer cell invasion. (A) Stable pools of HAI-2 knockdown CWR22Rv1 cells were established by G418 selection after the cells were transfected with shHAI-2 plasmids. The effects of HAI-2 silence on matriptase, cell invasion and migration were analyzed using the same methods as in Figure 4B and 4C. (B) Examination of the involvement of matriptase in increased cell migration and invasion by HAI-2 knockdown. HAI-2-knockdown CWR22Rv1 cells were infected with shMTX viral particles for 24 h and selected for three days. Cell lysates were analyzed by immunoblotting with anti-HAI-2, anti-matriptase (M32), anti-activated matriptase (M69) and anti-β-actin Abs. Infected cells were seeded at a number of 4 × 105 cells per chamber and cultured for 20 h. Invaded cells were stained, photographed, and measured as described in Figure 4C. (C) Effect of recombinant HAI-2 proteins on the HAI-2-knockdown-induced cancer cell invasion. The DNA fragment encoding the extracellular region of HAI-2 was cloned into a secretory vector pSecTag2. HEK293T cells were transfected with the plasmid or vector alone and the stable pools were selected by G418. The stable pools were seeded at a density of 3 × 106 cells in a 6-cm plate within a regular culture medium. The next day, the culture media were refreshed with serum-free RPMI1640 media. Twenty four hours after the refreshment, cell lysates (CL) and the conditioned media (CM) were collected. The protein concentrations in the cell lysates were determined by Protein Assays. Equal amounts of cell lysates and the conditioned media from the same cell numbers by normalizing to the whole cell lysates were used for SDS-PAGE and immunoblot analysis with anti-HAI-2 and anti-His Abs. The conditioned media were used for the cell invasion assays by adding to the upper and lower chambers. In the lower chambers, 10% FBS were supplemented as chemoattractant. The cell invasion was analyzed as the same methods as described previously. *, P< 0.05; **, P<0.01.
As described above, increased PCa cell migration and invasion caused by HAI-2 knockdown is associated with increased matriptase activation. This suggests the possibility that matriptase is a target serine protease for inhibition by HAI-2 to modulate PCa cell invasion and migration. To clarify this hypothesis, we analyzed whether the increased cell migration and invasion caused by HAI-2 knockdown could be antagonized by matriptase knockdown. Indeed, the result (Figure 5B) showed that knockdown of matriptase reduced the cell invasion promoted by HAI-2 knockdown, down to the level in control cells. Moreover, recombinant HAI-2 proteins in the conditioned media could also significantly reduce the cell invasion of the shHAI-2 cells (Figure 5C). Together, these data indicated that matriptase activity in human PCa cells can drive cell invasion and that HAI-2 serves as a potent gatekeeper modulating matriptase activity and the resulting cell migration and invasion.
Matriptase silencing or HAI-2 overexpression inhibits the tumorigenicity and metastasis of prostate cancer
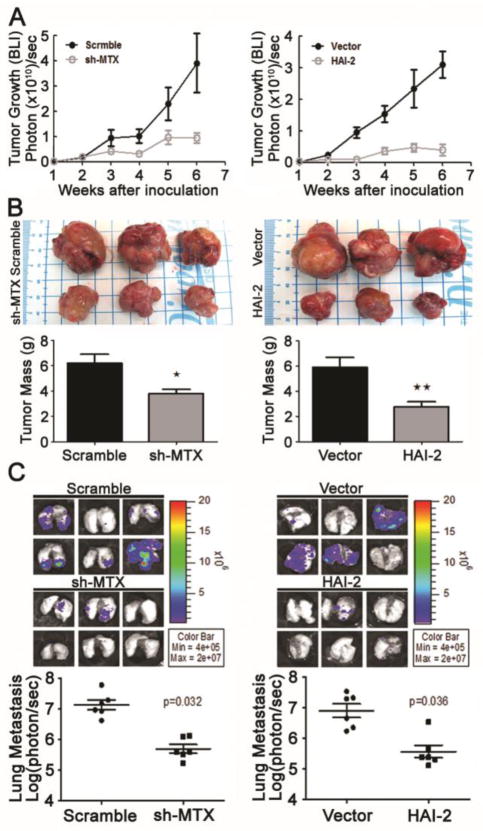
To further analyze the roles of matriptase and HAI-2 in the tumorigenicity and metastasis of PCa cells, we injected the stable pools of matriptase-silencing, HAI-2-overexpressing N2 and the respective control cells into the prostate glands of nude mice. As shown in Figure 6A, matriptase-silencing or HAI-2 overexpression in N2 cells strikingly reduced the tumor growth in nude mice. This result was confirmed by the primary tumor mass after euthanization (Figure 6B). Quantification of HAI-1, HAI-2 and matriptase expression using qPCR as well as the protein levels of matriptase and HAI-2 in the tumor tissues showed that matriptase knockdown or HAI-2 overexpression did not significantly affect the expression of the other two genes, and this silencing or overexpression in N2 cells remained stable during the in vivo experiments (Supplementary Figures 3A & 3B). Moreover, bioluminescence examination after euthanization revealed (Figure 6C) that matriptase silencing and HAI-2 overexpression could significantly reduce PCa metastasis to lung tissues in the mice. H&E staining (Supplementary Figure 3C) showed that metastatic lesions in lung tissues were significantly decreased in the mice injected with matriptase-silencing and HAI-2-overexpressing N2 cells. These data indicate that HAI-2 exerts an inhibitory effect on invasive tumor growth and metastasis of PCa.
Figure 6.
Analysis of the role of matriptase and HAI-2 in prostate tumor growth and metastasis in xenografted mice. Matriptase (MTX) knockdown (shMTX)/scramble RNA N2 cells and HAI-2-overexpressing/vector-transfected N2 cells were orthotopically xenografted into the anterior prostate of mice (six mice per group). (A) Tumor growth was statistically analyzed employing bioluminescence intensity after the measurement using the IVIS system (Xenogen) once a week after i.p. injection of D-luciferin at 150 mg/kg. (B) Six weeks after implantation, the tumors were excised, images and weighed. The average tumor masses from scramble RNA/shMTX N2, vector-transfected and HAI-2-overexpressing N2 xenografted mice were 6.201±0.7007, 3.643±0.2822, 5.879±0.801, and 2.781±0.3880 grams (n=6, mean±SE), respectively. Three images per group are presented to represent tumor sizes. The tumor masses in 6 xenografted mice were calculated and presented as mean±SD. *, P< 0.05; **, P<0.01. (C) Analysis of the metastatic lesions in xenografted mice. The metastatic lesions in lung tissues of mice were imaged by the IVIS system (Xenogen) after i.p. injection of D-luciferin at 150 mg/kg (upper panels) and their bioluminescence intensities were statistically plotted and calculated (lower panels).
Discussion
Metastasis to distant tissues or organs is a primary cause of human cancer mortality. Cell surface proteolysis has been strongly implicated in cancer cell dissemination and metastasis. In the current study we showed that the expression of HAI-2 was inversely correlated with the progression of PCa cells to a highly invasive and metastatic state. Moreover, the study also showed that in PCa, matriptase was primarily regulated by HAI-2. Reduction in HAI-2 expression resulted in an increase in matriptase activity, PCa cell migration and invasion. Re-establishing high levels of HAI-2 expression or silencing matriptase reduced matriptase activity, tumorigenicity and metastasis of PCa. Thus, HAI-2 may serve as an oncosuppressor protein for PCa progression.
To study the metastasis progression of human PCa, we established a metastatic progression model (103E, N1 and N2 cells) by serial orthotopic implantation and isolation of early metastatic cancer cells from nearby lymph nodes. The invasive capability of the cells in this progression series gradually increased throughout the selection. In parallel with the progression to a more invasive and metastatic phenotype, the expression of matriptase by the cells was increased, which mirrors the observation that increased expression of matriptase in human PCa is associated with advanced Gleason Scores (14). We suggest that increased matriptase expression is one factor responsible for enhanced protease activation and increased PCa cell invasion (Figure 3). Alternatively, the high level of activated matriptase observed in highly invasive PCa cells might result from the decrease in HAI-2 expression (Figure 5A). These data demonstrate that both matriptase upregulation and HAI-2 downregulation interact to shift the protease-inhibitor balance in a prostate tumor leading to a more aggressive phenotype. Furthermore, the increased cell invasion resulting from HAI-2 knockdown was significantly blocked by concomitant matriptase knockdown or recombinant HAI-2 proteins in the conditioned medium. This observation suggests that matriptase serves as a key target for HAI-2 in PCa cells, although it has also been proposed that HAI-2 may affect multiple targets in different cell types or developmental stages (25). In PCa, HAI-2 protein functioned as an inhibitor of cell invasion, tumorigenicity and metastasis, mainly through antagonism of matriptase activity. Moreover, since Figure 6 showed that matriptase silencing or HAI-2 overexpression significantly reduced tumorigenicity, it was possible that the primary effect of the matriptase/HAI-2 axis might be on tumor growth, and these two manipulations to reduce metastasis might be a secondary effect due to smaller primary tumors. In addition, the tumor masses (g) of matriptase-silencing (3.643±0.2822) and HAI-2-overexpressing (2.781±0.3880) N2 orthotopic xenografts were higher than the tumors mass (1.308±0.038) of 103E xenografts following the same procedure. Thus, although these two manipulations can significantly reduce N2 tumor masses, the “treated” N2 tumors are still bigger than the original 103E tumors. This implies that in addition to the matriptase/HAI-2 axis, there may exhibit some other pathways altered, which also contribute to the progression from 103E to N2 cells.
In addition to matriptase and HAI-2, prostate tissues also express HAI-1, which was the first matriptase inhibitor to be identified (11). We found that the expression of HAI-1 did not significantly change during the orthotopic growth of PCa cells and in HAI-2-overexpressing and matriptase-silencing N2 xenograft tumors (Supplemental Figure 3). Thus, these data suggest that the altered expression of HAI-2 and matriptase in PCa cells is independent of HAI-1 expression. This in turn suggests that HAI-2 may play a more important role than HAI-1 in a subset of PCa in modulating protease activation during the metastatic development, although HAI-1 levels have been shown to be inversely correlated with PCa progression (14).
In this study, we also found that HAI-2 overexpression in PCa cells reduced the levels of matriptase/HAI-1 complexes, a surrogate to indicate the activated level of matriptase (10). One possible reason for the apparent ability of HAI-2 to decrease complex formation between matriptase and HAI-1 might be that high levels of HAI-2 may complete with HAI-1 as an inhibitor for the formation of complexes with activated matriptase, resulting in the loss of matriptase/HAI-1 complexes, since both HAI-1 and HAI-2 have similar in vitro properties as catalytic inhibitors of matriptase (25). As non-covalent protein complexes may not be observed by immunoblot analysis, it is possible that matriptase-HAI-2 complexes may not be observed in SDS-PAGE in non-reduced and non-boiled conditions in the same way as matriptase-HAI-1 complexes. Thus, the immunoblots showed that HAI-2 overexpression decreased the levels of matriptase-HAI-1 complexes. Alternatively, it is possible that HAI-2 can suppress matriptase activation through some unknown mechanism. Without the proteolytic activation of matriptase, HAI-1 cannot form a stable complex with matriptase. The detailed mechanism underlying the ability of HAI-2 to reduce matriptase-HAI-1 complexes is the subject of ongoing investigation.
The decrease in HAI-2 expression during PCa progression may be due to DNA hypermethylation in the HAI-2 promoter, since treatment with 5′-Aza-dC could significantly increase HAI-2 expression in N2 cells (Supplementary Figure 4). This result is consistent with other reports that the HAI-2 gene is frequently hypermethylated and under-expressed in other malignant cancers (32–34). Thus, down-regulation of HAI-2 expression often occurs during cancer progression. Since some of these studies showed that decreased HAI-2 expression resulted in increased HGFA activity and HGF maturation, leading to cancer cell invasion in advanced PCa patients (45), we set out to examine whether HGFA might be a protease target in our PCa cell model; however, we were unable to detect mRNA expression of this protease in these cancer cells. In spite of no detection of HGFA in PCa cells, it is still possible that in vivo circulating HGFA may also be a target for HAI-2 in tumor lesions. In this study, the results from the PCa metastasis progression model and in vivo experiments suggest that matriptase is a key target of HAI-2 that modulates cancer cell invasion, tumor growth and metastasis. One mechanism underlying this modulation is HAI-2-mediated blocking of matriptase for HGF maturation (pro-HGF to HGF), leading to decreases of c-Met activity (Figure 4E), that might be expected to affect cancer cell invasion (Figure 4C), tumorigenicity and metastasis (Figure 6). Thus, loss of matriptase inhibition by down-regulated HAI-2 can thereby exacerbate PCa by promotion of cancer cell invasion, tumorigenicity and metastasis.
In summary, in this study we established a human PCa progression model through an in vivo selection approach that mimics PCa progression to an advanced stage with enhanced tumorigenicity, cancer cell invasion, and metastasis, that is at least in part due to down-regulation of HAI-2 and up-regulation of matriptase. The data also support the notion that dysregulation of the HAI-2/matriptase axis in favor of enhanced proteolysis contributes to prostate tumor growth, metastasis and malignancy. Thus, rebalancing the level of cell surface proteolysis either by HAI-2 expression or matriptase inactivation/silencing is tentatively suggested to be therapeutic strategy for PCa worth pursuing.
Materials and Methods
Cell culture
CWR22Rv1, 103E, N1, N2 and PC3 cells were maintained in RPMI1640 medium with 10% FBS (Hyclone, IL), 1% glutamine (Sigma-Aldrich, MO) and 1% Penicillin/Streptomycin (Invitrogen, CA).
Orthotopic xenograft of prostate cancer and isolation of the metastatic subline, N1 and N2
Athymic (nu/nu) nude mice (8 weeks old) were obtained from the National Laboratory Animal Center (Taiwan) and housed as described previously (37). The procedure for the establishment of N1 and N2 cells was described in the Supplementary Materials and Methods section.
Immunohistochemistry, HAI-2 cDNA cloning, western blotting, cell invasion and migration assays, and transfection and lentiviral particle preparation
Details are provided in the Supplementary Materials and Methods section.
Statistical analysis
Fisher’s exact test was used to evaluate the intensity differences (intensity score ≧2 vs. <2) of immunohistochemistry signals of matriptase or HAI-2 between tissues with higher and lower Gleason scores. A two-sided P value of less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This study was supported by Taiwan National Health Research Institutes Grants NHRI-EX101-9909BC and NHRI-EX102-9909BC, Taiwan National Science Council Grants NSC 97-2320-B-002-052-MY3, NSC 100-2628-B-002-004-MY4, and NSC 101-2324-B-002-015, and the Frontier and Innovative Research Grant of National Taiwan University 98R0305 and National Taiwan University Cutting-Edge Steering Research Project 10R71602C4 to M.S. Lee, Postdoctoral Fellowship from the Aim for Top University Program, National Taiwan University to T.S. Cheng, and grants from the National Science Council, Taiwan (NSC 98-2320-B-001-018-MY3), financial and instrumental support from the Agricultural Biotechnology Research Center and other institutes of Academia Sinica awarded to P.W. Hsiao.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Weiss L. Metastasis of cancer: a conceptual history from antiquity to the 1990s. Cancer Metastasis Rev. 2000;19:I–XI. 193–383. [PubMed] [Google Scholar]
- 2.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 4.Antalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochem J. 2010;428:325–346. doi: 10.1042/BJ20100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, et al. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–258. doi: 10.1023/a:1023003616848. [DOI] [PubMed] [Google Scholar]
- 6.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 7.Shi YE, Torri J, Yieh L, Wellstein A, Lippman ME, Dickson RB. Identification and characterization of a novel matrix-degrading protease from hormone-dependent human breast cancer cells. Cancer Res. 1993;53:1409–1415. [PubMed] [Google Scholar]
- 8.Lin CY, Anders J, Johnson M, Sang QA, Dickson RB. Molecular cloning of cDNA for matriptase, a matrix-degrading serine protease with trypsin-like activity. J Biol Chem. 1999;274:18231–18236. doi: 10.1074/jbc.274.26.18231. [DOI] [PubMed] [Google Scholar]
- 9.Oberst M, Anders J, Xie B, Singh B, Ossandon M, Johnson M, et al. Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo. Am J Pathol. 2001;158:1301–1311. doi: 10.1016/S0002-9440(10)64081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee MS, Kiyomiya K, Benaud C, Dickson RB, Lin CY. Simultaneous activation and hepatocyte growth factor activator inhibitor 1-mediated inhibition of matriptase induced at activation foci in human mammary epithelial cells. Am J Physiol Cell Physiol. 2005;288:C932–941. doi: 10.1152/ajpcell.00497.2004. [DOI] [PubMed] [Google Scholar]
- 11.Lin CY, Anders J, Johnson M, Dickson RB. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem. 1999;274:18237–18242. doi: 10.1074/jbc.274.26.18237. [DOI] [PubMed] [Google Scholar]
- 12.Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26:1546–1556. doi: 10.1038/sj.onc.1209966. [DOI] [PubMed] [Google Scholar]
- 13.Kang JY, Dolled-Filhart M, Ocal IT, Singh B, Lin CY, Dickson RB, et al. Tissue microarray analysis of hepatocyte growth factor/Met pathway components reveals a role for Met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node-negative breast cancer. Cancer Res. 2003;63:1101–1105. [PubMed] [Google Scholar]
- 14.Saleem M, Adhami VM, Zhong W, Longley BJ, Lin CY, Dickson RB, et al. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev. 2006;15:217–227. doi: 10.1158/1055-9965.EPI-05-0737. [DOI] [PubMed] [Google Scholar]
- 15.Wu SR, Cheng TS, Chen WC, Shyu HY, Ko CJ, Huang HP, et al. Matriptase is involved in ErbB-2-induced prostate cancer cell invasion. Am J Pathol. 2010;177:3145–3158. doi: 10.2353/ajpath.2010.100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, et al. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 2005;19:1934–1950. doi: 10.1101/gad.1300705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin CY, Tseng IC, Chou FP, Su SF, Chen YW, Johnson MD, et al. Zymogen activation, inhibition, and ectodomain shedding of matriptase. Front Biosci. 2008;13:621–635. doi: 10.2741/2707. [DOI] [PubMed] [Google Scholar]
- 18.Lee MS. Matrix-degrading type II transmembrane serine protease matriptase: Its role in cancer development and malignancy. J Cancer Molecules. 2006;2:183–190. [Google Scholar]
- 19.Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem. 2000;275:36720–36725. doi: 10.1074/jbc.M007802200. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 2000;275:26333–26342. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- 21.Jin X, Yagi M, Akiyama N, Hirosaki T, Higashi S, Lin CY, et al. Matriptase activates stromelysin (MMP-3) and promotes tumor growth and angiogenesis. Cancer Sci. 2006;97:1327–1334. doi: 10.1111/j.1349-7006.2006.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satomi S, Yamasaki Y, Tsuzuki S, Hitomi Y, Iwanaga T, Fushiki T. A role for membrane-type serine protease (MT-SP1) in intestinal epithelial turnover. Biochem Biophys Res Commun. 2001;287:995–1002. doi: 10.1006/bbrc.2001.5686. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi T, Qin L, Shimomura T, Kondo J, Matsumoto K, Denda K, et al. Purification and cloning of hepatocyte growth factor activator inhibitor type 2, a Kunitz-type serine protease inhibitor. J Biol Chem. 1997;272:27558–27564. doi: 10.1074/jbc.272.44.27558. [DOI] [PubMed] [Google Scholar]
- 24.Marlor CW, Delaria KA, Davis G, Muller DK, Greve JM, Tamburini PP. Identification and cloning of human placental bikunin, a novel serine protease inhibitor containing two Kunitz domains. J Biol Chem. 1997;272:12202–12208. doi: 10.1074/jbc.272.18.12202. [DOI] [PubMed] [Google Scholar]
- 25.Szabo R, Hobson JP, List K, Molinolo A, Lin CY, Bugge TH. Potent inhibition and global co-localization implicate the transmembrane Kunitz-type serine protease inhibitor hepatocyte growth factor activator inhibitor-2 in the regulation of epithelial matriptase activity. J Biol Chem. 2008;283:29495–29504. doi: 10.1074/jbc.M801970200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinz-Erian P, Muller T, Krabichler B, Schranz M, Becker C, Ruschendorf F, et al. Mutations in SPINT2 cause a syndromic form of congenital sodium diarrhea. Am J Hum Genet. 2009;84:188–196. doi: 10.1016/j.ajhg.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang HP, Chang MH, Chen YT, Hsu HY, Chiang CL, Cheng TS, et al. Persistent elevation of hepatocyte growth factor activator inhibitors in cholangiopathies affects liver fibrosis and differentiation. Hepatology. 2012;55:161–172. doi: 10.1002/hep.24657. [DOI] [PubMed] [Google Scholar]
- 28.Szabo R, Hobson JP, Christoph K, Kosa P, List K, Bugge TH. Regulation of cell surface protease matriptase by HAI2 is essential for placental development, neural tube closure and embryonic survival in mice. Development. 2009;136:2653–2663. doi: 10.1242/dev.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parr C, Watkins G, Mansel RE, Jiang WG. The hepatocyte growth factor regulatory factors in human breast cancer. Clin Cancer Res. 2004;10:202–211. doi: 10.1158/1078-0432.ccr-0553-3. [DOI] [PubMed] [Google Scholar]
- 30.Bergum C, List K. Loss of the matriptase inhibitor HAI-2 during prostate cancer progression. Prostate. 2010;70:1422–1428. doi: 10.1002/pros.21177. [DOI] [PubMed] [Google Scholar]
- 31.Muller-Pillasch F, Wallrapp C, Bartels K, Varga G, Friess H, Buchler M, et al. Cloning of a new Kunitz-type protease inhibitor with a putative transmembrane domain overexpressed in pancreatic cancer. Biochim Biophys Acta. 1998;1395:88–95. doi: 10.1016/s0167-4781(97)00129-2. [DOI] [PubMed] [Google Scholar]
- 32.Kongkham PN, Northcott PA, Ra YS, Nakahara Y, Mainprize TG, Croul SE, et al. An epigenetic genome-wide screen identifies SPINT2 as a novel tumor suppressor gene in pediatric medulloblastoma. Cancer Res. 2008;68:9945–9953. doi: 10.1158/0008-5472.CAN-08-2169. [DOI] [PubMed] [Google Scholar]
- 33.Morris MR, Gentle D, Abdulrahman M, Maina EN, Gupta K, Banks RE, et al. Tumor suppressor activity and epigenetic inactivation of hepatocyte growth factor activator inhibitor type 2/SPINT2 in papillary and clear cell renal cell carcinoma. Cancer Res. 2005;65:4598–4606. doi: 10.1158/0008-5472.CAN-04-3371. [DOI] [PubMed] [Google Scholar]
- 34.Fukai K, Yokosuka O, Chiba T, Hirasawa Y, Tada M, Imazeki F, et al. Hepatocyte growth factor activator inhibitor 2/placental bikunin (HAI-2/PB) gene is frequently hypermethylated in human hepatocellular carcinoma. Cancer Res. 2003;63:8674–8679. [PubMed] [Google Scholar]
- 35.Parr C, Jiang WG. Hepatocyte growth factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced invasion of human breast cancer cells. Int J Cancer. 2006;119:1176–1183. doi: 10.1002/ijc.21881. [DOI] [PubMed] [Google Scholar]
- 36.Kirchhofer D, Peek M, Lipari MT, Billeci K, Fan B, Moran P. Hepsin activates pro-hepatocyte growth factor and is inhibited by hepatocyte growth factor activator inhibitor-1B (HAI-1B) and HAI-2. FEBS Lett. 2005;579:1945–1950. doi: 10.1016/j.febslet.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 37.Tsai CH, Lin FM, Yang YC, Lee MT, Cha TL, Wu GJ, et al. Herbal extract of Wedelia chinensis attenuates androgen receptor activity and orthotopic growth of prostate cancer in nude mice. Clin Cancer Res. 2009;15:5435–5444. doi: 10.1158/1078-0432.CCR-09-0298. [DOI] [PubMed] [Google Scholar]
- 38.Chen CJ, Wu BY, Tsao PI, Chen CY, Wu MH, Chan YL, et al. Increased matriptase zymogen activation in inflammatory skin disorders. American journal of physiology Cell physiology. 2011;300:C406–415. doi: 10.1152/ajpcell.00403.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oberst MD, Singh B, Ozdemirli M, Dickson RB, Johnson MD, Lin CY. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem. 2003;51:1017–1025. doi: 10.1177/002215540305100805. [DOI] [PubMed] [Google Scholar]
- 40.List K, Bugge TH, Szabo R. Matriptase: potent proteolysis on the cell surface. Mol Med. 2006;12:1–7. doi: 10.2119/2006-00022.List. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhland K. Matriptase and its putative role in cancer. Cell Mol Life Sci. 2006;63:2968–2978. doi: 10.1007/s00018-006-6298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng TS, Chen WC, Lin YY, Tsai CH, Liao CI, Shyu HY, et al. Curcumin-targeting pericellular serine protease matriptase role in suppression of prostate cancer cell invasion, tumor growth, and metastasis. Cancer Prev Res. 2013;6:495–505. doi: 10.1158/1940-6207.CAPR-12-0293-T. [DOI] [PubMed] [Google Scholar]
- 43.Lee MS, Tseng IC, Wang Y, Kiyomiya K, Johnson MD, Dickson RB, et al. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol. 2007;293:C95–105. doi: 10.1152/ajpcell.00611.2006. [DOI] [PubMed] [Google Scholar]
- 44.Szabo R, Rasmussen AL, Moyer AB, Kosa P, Schafer JM, Molinolo AA, et al. c-Met-induced epithelial carcinogenesis is initiated by the serine protease matriptase. Oncogene. 2011;30:2003–2016. doi: 10.1038/onc.2010.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagakawa O, Yamagishi T, Fujiuchi Y, Junicho A, Akashi T, Nagaike K, et al. Serum hepatocyte growth factor activator (HGFA) in benign prostatic hyperplasia and prostate cancer. Eur Urol. 2005;48:686–690. doi: 10.1016/j.eururo.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Oberst MD, Williams CA, Dickson RB, Johnson MD, Lin CY. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem. 2003;278:26773–26779. doi: 10.1074/jbc.M304282200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.