Abstract
Objective
Using a validated swine model of human scar formation, hyper- and hypopigmented scar samples were examined for their histological and optical properties to help elucidate the mechanisms and characteristics of dyspigmentation.
Methods
Full thickness wounds were created on the flanks of red Duroc pigs and allowed to heal. Biopsies from areas of hyperpigmentation, hypopigmentation, and uninjured tissue were fixed and embedded for histological examination using Azure B and primary antibodies to S100B, HMB45, and α-MSH. Spatial Frequency Domain Imaging (SFDI) was then used to examine the optical properties of scars.
Results
Hyperpigmentation was first noticeable in healing wounds around weeks 2–3, gradually becoming darker. There was no significant difference in S100B staining for the presence of melanocytes between hyperpigmented and hypopigmented scar samples. Azure B staining of melanin was significantly greater in histological sections from hyperpigmented areas than sections from both uninjured skin and hypopigmented scar (p<0.0001). There was significantly greater staining for α-MSH in hyperpigmented samples compared to hypopigmented samples (p=0.0121) and HMB45 staining was positive for melanocytes in hyperpigmented scar. SFDI at a wavelength of 632 nm resulted in an absorption coefficient map correlating with visibly hyperpigmented areas of scars.
Conclusions
In a red Duroc model of hypertrophic scar formation, melanocyte number is similar in hyper- and hypopigmented tissues. Hyperpigmented tissues, however, show a greater amount of melanin and α-MSH, along with immunohistochemical evidence of stimulated melanocytes. These observations encourage further investigation of melanocyte stimulation and the inflammatory environment within a wound that may influence melanocyte activity. Additionally, SFDI can be used to identify areas of melanin content in mature, pigmented scars, which may lead to its usefulness in wounds at earlier time points before markedly apparent pigmentation abnormalities.
Introduction
Abnormal pigmentation in scars presents a challenge to clinicians and can be a source of distress to many patients1. These types of scars are common after cutaneous trauma and especially prevalent and psychosocially impactful in individuals recovering from burn injury2,3. While normal skin pigmentation is fairly well-understood4, the mechanisms that lead to both hypo- and hyperpigmentation in scar are less clear5. In order to target and improve abnormal pigmentation as a sequela of burn injury, its role in hypertrophic scar must be better understood.
It is generally accepted that baseline skin pigmentation as well as sun-tanning are not a function of melanocyte quantity, but rather a function of the activity of these melanocytes, either in the ratio of eumelanin (brown-black) to pheomelanin (red-yellow) or in the amount of melanin an activated melanocyte produces. Histologically, melanocytes appear as fusiform dendritic cells located in the basal layer of the epidermis, and one melanocyte is typically associated with 30–40 keratinocytes 6,7. Melanosomes, or melanin-containing granules, are exported from melanocytes to nearby keratinocytes, leading to visible pigment. Once melanosomes have entered keratinocytes they are normally distributed toward the superficial side of the nucleus to shield it from UV radiation4. Differences in the quantity, content, and distribution of these melanosomes lead to differences in pigmentation, whereas pure numbers of melanocytes are less variable between individuals. The abnormal behavior of melanocytes has largely been studied with respect to melanoma, and their behavior in the dyspigmentation of scar is still inadequately understood.
Melanocytes and their secretion products have been associated with components of different fibroproliferative processes. Areas of the human body more prone to keloid formation have been found to have higher melanocyte concentrations, while in-vitro studies have shown that fibroblasts in co-culture with melanocytes proliferate more quickly than those without melanocytes, exhibiting increased mRNA expression levels for type I collagen and TGF-β18. Alpha-melanocyte stimulating hormone (α-MSH), a key component of melanogenesis, has been shown to be present in the migrating epithelial edge and wound bed of early full thickness excisional murine wounds, as well as in the sebaceous glands and epidermis of uninjured tissue adjacent to wounds9. In human burn wounds, α-MSH is present in dermal fibroblasts and epidermal keratinocytes early in the healing process, and later, can be identified in dermal fibroblasts and epidermal keratinocytes of hypertrophic scar9.
Duroc swine are known to scar with varying degrees of abnormal pigmentation, but prior to injury possesses uniformly colored red-brown to brown-black skin and provides an excellent model for human wound healing10–12. In the current study, characteristics of hypo- and hyper-pigmented scar as well as the optical properties of melanin were investigated in a validated model of hypertrophic scar. The primary aim was to evaluate whether quantifiable differences exist in factors hypothesized to result in abnormal pigmentation in hyper-and hypopigmented scar. To this end, melanocyte quantity, melanocyte activation, melanin content, and alpha-melanocyte stimulating hormone were examined.
Methods
Animal Model and Wound Creation
All animal work described was reviewed and approved by the MedStar Health Research Institute’s Institutional Animal Care and Use Committee (IACUC). Juvenile castrated male Duroc swine (30–55kg) were received and handled according to facility standard operating procedures under an animal care and use program accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and Public Health Service (PHS) Animal Welfare assured.
On the day of surgery, animals were anesthetized with a combination of ketamine and xylazine that were delivered intramuscularly. Animals were intubated, maintained on isoflurane, placed on a warming blanket, and ventilated while heart rate, blood pressure, peripheral oxygen saturation, end-tidal carbon dioxide saturation, and core body temperature were continuously monitored by trained research staff. The body hair was clipped and the skin was prepped with chlorhexidine gluconate scrub. To create full-thickness wounds, one 4 inch × 4 inch (10.16 cm × 10.16 cm) square was excised over the rib cage on each side of the animal, at a total depth of 0.090 inches (0.030 in × 3 passes) using a Zimmer dermatome (Zimmer, Ltd., Swindon, UK). In previous experience, this wound creation technique consistently results in hypertrophic scars13. Punch biopsies (3mm) were taken at baseline (pre-excision) and immediately post-excision and placed in formalin for subsequent histology. Mepilex® Ag dressings (Monlylke, Gothenburg, Sweden) were applied to wounds after wound creation and at each dressing change. Animals were fitted with custom-made 2–3 mm thick neoprene vests14, which completely covered the applied dressings while allowing mobility of the animal. Buprenorphine and fentanyl were administered for pain control at the end of each surgical procedure. Animals returned to the operating room every 7 days until day 133 post-wound creation for examinations, imaging, dressing changes, and biopsies. The animals were examined at least twice daily to monitor animal health and to identify any signs of pain, wound infection, or distress.
Spectral Imaging
The Spatial Frequency Domain Imaging (SFDI) module of a spectropolarimetric system previously described15–17 was used to image the absorption and scattering coefficients of re-epithelialized scars at the described weekly timepoints, beginning at re-epithelialization of wounds. Images were subsequently processed to develop optical properties calculations that were related to pathophysiology of the scar tissue. Briefly, wound optical properties μa and μs at different wavelengths are obtained, as well as the rough surface signature as a function of the incident illumination angle. From the total μa the concentration of melanin (M), total hemoglobin (Vblood), and water (W) are calculated using a minimization algorithm, Eq. 1 below, and tabulated values of oxygenated and deoxygentated hemoglobin (μa_oxy and μa_deoxy), water (μa_water) and melanin (μa_melanin) at the selecet wavelengths18,19.
| Eq. 1 |
Therefore, the minimization algorithm in combination with tabulated data of melanin, oxygenated and deoxygenated hemoglobin, and water absorption coefficient were used to reconstruct maps of melanin volume fraction. Ultimately this information was used to quantify abnormal melanin concentration in normal skin, hypo-, and hyperpigmented scar.
Histology
Punch biopsies taken from areas of hyper- and hypopigmentation were fixed in 10% formalin and embedded in paraffin. Paraffin blocks were sectioned at a thickness of 5 μm and baked at 60 °C. After cooling, slides were deparaffinized and rehydrated with PBS. Antigen retrieval was performed in Tris-EDTA buffer at 95–100 °C for 20 minutes. Slides were then cooled for 5 minutes in running cold water. Slides were washed in 0.025% Triton X-100 for 5 minutes × 2. Slides were blocked in 5% nonfat milk, 1% BSA, in PBS for 1 hour. After blocking, slides were incubated with monoclonal mouse anti-S100 beta (Abcam, Cambridge, UK), polyclonal rabbit anti-alpha-melanocyte stimulating hormone (α-MSH) (Thermo Fisher Scientific Inc., Waltham, MA), or monoclonal mouse anti-HMB45 (Abcam, Cambridge, UK) primary antibody diluted in PBS-0.05% Tween-20 at 4°C overnight. Negative control slides were incubated with PBS-Tween 20 only. After overnight incubation with primary antibody, slides were rinsed with 0.025% Triton-X-100 for 5 minutes × 2, then treated according to methods described for immunofluorescent immunohistochemistry or immunohistochemistry with Azure B staining, below.
Following the above methods for histology, polyclonal goat anti-mouse IgG-FITC conjugated (EMD Millipore, Billerica, MA) or polyclonal goat anti-rabbit IgG-CY3 conjugated (Abcam, Cambridge, UK) secondary antibody diluted in PBS-0.05% Tween-20 was applied for 1 hour at room temperature. Slides were rinsed with PBS for 5 minutes three times, then counterstained with DAPI (Santa Cruz Biotechnology, Dallas, TX) for 10 minutes. Slides were then viewed with a Zeiss Axioimager microscope and multichannel black and white camera equipped with fluorescence filters (Carl Zeiss, Oberkochen, Ger).
Following the above methods for histology, endogenous peroxidase activity was quenched with 15 minute incubation in 0.3% H2O2. Goat polyclonal anti-mouse IgG+IgM-HRP (Abcam, Cambridge, UK) secondary antibody diluted in PBS-0.05% Tween-20 was applied for 2 hours at room temperature. Slides were rinsed with PBS for 5 minutes × 3, then developed with DAB peroxidase substrate (Vector Laboratories, Burlingame, CA) for 10 minutes. After 5 minute rinse with running tap water, slides were stained with Azure B (Sigma-Aldrich, St. Louis, MO) for 30 minutes, then rinsed in running tap water. Lastly, slides were counterstained with hematoxylin, cleared, and mounted, then viewed with a Zeiss Axioimager microscope and brightfield color camera (Carl Zeiss, Oberkochen, Ger).
Image Analysis
Hyperpigmented and hypopigmented scar samples for all time points after the appearance of pigmentation were stained and imaged in duplicate. Immunofluorescent images stained for S100B were examined at 400x magnification and after the confirmation of a lack of staining in negative controls, the number of positively stained cells per 400x field were manually counted and recorded. Immunofluorescent images stained for α-MSH were examined at 100x magnification, and after the recording of background CY3 staining levels in negative control images, positively stained images were analyzed for CY3 expression levels using Zeiss Zen software (Carl Zeiss, Oberkochen, Ger). Control background staining levels were subtracted from CY3 levels of positively stained slides, then CY3 expression levels were calculated normalized to DAPI expression. Azure-B and HMB45/DAB stained images were examined at 400x magnification and after the confirmation of a lack of staining in negative controls, Azure-B-stained melanin was quantified as a percentage of the imaged area using Image J software (available from the National Institutes of Health) with a blue image stack threshold set at 160. HMB45-positively stained cells were manually counted per 400x viewing field. All image analysis data were checked for outliers with Grubbs’ test with an alpha set at 0.05. Averages, standard deviations, and statistical significance with a two-tailed, unpaired t-test were calculated. Statistical significance was set at p<0.05. Prism GraphPad Software (GraphPad Software, Inc., LaJolla, CA) was used for graphical representations of results.
Results
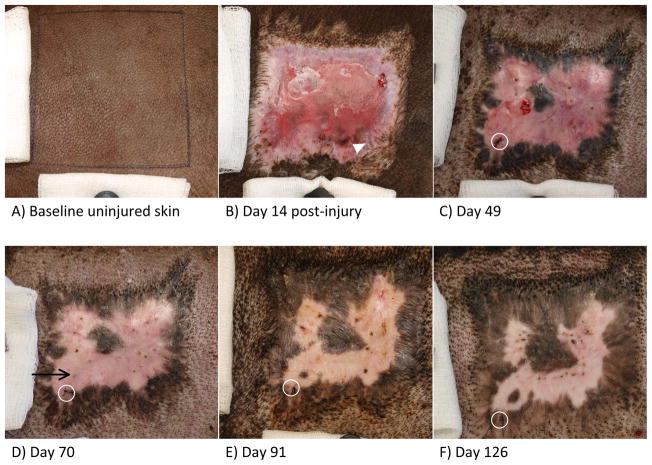
Peripheral hyperpigmentation directly adjacent to normal uninjured skin began to appear as early as day 14 after injury (Figure 1B, arrowhead). Early after the completion of epithelialization, peripheral hyperpigmentation continued to increase in area and darkness of color, with the additional appearance of an island of hyperpigmentation surrounded by erythematous, hypopigmented skin (Figure 1C). A firm, hypertrophic scar is usually apparent in this model by day 7013, with fading erythema and increasing areas of peripheral hyperpigmentation. Additionally, a new island of hyperpigmentation surrounded by hypopigmented tissue appeared around day 70 in the animal pictured in Figure 1 (see image D). Figure 1E depicts day 91 after wound creation, with continued firm hypertrophic scar and largely resolved erythema. Additionally, the central island of hyperpigmentation is shown merged with the superior periphery of hyperpigmentation, and the newer area of hyperpigmentation first noticeable at day 70 has greatly increased in size and darkness of color. Day 126, or week 18, after wound healing is shown in Figure 1F, with continued increases in hyperpigmentation, but the maintenance of a border of hypopigmented scar surrounding the hyperpigmented island seen in the left lower corner. The areas of hyperpigmentation generally increased in size and pigmentation over time, with peripheral hyperpigmentation showing the appearance of pigment migration or spread toward the center of the wound/scar (Figure 2A). Additionally, while some islands of hyperpigmented tissue not associated with uninjured skin or hair follicles eventually merged with peripheral areas of hyperpigmentation, as shown in Figure 1E, others remained surrounded by hypopigmented scar for the duration of the 133-day observation period (Figure 1D–F, Figure 2B). Erythema in hypopigmented scar faded with time, but interestingly, these areas remained substantially lighter in color than the surrounding uninjured skin or baseline uninjured skin (Figure 1).
Figure 1.
Macroscopic images of Duroc uninjured skin, wound, and scar. Images taken at a distance of 25cm from the skin surface. A) Baseline uninjured skin. B) Wound 14 days post injury with incomplete epithelialization. Arrowhead points to early hyperpigmented areas associated with surrounding uninjured skin and hair follicles. C) Day 49 after wound creation, early after completion of epithelialization. Circle indicates imaging reference suture. D) Day 70 after wound creation. Black arrow points to first indication of new island of hyperpigmented scar. Circle indicates imaging reference suture. E) Day 91 after wound creation. Circle indicates imaging reference suture. F) Day 126 after wound creation. Circle indicates imaging reference suture.
Figure 2.

Magnified macroscopic images of Duroc hyper- and hypopigmented scar. Peripheral areas of hyperpigmentation exhibited a pattern of expansion toward the center of the wound/scar, as seen in A, with the direction of expansion indicated by the arrow. Islands of hyperpigmented scar also arose that were completely surrounded by hypopigmented scar, as seen in B. These islands were not associated with hair follicles or normal, uninjured skin. Circle points out the reference suture used for spectral imaging.
During the period of scar observation, regions of interest were imaged with the Spatial Frequency Domain Imaging module of a spectropolarimetric system15–17. Reference sutures were used to recreate the same imaging region of interest from week to week, which can be seen encircled in Figures 1 and 2. One area of imaging included the hyperpigmented island seen in Figure 1D–F and Figure 2B. As described, a minimization algorithm using melanin, oxygenated and deoxygenated hemoglobin, as well as water absorption coefficient data was used to reconstruct maps of melanin volume fraction, represented visually in Figure 3. This information was used to quantify melanin concentration within the imaged region of interest. The trend in melanin concentration within the region is represented graphically in Figure 4, with hyper- and hypopigmented regions graphed over time, and the general volume fraction of melanin in normal uninjured skin represented for reference. Prior to approximately week 13 (day 91), the hyperpigmented region is analyzed to contain a melanin volume fraction less than that of normal uninjured skin, though it continued to increase in the following weeks. The data points for hyper- and hypopigmentation at week 18 correspond to the images seen in Figure 1F, 2B, and 3, which were also captured at week 18.
Figure 3.
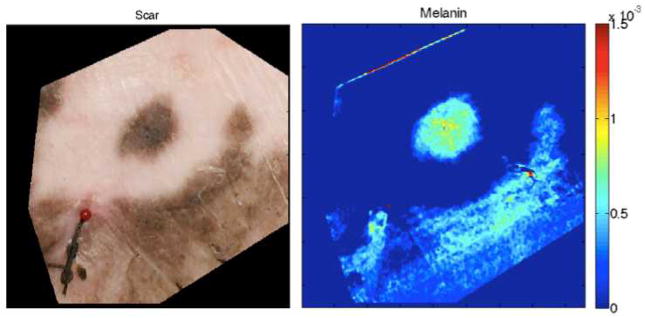
Photographic region of interest and melanin volume fraction map from Spatial Frequency Domain Imaging. Reference sutures were used to recreate the same imaging region of interest from week to week, seen in the left lower corner of both images. Dark blue in the melanin volume fraction map corresponds to a melanin volume fraction of zero. These images correspond to week 18 in Figure 4.
Figure 4.
Graphical representation of melanin volume fraction maps created with Spatial Frequency Domain Imaging. Upper line with open triangles represents the melanin volume fraction of hyperpigmented areas within the region of interest. Lower line with open circles represents the melanin volume fraction of hypopigmented areas within the region of interest. Dashed line lies at the typical melanin volume fraction found in normal uninjured Duroc skin. Week 18 corresponds to the images shown in Figure 3.
S100B is a known immunohistologic marker for melanocytes20 and also known to be absent in keratinocytes and fibroblasts21. Primary antibody to was used to stain for melanocytes in hyperpigmented and hypopigmented tissue samples over the course of scar imaging and observation. A FITC-tagged anti-mouse secondary antibody was used to visualize stained cells by immunofluorescence. Both hyper- and hypopigmented tissue samples contained S100B-positive melanocytes, at an average of 1–2 per 400x imaging field. S100B positive melanocytes in hyperpigmented skin can be visualized in Figure 5A, and S100B positive melanocytes in hypopigmented skin can be visualized in Figure 5B. Positively-stained cells were counted and revealed that there was no significant difference in the average number of melanocytes per 400x imaging field, as shown in Figure 6 (p=0.5203, n=36).
Figure 5.
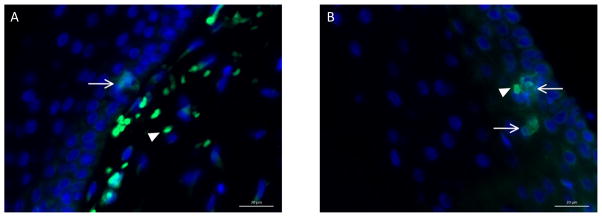
Representative immunofluorescent photomicrographs of S-100B-stained hyperpigmented and hypopigmented scar samples. An FITC-tagged anti-mouse secondary antibody was used to visualize stained cells by immunofluorescence. There was no difference in the average number of S100-positive cells between hyper- and hypopigmented scar samples within a 400x viewing field. Negative controls received secondary antibody only. DAPI counterstained nuclei. Scale bar = 20μm. 400x magnification. A) Hyperpigmented scar. Arrow points to positively-stained melanocyte. Arrowhead points to autofluorescent red blood cells. B) Hypopigmented scar. Arrows point to positively-stained melanocytes. Arrowhead points to autofluorescent red blood cell.
Figure 6.
Hyperpigmented and hypopigmented scar samples were stained with primary antibody to S100B. An FITC-tagged anti-mouse secondary antibody was used to visualize stained cells by immunofluorescence. There was no difference in the average number of S100-positive cells within a 400x viewing field between hyper- and hypopigmented scar samples. By two-tailed unpaired Students’ t-test, p=0.5205, n=36. Error bars represent standard deviations of the mean.
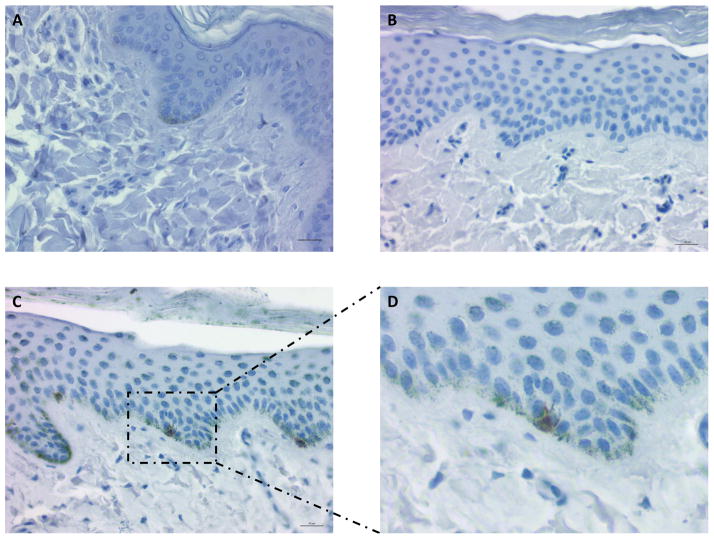
Azure B is a useful tool in evaluating melanocytic lesions immunohistochemically, as it counter-stains melanin a green-blue color, allowing the brown chromogen of DAB to stand out from otherwise brown melanosomes22. Azure B stain was used to stain melanosomes green-blue for melanin-quantification and for contrast with DAB-stained activated melanocytes. Consistent with spectral imaging, there was a clear increase in the amount of melanin staining of hyperpigmented scar as compared to both uninjured skin and hypopigmented scar (Figures 7C, A, and B, respectively). Uninjured skin also appeared to contain a greater amount of melanin than hypopigmented scar (Figure 7A vs B). Analysis of images quantifying Azure B staining showed that hyperpigmented scar sample images contained positive melanin staining over average of 15.3±10.3% of the imaged area, whereas uninjured skin and hypopigmented scar samples showed positive staining over 3.7±3.0% and 2.0±2.2% of the imaged areas, respectively (Figure 8). Hyperpigmented scars showed significantly greater staining than both hypopigmented scar and normal skin (p<0.0001, n=68). Interestingly, normal skin also showed significantly greater staining for melanin than hypopigmented scar (p= 0.0043, n=30).
Figure 7.
Representative photomicrographs of samples stained with Azure B and primary antibody to HMB45 with DAB. Scale bar = 20μm. A–C pictured at 400x magnification. A) Uninjured Duroc skin showing some melanin staining at the base of the epidermis. No HMB45-positive melanocytes. B) Hypopigmented scar showing minimal melanin staining. No HMB45-positive melanocytes. C) Hyperpigmented scar showing widespread melanin staining from basal layer of epidermis up to keratin layer. Occasional HMB45-positive melanocytes. Rectangle borders image enlarged in D. D) Enlarged portion of image C. HMB45-positive melanocyte with dendritic projections toward neighboring keratinocytes.
Figure 8.
Azure B staining of melanin was quantified using Image J as a percentage of imaged area positively-stained. Normal skin showed an average of 3.7±3.0%, hypopigmented scar showed an average of 2.0±2.2%, and hyperpigmented scar showed an average of 15.3 ±10.3%. Statistically significant differences were assessed using a two tailed, unpaired Students’ t-test, and are denoted by p-values. N=30 for uninjured skin samples, n=68 for hyperpigmented samples, and n=53 for hypopigmented samples. Error bars represent standard deviations of the mean.
Primary antibody to HMB45 was used in combination with the Azure B staining above to identify active melanocytes, as it is known that in human tissue, HMB45 detects immature melanosomes in early-stage melanocytes and re-activated adult melanocytes, but does not stain normal adult epidermal melanocytes11,23. HMB45-positive melanocytes were identified in hyperpigmented scar at an average frequency of 1–2 per 400x field of view, similar to that seen with S100B staining (Figure 7C). There were no HMB45-positive melanocytes identified in hypopigmented scar or normal uninjured skin. In some HMB45-stained melanocytes, dendritic projections toward neighboring keratinocytes could be visualized as well (Figure 7D).
Alpha-MSH is known to bind the melanocortin 1 receptor (MC1R) leading to downstream expression of tyrosinase, required for the production of melanin24. To investigate melanocyte-stimulating activity, primary antibody to α-MSH was used in immunofluorescent staining of hyper- and hypopigmented scar samples. A CY3-labeled anti-rabbit secondary antibody was used and cell nuclei were counterstained with DAPI (Figure 9). CY3 expression was measured and quantified relative to DAPI expression across samples. Hyperpigmented scar samples showed a greater average CY3 expression than hypopigmented samples (4.42±3.60 vs 2.49±2.53, p=0.0121, n=42) (Figure 10).
Figure 9.

Representative immunofluorescent photomicrographs of α-MSH-stained hyperpigmented and hypopigmented scar samples. A CY3-tagged anti-rabbit secondary antibody was used to visualize stained cells by immunofluorescence. Hyperpigmented scars showed stronger staining for α-MSH on average than hypopigmented scars. Negative controls received secondary antibody only. DAPI counterstained nuclei. Scale bar = 50μm. 100x magnification. A) Hyperpigmented scar. Arrowhead points to autofluorescent red blood cells. B) Hypopigmented scar. Arrowhead points to autofluorescent red blood cells.
Figure 10.
Immunofluorescent α-MSH staining of scar samples was quantified using Zeiss Zen software. Relative CY3 expression as a function of DAPI expression is represented graphically. Hyperpigmented scar samples showed an average relative CY3 expression of 4.42±3.60 and hypopigmented scar samples showed an average expression of 2.49±2.53 (p=0.0121, n=42). Error bars represent standard deviations of the mean.
Discussion
Scar, especially hypertrophic scar, remains a challenging clinical issue. When scars are abnormally pigmented, they become even more distressing to patients and providers25. The continued investigation into hypertrophic scar and potential therapeutic targets should include the exploration of scar pigmentation, and its pathophysiologic development.
It is well-documented that melanocyte stem cells are located in the bulge region of hair follicles26. Melanocyte stem cells that transform into differentiated melanocytes have the capacity to form melanin when migrating either to hair bulbs or the surrounding epidermis4,26. Both epithelialization and pigmentation have previously been observed to increase radially from hair follicles, supporting the purported activity of these stem cells residing in the bulge area4,27. Melanocytes in association with hair follicles have additionally been implicated in various pigmentation of scar. The loss or destruction of hair follicles have been suggested as a cause of the common hypopigmentation seen in incisional epidermal scars26, while wound repigmentation studies consistently point to hair follicles as a source of melanocytes for restoring color to a reepithelialized wound25,28. The hypertrophic scars in the present study indeed appeared to have repigmentation associated spatially with normal uninjured skin, and consequently, hair follicles. The hypopigmented areas may potentially be explained, in part, by a lack of hair follicles present in the deep, large wounds created, and if these scars had been observed indefinitely, the pattern demonstrated in Figure 1 suggests that the entire scar may have eventually become hyperpigmented. The more perplexing components of the pigmentation patterns of these scars are twofold: 1) the intensity of hyperpigmentation and 2) the isolated islands of hyperpigmentation observed. Even the hair-follicle-adjacent periphery of these scars were not simply re-pigmented to a near-normal color, rather, they accumulated much darker pigmentation than pre-injury, observed both grossly and microscopically with Azure B staining. Beyond this, the isolated islands of hyperpigmentation, which developed over time, were not associated with normal adjacent skin or hair follicles, yet these darkened with the same intensity, and in some cases, coalesced with the surrounding areas of hyperpigmentation. It is possible that the factors influencing the intensity of repigmentation to hyperpigmentation of the peripheries of these scars were also responsible for the more remote areas of hyperpigmentation.
The number of melanocytes between hyperpigmented and hypopigmented samples did not significantly differ in the current study, while the number of stimulated melanocytes, as evidenced by positive HMB45 staining, did. Those samples with positive HMB45-stained melanocytes also possessed large amounts of melanin, with clear evidence of melanosome transfer from melanocyte to keratinocyte and up superficially to the keratin layer of the skin (Figure 7C, D). Postinflammatory effects have been suggested by previous investigators as a potential mechanism for hyperpigmentation of scars, acting in similar ways to the hyperpigmentation seen after UV-radiation damage of skin, increasing the production of melanin and export of melanosomes to neighboring keratinocytes25. Additionally, stimulated melanocytes release a number of factors, many of which are important in inflammation; IL-1α, IL-2, IL-3, IL-6, IL-10, TNF-α, IL-8, CCL2, TGF-β, catecholamines, eicosanoids, serotonin, nitric oxide, and α-MSH are among the many products of melanocytes6,29. Some of these secretion factors act in an autocrine fashion, with melanocyte inhibition occurring via IL-1, IL-6, and TNF-α, but stimulation via eicosanoids and α-MSH6,30. Alpha-MSH acts on MC1R, which activates micropthalmia-associated transcription factor, leading to the expression of tyrosinase, a necessary enzyme for melanin production24. Activation of the MC1R/α-MSH signaling pathway has been associated with regulation of both inflammation and the extracellular matrix environment9. Alpha-MSH itself has been described as anti-inflammatory, downregulating the production of IL-1, IL-4, IL-6, IFN-γ, and TNF-α31, while increasing the production of IL-109,32,33, adding further complexity to the feedback loops that appear to exist between melanocytes, their products, and the inflammatory processes inherent to wound healing.
Inflammation was certainly a contributing factor in this swine model, simply by the nature of healing a large, full-thickness wound and the resultant fibroproliferative processes. Alpha-MSH was found to be increased in those areas of scar that were hyperpigmented compared to those that were hypopigmented, however, it is unlikely that this one factor alone explains not only the intensity of hyperpigmentation seen, but the curious pattern of its appearance as well. Evidence exists that α-MSH possesses some activities independent of MC1R and reduces TGF-β1-induced synthesis of collagens I and III in fibroblasts9,34. The continued effort to understand the myriad of actors in the processes of inflammation, wound healing, fibrosis, and scar formation should likely include additional questions about α-MSH and special attention to the problems of both hyper- and hypopigmentation, as these appear to not be simply explained by hair follicle proximity.
Finally, the current study of the characteristics of abnormally pigmented hypertrophic scars employed a spectral imaging system whose data output correlated with the observed macroscopic and microscopic appearances of scars. Furthermore, this unique imaging modality provided a method for estimating melanin concentration noninvasively. The continued use of this tool in concert with further elucidation of the characteristics of dyspigmented scar may allow for future predictive capabilities and therapeutic interventions prior to clinically apparent abnormal skin pigmentation.
Conclusions
In a red Duroc model of human scar formation, melanocyte quantity does not appear to influence pigmentation, while the production of melanin does correlate with the pigmented appearances of skin and scars. Hyperpigmented scars appear to contain activated melanocytes, as evidenced by HMB45 staining, and increased levels of α-MSH. These observations open the door to investigating melanocyte stimulation and the inflammatory environment within a wound that may influence melanocyte activity. SFDI can be used to identify areas of melanin content in mature, pigmented scars, which may lead to its usefulness in wounds at earlier time points before markedly apparent pigmentation abnormalities.
Acknowledgments
Funding Source: NIH R15EB013439 and The DC Firefighters’ Burn Foundation.
Footnotes
Conflicts of Interest: None
References
- 1.Tyack ZF, Pegg S, Ziviani J. Postburn dyspigmentation: its assessment, management, and relationship to scarring--a review of the literature. The Journal of burn care & rehabilitation. 1997;18:435–40. doi: 10.1097/00004630-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Wisely JA, Hoyle E, Tarrier N, Edwards J. Where to start? Attempting to meet the psychological needs of burned patients. Burns: journal of the International Society for Burn Injuries. 2007;33:736–46. doi: 10.1016/j.burns.2006.10.379. [DOI] [PubMed] [Google Scholar]
- 3.Zeitlin RE. Long-term psychosocial sequelae of paediatric burns. Burns: journal of the International Society for Burn Injuries. 1997;23:467–72. doi: 10.1016/s0305-4179(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 4.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–50. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 5.Velangi SS, Rees JL. Why are scars pale? An immunohistochemical study indicating preservation of melanocyte number and function in surgical scars. Acta dermato-venereologica. 2001;81:326–8. doi: 10.1080/000155501317140016. [DOI] [PubMed] [Google Scholar]
- 6.Cichorek M, Wachulska M, Stasiewicz A, Tyminska A. Skin melanocytes: biology and development. Postepy dermatologii i alergologii. 2013;30:30–41. doi: 10.5114/pdia.2013.33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzpatrick TB, Breathnach AS. the Epidermal Melanin Unit System. Dermatologische Wochenschrift. 1963;147:481–9. [PubMed] [Google Scholar]
- 8.Gao FL, Jin R, Zhang L, Zhang YG. The contribution of melanocytes to pathological scar formation during wound healing. International journal of clinical and experimental medicine. 2013;6:609–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Muffley LA, Zhu KQ, Engrav LH, Gibran NS, Hocking AM. Spatial and temporal localization of the melanocortin 1 receptor and its ligand alpha-melanocyte-stimulating hormone during cutaneous wound repair. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2011;59:278–88. doi: 10.1369/0022155410397999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 11.Chadwick SL, Yip C, Ferguson MW, Shah M. Repigmentation of cutaneous scars depends on original wound type. Journal of anatomy. 2013;223:74–82. doi: 10.1111/joa.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallant-Behm CL, Olson ME, Hart DA. Cytokine and growth factor mRNA expression patterns associated with the hypercontracted, hyperpigmented healing phenotype of red duroc pigs: a model of abnormal human scar development? Journal of cutaneous medicine and surgery. 2005;9:165–77. doi: 10.1007/s10227-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 13.Travis T, Mino MJ, Mauskar NA, Jo DY, Ghassemi P, Moffatt LT, Jordan MH, Ramella-Roman JC, Shupp JW. A novel and reproducible porcine scar model for testing the effects of pressure therapy while correlating non-invasive imaging metrics to molecular and histological changes. 45th Annual Meeting of the American Burn Association; 2013; Palm Springs, CA.. [Google Scholar]
- 14.Mino MJ, Mauskar NA, Matt SE, et al. A fitted neoprene garment to cover dressings in swine models. Lab animal. 2012;42:23–5. doi: 10.1038/laban.164. [DOI] [PubMed] [Google Scholar]
- 15.Ghassemi P, Shupp JW, Moffatt LT, Ramella-Roman JC. A novel spectral imaging system for quantitative analysis of hypertrophic scar. Proceedings of SPIE; 2013. [Google Scholar]
- 16.Ghassemi P, Travis TE, Shupp JW, Moffatt LT, Ramella-Roman JC. Monitoring the influence of compression therapy on pathophysiology and structure of a swine scar model using a multispectral imaging system. Proceedings of SPIE; 2014. [Google Scholar]
- 17.Nguyen TT, Ramella-Roman JC, Moffatt LT, Ortiz RT, Jordan MH, Shupp JW. Novel application of a spatial frequency domain imaging system to determine signature spectral differences between infected and noninfected burn wounds. Journal of burn care & research: official publication of the American Burn Association. 2013;34:44–50. doi: 10.1097/BCR.0b013e318269be30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale GM, Brown RE, Jarmie N. Pole structure of the J pi =3/2(+) resonance in 5He. Physical review letters. 1987;59:763–6. doi: 10.1103/PhysRevLett.59.763. [DOI] [PubMed] [Google Scholar]
- 19.Takatani H, Oka M, Fukuda M, et al. Gene mutation analysis and quantitation of DNA topoisomerase I in previously untreated non-small cell lung carcinomas. Japanese journal of cancer research: Gann. 1997;88:160–5. doi: 10.1111/j.1349-7006.1997.tb00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrestha P, Muramatsu Y, Kudeken W, et al. Localization of Ca(2+)-binding S100 proteins in epithelial tumours of the skin. Virchows Archiv: an international journal of pathology. 1998;432:53–9. doi: 10.1007/s004280050134. [DOI] [PubMed] [Google Scholar]
- 21.Cheong KA, Noh M, Kim CH, Lee AY. S100B as a Potential Biomarker for the detection of Cytotoxicity of Melanocytes. Experimental dermatology. 2014 doi: 10.1111/exd.12332. [DOI] [PubMed] [Google Scholar]
- 22.Kamino H, Tam ST. Immunoperoxidase technique modified by counterstain with azure B as a diagnostic aid in evaluating heavily pigmented melanocytic neoplasms. Journal of cutaneous pathology. 1991;18:436–9. doi: 10.1111/j.1600-0560.1991.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 23.Smoller BR, Hsu A, Krueger J. HMB-45 monoclonal antibody recognizes an inducible and reversible melanocyte cytoplasmic protein. Journal of cutaneous pathology. 1991;18:315–22. doi: 10.1111/j.1600-0560.1991.tb01542.x. [DOI] [PubMed] [Google Scholar]
- 24.Wen KC, Chang CS, Chien YC, et al. Tyrosol and its analogues inhibit alpha-melanocyte-stimulating hormone induced melanogenesis. International journal of molecular sciences. 2013;14:23420–40. doi: 10.3390/ijms141223420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chadwick S, Heath R, Shah M. Abnormal pigmentation within cutaneous scars: A complication of wound healing. Indian journal of plastic surgery: official publication of the Association of Plastic Surgeons of India. 2012;45:403–11. doi: 10.4103/0970-0358.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gola M, Czajkowski R, Bajek A, Dura A, Drewa T. Melanocyte stem cells: biology and current aspects. Medical science monitor: international medical journal of experimental and clinical research. 2012;18:RA155–9. doi: 10.12659/MSM.883475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the “hair growth-wound healing connection”: anagen phase promotes wound re-epithelialization. The Journal of investigative dermatology. 2011;131:518–28. doi: 10.1038/jid.2010.291. [DOI] [PubMed] [Google Scholar]
- 28.Snell RS. A study of the melanocytes and melanin in a healing deep wound. Journal of anatomy. 1963;97:243–53. [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y, Zhu WY, Tan C, Yu GH, Gu JX. Melanocytes are potential immunocompetent cells: evidence from recognition of immunological characteristics of cultured human melanocytes. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2002;15:454–60. doi: 10.1034/j.1600-0749.2002.02065.x. [DOI] [PubMed] [Google Scholar]
- 30.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiological reviews. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 31.Brzoska T, Luger TA, Maaser C, Abels C, Bohm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocrine reviews. 2008;29:581–602. doi: 10.1210/er.2007-0027. [DOI] [PubMed] [Google Scholar]
- 32.Bhardwaj RS, Schwarz A, Becher E, et al. Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. Journal of immunology. 1996;156:2517–21. [PubMed] [Google Scholar]
- 33.Redondo P, Garcia-Foncillas J, Okroujnov I, Bandres E. Alpha-MSH regulates interleukin-10 expression by human keratinocytes. Archives of dermatological research. 1998;290:425–8. doi: 10.1007/s004030050330. [DOI] [PubMed] [Google Scholar]
- 34.Bohm M, Raghunath M, Sunderkotter C, et al. Collagen metabolism is a novel target of the neuropeptide alpha-melanocyte-stimulating hormone. The Journal of biological chemistry. 2004;279:6959–66. doi: 10.1074/jbc.M312549200. [DOI] [PubMed] [Google Scholar]