Abstract
Objective
To determine whether maternal body mass index (BMI) influences the beneficial effects of diabetes treatment in women with gestational diabetes (GDM).
Study Design
Secondary analysis of a multicenter randomized treatment trial of women with GDM. Outcomes of interest were elevated umbilical cord c-peptide levels (>90th percentile 1.77 ng/mL), LGA birth weight (>90th percentile), and neonatal fat mass (g). Women were grouped into five BMI categories adapted from the WHO International Classification of normal, overweight, and obese adults. Outcomes were analyzed according to treatment group assignment.
Results
A total of 958 women were enrolled (485 treated and 473 controls). Maternal BMI at enrollment was not related to umbilical cord c-peptide levels. However, treatment of women in the overweight, Class I, and Class II obese categories was associated with a reduction in both LGA birth weight and neonatal fat mass. Neither measure of excess fetal growth was reduced with treatment in normal weight (BMI <25) or Class III (BMI ≥ 40) obese women.
Conclusion
There was a beneficial effect of treatment on fetal growth in women with mild GDM who were overweight or Class I and II obese. These effects were not apparent for normal weight and very obese women.
Keywords: Gestational diabetes, BMI, Treatment effect
Introduction
In women identified with gestational diabetes (GDM), risk of excessive fetal growth is commonly attributed to maternal hyperglycemia. However, maternal obesity and weight gain during pregnancy also have a significant impact on the development of overgrown infants.1–4 Recent analyses of the results of the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study demonstrate that both maternal body mass index (BMI) and glucose levels have strong independent associations with delivery of large for gestational age (LGA) infants.5 Neonatal fat mass and umbilical cord serum c-peptide, a surrogate for fetal hyperinsulinemia, are also increased in offspring of women with glucose intolerance identified during pregnancy.6,7 A secondary analysis of the HAPO study as well as other studies have found that neonatal fat mass, fetal hyperinsulinemia, and neonatal hypoglycemia are positively related to maternal size after adjustment for maternal glycemia.5,8,9,10
In the original HAPO observational study, LGA birth weight, umbilical cord serum c-peptide, and neonatal fat mass were all increased in a continuous fashion with maternal glucose values. This was true even at maternal glucose levels below thresholds typically used for diagnosis of diabetes during pregnancy.7, 11 Both the Australian Carbohydrate Intolerance Study (ACHOIS) and the Eunice Kennedy Shriver NICHD Maternal Fetal Medicine Units (MFMU) Network trial have established that treatment of women with mild degrees of hyperglycemia during pregnancy results in fewer large infants and consequent traumatic delivery. 12, 13 Taken together, these findings have led to the proposal of new thresholds for the diagnosis of GDM by the International Association of Diabetes and Pregnancy Study Groups (IADPSG).11, 14 It is estimated that these new thresholds will result in a dramatic increase in the number of pregnant women identified with mild GDM. In this secondary analysis of the MFMU Network trial, we sought to determine whether maternal BMI might alter the impact of therapy on fetal growth in women with mild GDM.12,13
Materials and Methods
This is a secondary analysis of a multi-center randomized clinical trial of treatment in women with mild GDM.13 Women with a 50-gram glucose screen administered between 24 and 30 weeks 6 days gestation and serum glucose value between 135 and 200 mg per deciliter were eligible for a 3-hour oral glucose tolerance test. Mild GDM was defined as a fasting serum glucose value less than 95 mg/dl with two or more glucose measurements that met or exceeded established thresholds after a 100-gram oral glucose load: 1 hour ≥ 180 mg/dl; 2-hour ≥ 155 mg/dl; 3-hour ≥ 140 mg/dl.15 Women were randomized to treatment versus control group at each clinical center. Treated women received formal nutritional counseling and diet therapy. They also performed daily self-monitoring of their blood glucose including fasting and 2 hour postprandial measurements. Insulin was prescribed if the majority of fasting or postprandial values met or exceeded 95 mg% and 120 mg% respectively. Control women received usual prenatal care.
For this analysis, all women were grouped into five BMI (kg/m2) categories adapted from the WHO International Classification of normal, overweight, and obese adults. Normal weight women were those with a BMI of less than 25 kg/m2 at enrollment. Overweight women had a BMI from 25.0 to 29.9 kg/m2 and obese women with a BMI greater than or equal to 30 kg/m2 were subcategorized into three groups. Those with a BMI of 30.0 to 34.9 kg/m2 were classified as Class I obese; those with a BMI of 35.0 to 39.9 kg/m2 were identified as Class II obese; and those with a BMI greater than or equal to 40 kg/m2 were identified as Class III or morbidly obese. Outcomes of interest for this secondary analysis were LGA birth weight, umbilical cord serum c-peptide levels, and neonatal fat mass. LGA birth weight was defined as birth weight above the 90th percentile for gestational age according to established national thresholds.16 Umbilical cord serum c-peptide was determined at a central laboratory. Fetal hyperinsulinemia was defined as cord c-peptide level greater than 1.77 ng/mL (95th percentile from an unselected obstetrical population of women in the Maternal-Fetal Medicine Units (MFMU) Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development). Shortly after birth, trained research personnel measured the infant’s length, head and upper mid-arm circumferences, and flank skinfold. Neonatal fat mass was calculated according to the technique described by Catalano and colleagues.17 Outcomes were analyzed according to treatment group assignment and BMI category.
Statistical analysis included Chi square or Fisher’s exact test for categorical variables, where appropriate, and the Wilcoxon Rank Sum test for continuous variables. A stratified analysis was used to examine the association between treatment group and each outcome of interest, stratified by BMI category. Statistical analysis was conducted with SAS software (SAS Institute, Cary, NC). A nominal two-sided P value less than 0.05 was considered to indicate statistical significance and no adjustments were made for multiple comparisons.
Results
Between October 2002 and November 2007, 958 women were identified with mild GDM at 16 clinical centers and randomized to either treatment (n=485) or usual prenatal care (n=473).13 Shown in Table 1 are the baseline maternal characteristics of all women enrolled in the randomized trial according to treatment group. Eighty-five percent of women with mild GDM were either overweight or obese. Moreover, almost half were obese at the time of enrollment with 39 (4%) categorized as morbidly obese. There were no significant differences in demographic variables, oral glucose tolerance test results, or BMI category between the 485 treated and the 473 control women.
Table 1.
Baseline maternal characteristics of 958 women identified with mild gestational diabetes and randomized to treatment or usual prenatal care.
| Characteristic | Treated n = 485 |
Control n = 473 |
p-value |
|---|---|---|---|
| Age, yrs | 29 ± 5.1 | 29 ± 5.6 | 0.86 |
| Primigravida | 104 (21) | 123 (26) | 0.10 |
| Race | 0.55 | ||
| Black | 56 (12) | 54 (12) | |
| White | 123 (25) | 119 (25) | |
| Hispanic | 281 (58) | 265 (56) | |
| Other | 25 (5) | 35 (7) | |
| Gestational age at randomization, wks | 29 ± 0.6 | 29 ± 1.5 | 0.13 |
| BMI category, kg/m2 | 1.0 | ||
| Normal weight (< 25) | 73 (15) | 70 (15) | |
| Overweight (25 – 29.9) | 187 (38) | 181 (38) | |
| Class I Obese (30 – 34.9) | 153 (32) | 151 (32) | |
| Class II Obese (35 – 39.9) | 53 (11) | 57 (11) | |
| Class III Obese (≥ 40) | 19 (4) | 20 (4) | |
| 50 mg challenge, mg% | 159 ± 15.3 | 160 ± 15.5 | 0.50 |
| 100 gm test: | |||
| Fasting, mg% | 87 ± 5.7 | 86 ± 5.7 | 0.34 |
| 1 hr, mg % | 192 ± 21.9 | 193 ± 19.3 | 0.11 |
| 2 hr, mg% | 174 ± 21.8 | 173 ± 19.6 | 0.84 |
| 3 hr, mg% | 137 ± 29.0 | 134 ± 31.5 | 0.14 |
Data presented as n(%) or mean ± SD.
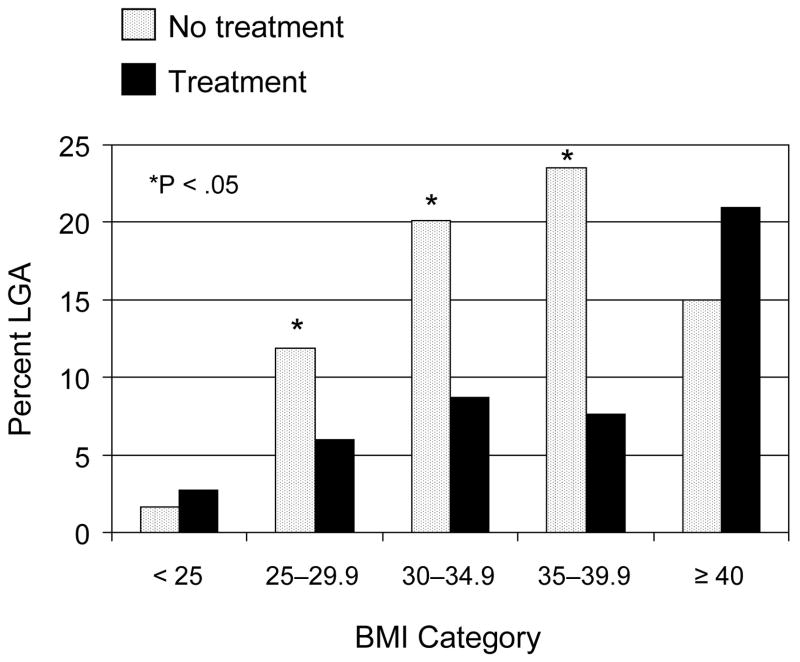
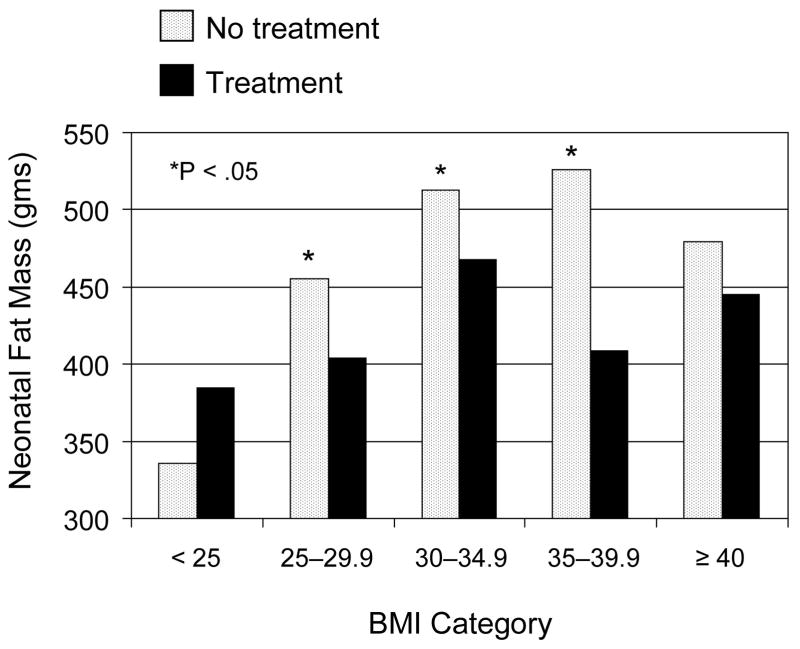
LGA birth weight, neonatal fat mass, and umbilical cord serum c-peptide levels were evaluated according to maternal BMI category and treatment group. The beneficial effects of treatment on the percent of women delivering an LGA birth weight infant are depicted in Figure 1. There were significant reductions in LGA birth weight in treated women with a BMI between 25 and 40 kg/m2 at enrollment. For example, 13 (9%) of the Class I obese women who received formal nutritional counseling and diet therapy delivered an overgrown infant compared to 29 (20%) of those who received routine prenatal care (P = .005). However, in women categorized as normal weight (n= 143) or in those who were morbidly obese (n=39), the beneficial treatment effect on excessive neonatal size was no longer evident. Likewise, as shown in Figure 2, women in the lowest (< 25 kg/m2) or highest (≥ 40 kg/m2) BMI categories delivered infants with similar neonatal fat mass regardless of treatment assignment. Treated normal weight women delivered infants whose mean fat mass was calculated to be 385 ± 139 g compared to 336 ± 180 g in normal weight women who received routine care. In contrast, neonatal fat mass was significantly reduced with diet therapy and routine glucose monitoring in women with a BMI between 25–40 kg/m2. Overweight treated women delivered infants with a mean fat mass of 404 ± 189 g compared to 455 ± 210 g for women who received routine care. Umbilical cord serum c-peptide was elevated in 20 percent of the entire cohort. However, consideration of maternal BMI at enrollment did not modify the small but statistically insignificant treatment effect previously reported from this randomized trial (P = 0.16, data not shown).13
Figure 1.
Percent LGA according to maternal body mass index (BMI) and treatment group in women with mild gestational diabetes who were randomized to either diet therapy and glucose monitoring or routine prenatal care. The X2 or Fisher exact test was used to compare treatment groups within BMI category.
Figure 2.
Neonatal Fat Mass (grams) according to maternal body mass index (BMI) and treatment group in women with mild gestational diabetes that were randomized to either diet therapy and glucose monitoring or routine prenatal care. The Wilcoxon rank sum test was used to compare treatment groups within BMI category.
Discussion
The most noteworthy finding in this secondary analysis of a large multicenter randomized treatment trial of women with mild GDM is that the benefits of treatment concerning fetal overgrowth varies by maternal BMI at enrollment. As evidenced by reductions in LGA birth weight and neonatal fat mass, infants of women classified as overweight, Class I, or Class II obese clearly benefited from formal nutritional counseling, diet therapy, and daily glucose monitoring. However, normal weight and morbidly obese women who were treated did not demonstrate similar significant reductions in these outcomes. Importantly, umbilical cord serum c-peptide (fetal hyperinsulinemia) did not appear to be related to maternal BMI.
In the latest Centers for Disease Control and Prevention report, approximately 56.7% of women of reproductive age were determined to be at least overweight. The prevalence of obesity was 30.2 %.18 The number of overweight and obese women at enrollment in the GDM study was remarkably higher (85%). This is not surprising when one considers that the pathophysiology of GDM is intimately linked to maternal obesity. However, the differential contribution of maternal obesity and maternal hyperglycemia to outcomes frequently associated with diabetes (i.e. LGA birth weight) is not easily sorted out. In a recent analysis of more than 23,000 women enrolled in the HAPO study, the likelihood of delivering an LGA infant was almost 5 times higher in women who were considered overweight or obese.5 After adjustment for fasting maternal glucose levels and numerous other maternal and fetal characteristics, there was a persistent 3-fold increased risk for LGA birth weight for the same BMI categories. The idea that maternal obesity is a more significant risk factor for delivery of an LGA infant when compared to maternal glucose intolerance has been reported in the past.3,4,19,20
The complex process of fetal growth is largely determined by transfer of nutrients across the placenta and is dependent on several factors, including maternal nutrient levels, maternal hormone levels, placental size and blood flow, and placental nutrient transporters. Together, these genetic and environmental factors can modify and differentially affect fetal growth.21 It is also widely held that in pregnancies complicated by diabetes, maternal hyperglycemia leads to fetal hyperinsulinemia and subsequent fetal overgrowth.22 This simple view however, does not explain the persistent increased risk of LGA infants in women with GDM who demonstrate good glycemic control.23 Along these lines, we were unable to demonstrate a significant reduction in LGA birth weight or neonatal fat mass in morbidly obese women treated for mild GDM. One explanation for these findings is that factors other than maternal hyperglycemia contribute to excess fetal growth in these cases. For example, maternal obesity has been linked with low-grade inflammation that leads to a shift in commitment of fetal mesenchymal cells from myogenesis to adipogenesis.24 This initial change in the properties of fetal muscle cells in offspring of obese mothers may lead to a cascade of events that is first recognized as excessive fetal size regardless of glucose tolerance or control. Alterations in expression of genes related to fetoplacental lipid homeostasis in obese women with GDM is another plausible mechanism for fetal overgrowth unrelated to maternal glycemia.25 Therefore, we believe that the lack of demonstrable treatment benefit in morbidly obese women indicate that there may be a limit to improvements achievable through interventions directed toward maternal glucose levels alone.
Similar to our findings in morbidly obese women, we were unable to reduce infant LGA birth weight or neonatal fat mass with nutritional counseling and diet therapy in 143 normal weight women with mild GDM. Actually, treated women had higher rates of both outcomes. When considering potential opportunities for improved outcomes in such women however, it is important to acknowledge that the number of overgrown infants delivered to such women is relatively small. In fact there were only 3 (2%) LGA infants delivered in this normal weight cohort. For comparison, one large population study of over 146,000 births, including women with diabetes, reported the rate of LGA birth weight in normal women as 8.7% in contrast to 16.4% in obese women.26 Classification of body composition for our study was based on weight at the time of enrollment and this could be one reason the absolute LGA rate was lower in these normal weight women with mild GDM. Nonetheless, we are of the view that the lack of benefit in these women suggests that there may be limited opportunity for improvement in outcomes in normal weight women with mild GDM. Indeed, current screening recommendations from the Fifth International Workshop-Conference on Gestational Diabetes Mellitus exclude normal weight women from a risk-based, selective screening approach.27 To further illustrate this point, based on the data now reported, we estimate that over 5,000 normal weight women with mild GDM would have to be randomized in order to determine if treatment was beneficial in this subgroup. Over the span of 5 years, the MFMU Network identified and randomized 143 normal weight women to treatment, making such a study in normal weight women not feasible.13
Our findings of diminished treatment effect on fetal growth at the extremes of maternal body composition should be viewed with caution. First, given that this study was performed in women with only mild degrees of glucose intolerance, the potential absolute risk reduction for fetal overgrowth is smaller.23 There were also very few randomized women who were considered morbidly obese. This is true despite the fact that women were classified according to weight at enrollment and this is plausibly overestimated. While the finding of no difference in LGA birthweight in morbidly obese women may arguably be related to these small numbers, we believe the lack of any reduction associated with treatment suggests a limitation to diet therapy in these women. Another potential weakness of this study is that we did not consider weight gain during pregnancy. However, in women weighing more than 135% of ideal body weight for height, it has been reported that there is no correlation between weight gain and birth weight.28 Despite these shortcomings, it seems apparent that diabetes-related fetal overgrowth is a marginal problem in normal weight women identified with mild GDM and that excess fetal growth in morbidly obese women with GDM cannot be simply addressed with interventions aimed at maternal glucose control. It also seems unlikely that a randomized trial addressing these subgroups of women with GDM is feasible. With consideration of the less stringent criteria for the diagnosis of GDM currently proposed by the IADPSG and the associated disproportionate increase in the number of women with mild GDM, we believe our findings could help guide resource allocation or therapeutic interventions and moderate expectations for treatment benefit in women with GDM at the extremes of BMI. 11
Acknowledgments
The authors thank the following subcommittee members who participated in protocol development and coordination between clinical research centers (Jo-Ann Tillinghast, R.N., M.S.N., and Francee Johnson, R.N., B.S.N.), and protocol/data management and statistical analysis (Elizabeth Thom, Ph.D.).
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD27915, HD34116, HD40485, HD34208, HD27869, HD40500, HD40560, HD34136, HD40544, HD27860, HD40545, HD53097, HD21410, HD27917, HD40512, HD53118, HD36801], General Clinical Research Centers Grant [M01-RR00034] and the National Center for Research Resources [UL1-RR024989, M01-RR00080, UL1-RR025764, C06-RR11234 ] and does not necessarily represent the official views of the NICHD or NIH.
]In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
University of Texas Southwestern Medical Center — K. Leveno, L. Moseley, J. Gold, D. Bradford, L. Fay, M. Garcia, F. Capellan
Columbia University — M. Miodovnik, F. Malone, S. Bousleiman, H. Husami, V. Carmona, N. Fredericks, E. Gantioqui, B. Greenspan, M. Williams
University of Utah — K. Anderson, P. Ashby, and S. McAllister (University of Utah Health Sciences Center), S. Quinn and F. Castinella (LDS Hospital), A. Guzman and J. Steiner (McKay-Dee Hospital), J. Parker (Utah Valley Regional Medical Center)
University of Alabama at Birmingham — J. Sheppard, J. Tisdale, A. Northen, W. Andrews
Brown University — M. Carpenter, D. Catlow, D. Allard, M. Seebeck, J. Tillinghast
The Ohio State University — J. Iams, F. Johnson, C. Latimer, E. Weinandy, B. Maselli
University of North Carolina at Chapel Hill — K. Dorman, S. Brody, S. Timlin, J. Bernhardt
Drexel University — A. Sciscione, M. Hoffman, E. Guzman, M. Talucci, T. Grossman, C. Perez, L. Zeghibe, P. Tabangin
Case Western Reserve University-MetroHealth Medical Center — B. Mercer, B. Stetzer, C. Milluzzi, W. Dalton, S. Pichette
Wake Forest University Health Sciences — M. Swain, P. Meis, J. White
The University of Texas Health Science Center at Houston-Children’s Memorial Herman Hospital — L. Gilstrap, K. Cannon, J. Martinez, D. Dusek
University of Texas Medical Branch — J. Moss, J. Brandon, A. Jackson, G. Hankins, D. Sharp
University of Pittsburgh — S. Caritis, M. Bickus, H. Birkland, M. Cotroneo, N. Cuddy
Wayne State University — G. Norman, P. Lockhart, S. Blackwell, L. Quast
Northwestern University — P. Simon, G. Mallett
Oregon Health & Science University — J. Tolosa, L. Davis, E. Lairson, C. Cromett, C. Naze, M. Blaser
The George Washington University Biostatistics Center — E. Thom, J. Zachary, B. Getachew, C. Cobb, L. Leuchtenburg, S. Gilbert
Eunice Kennedy Shriver National Institute of Child Health and Human Development — S. Tolivaisa, K. Howell
MFMU Network Steering Committee Chair (University of Texas Medical Branch, Galveston, TX) — G.D. Anderson, M.D.
Footnotes
The authors report no conflict of interest.
References
- 1.Schaefer-Graf UM, Heuer R, Kilavuz O, Pandura A, Henrich W, Vetter K. Maternal obesity not maternal glucose values correlates best with high rates of fetal macrosomia in pregnancies complicated by gestational diabetes. J Perinat Med. 2002;30:313–21. doi: 10.1515/JPM.2002.046. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191:964–8. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 3.Schaffer-Graf UM, Kjos SL, Kilavuz O, et al. Determinants of fetal growth at different periods of pregnancies complicated by gestational diabetes mellitus or impaired glucose tolerance. Diabetes Care. 2003;26:193198. doi: 10.2337/diacare.26.1.193. [DOI] [PubMed] [Google Scholar]
- 4.Voldner N, Qvigstad E, Froslie KF, et al. Increased risk of macrosomia among overweight women with high gestational rise in fasting glucose. J Maternal Fetal and Neonate Med. 2010;23(1):74–81. doi: 10.3109/14767050903121472. [DOI] [PubMed] [Google Scholar]
- 5.HAPO Cooperative Research Group. Hyperglycaemia and adverse pregnancy outcome (HAPO) study. BJOG. 2010;175:575–84. doi: 10.1111/j.1471-0528.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- 6.Durnwald C, Huston-Presley L, Amini S, Catalano P. Evaluation of body composition of large-for-gestational-age infants of women with gestational diabetes mellitus compared with women with normal glucose tolerance levels. Am J Obstet Gynecol. 2004;191:804–8. doi: 10.1016/j.ajog.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 7.HAPO Study Cooperative Research Group. Metzger BE, et al. Hyperglycemia and adverse pregnancy outcomes. N Eng J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 8.Hull H, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol. 2008;198:416.e1–e6. doi: 10.1016/j.ajog.2007.10.796. [DOI] [PubMed] [Google Scholar]
- 9.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–03. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Patterson A, Aulinas A, Maria MA, et al. Maternal body mass index is a predictor of neonatal hypoglycemia in gestational diabetes mellitus. J Clin Endocrinol Metab. 2012;97:1623–1628. doi: 10.1210/jc.2011-3425. [DOI] [PubMed] [Google Scholar]
- 11.Coustan DR, Lowe LP, Metzger BE, Dyer AR. The hyperglycemia and adverse pregnancy outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol. 2010;202:654.e1–e6. doi: 10.1016/j.ajog.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Eng J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 13.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial for treatment for mild gestational diabetes. N Eng J Med. 2009;361:1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadar E, Hod M. Establishing consensus criteria for the diagnosis of diabetes in pregnancy following the HAPO study. Ann N Y Acad Sci. 2010;1205:88–93. doi: 10.1111/j.1749-6632.2010.05671.x. [DOI] [PubMed] [Google Scholar]
- 15.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21 (Suppl 2):B161–7. [PubMed] [Google Scholar]
- 16.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin and gender. Maternal Child Health J. 1999;3:225–31. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 17.Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol. 1995;173:1176–81. doi: 10.1016/0002-9378(95)91348-3. [DOI] [PubMed] [Google Scholar]
- 18.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007;109:419–33. doi: 10.1097/01.AOG.0000253311.44696.85. [DOI] [PubMed] [Google Scholar]
- 19.Spellacy W, Miller S, Winegar A, Peterson PQ. Macrosomia-maternal characteristics and infant complications. Obstet Gynecol. 1985;66:158–61. [PubMed] [Google Scholar]
- 20.Lucas MJ, Lowe TW, Bowe L, McIntire DD. Class A1 Gestational diabetes: a meaningful diagnosis. Obstet Gynecol. 1993;82:260–65. [PubMed] [Google Scholar]
- 21.Catalano PM, Drago NM, Amini SB. Factors affecting fetal growth and body composition. Am J Obstet Gynecol. 1995;172:1459–63. doi: 10.1016/0002-9378(95)90478-6. [DOI] [PubMed] [Google Scholar]
- 22.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204:479–87. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brody SC, Harris R, Lohr K. Screening for gestational diabetes: a summary of the evidence for the U.S. Preventive Services Task Force. Obstet Gynecol. 2003;101:380–92. doi: 10.1016/s0029-7844(02)03057-0. [DOI] [PubMed] [Google Scholar]
- 24.Jansson T, Cetin I, Powell TL, Desoye G, Radaelli T, Ericsson A, et al. Placental transport and metabolism in fetal overgrowth – a workshop report. Placenta. 2006;27:109–13. doi: 10.1016/j.placenta.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Radaelli T, Lepercq J, Varastehpour A, Basu S, Catalano PM, Hauguel-De Mouzon S. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am J Obstet Gynecol. 2009;201:209.e1–10. doi: 10.1016/j.ajog.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Getahun D, Ananth CV, Peltier MR, Salihu HM, Scorza WE. Changes in pregnancy body mass index between the first and second pregnancies and risk of large-for-gestational-age birth. Am J Obstet Gynecol. 2007;196:530.e1–e8. doi: 10.1016/j.ajog.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 27.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30:S251–60. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 28.Abrams BF, Laros RK. Prepregnancy weight, weight gain, and body birthweight. Am J Obstet Gynecol. 1986;154:503–09. doi: 10.1016/0002-9378(86)90591-0. [DOI] [PubMed] [Google Scholar]