SUMMARY
Adaptive immune responses begin when naive CD4+ T cells engage peptide+major histocompatibility complex class II and co-stimulatory molecules on antigen-presenting cells (APCs). Notch signaling can influence effector functions in differentiated CD4+ T helper and T regulatory cells. Whether and how ligand-induced Notch signaling influences the initial priming of CD4+ T cells has not been addressed. We have found that Delta Like Ligand 4 (DLL4)-induced Notch signaling potentiates phosphatidylinositol 3-OH kinase (PI3K)-dependent signaling downstream of the T cell receptor+CD28, allowing naive CD4+ T cells to respond to lower doses of antigen. In vitro, DLL4-deficient APCs were less efficient stimulators of CD4+ T cell activation, metabolism, proliferation, and cytokine secretion. With deletion of DLL4 from CD11c+ APCs in vivo, these deficits translated to an impaired ability to mount an effective CD4+-dependent anti-tumor response. These data implicate Notch signaling as an important regulator of adaptive immune responses.
INTRODUCTION
Adaptive immune responses begin when a naive T cell encounters peptide+major histocompatibility complex (pMHC) on antigen-presenting cells (APCs) via its antigen-specific receptor (TCR). Any given pMHC combination is displayed at low density on an APC, making it essential that T cells become activated at low TCR occupancy (Lanzavecchia et al., 1999). One mechanism that allows naive T cells to become activated in response to lower doses of antigen (Ag) is co-stimulation (Chen and Flies, 2013). CD28 is the primary co-stimulatory molecule for naive T cells. Constitutive expression of the CD28 ligand CD80 is a feature that distinguishes “professional APCs” from other pMHCII-bearing cells.
Generation of fully differentiated CD4+ T effector cells from naive T cells takes place over the course of approximately 1.5 days, which can be functionally divided into three phases: priming, proliferation, and differentiation (Jelley-Gibbs et al., 2008). The priming phase encompasses the time of initial pMHC:TCR engagement until the first cell division. During priming, sustained signaling by TCR+CD28 leads to expression of early activation antigens, secretion of interleukin-2 (IL-2), and preparation to enter the cell cycle. The later phases of proliferation and differentiation are characterized by cell division, which is initially driven by TCR and becomes increasingly Ag independent. Cytokine and Notch receptors are major influencers of gene expression in CD4+ T helper (Th) and T regulatory (Treg) cells and in CD8+ cytotoxic T cells during the differentiation phase (Radtke et al., 2013). However, little is known about the effects of Notch signaling in CD4+ T cells during the first few hours after Ag encounter.
In the priming phase, CD4+ T cells undergo activation-induced metabolic changes to meet the demands of proliferation and effector function (MacIver et al., 2013). The phosphatidylinositol-3-OH kinase (PI3K) and mammalian target of rapamycin (mTOR) pathways have emerged as key regulators of T cell priming. Activation of these pathways downstream of TCR+CD28 increases nutrient uptake and stimulates synthesis of proteins, lipids, and nucleotides, which are prerequisites for IL-2 secretion and cell division. A positive correlation between Notch, PI3K, and mTOR activation in T cell acute lymphoblastic leukemia cells (T-ALL) (Chan et al., 2007) led us to hypothesize that Notch signaling might enhance CD4+ T cell priming.
Signaling through mammalian Notch receptors begins with binding of a Notch receptor to a ligand (NotchL) of the Delta-like or Jagged family. Ligand binding initiates cleavages that release the intracellular domain of Notch (NICD) and allow its nuclear translocation. In the nucleus, NICD, RBPJ, and co-activators form a transcriptional complex. The promoters of true “Notch target” genes contain binding sites that are directly bound by NICD+RBPJ, for example Hes1 (Fischer and Gessler, 2007). Many direct target genes are transcription factors that regulate a second tier of “Notch-responsive” genes, which in thymocytes include the genes encoding glucose transporter-1 (Glut1) (Slc2a1), CD71 (Tfrc), and CD98 (Slc3a2) (Palomero et al., 2007; O’Donnell et al., 2006). Notch can also act via non-canonical pathways, although the mechanisms are less well understood (Minter and Osborne, 2012).
We have developed model systems in which Notch signaling was manipulated in wild-type (WT) T cells indirectly, by controlling the availability of an activating NotchL on APCs. To assess the activation requirements of naive T cells necessitates the use of T cells that have not been previously activated (Inaba and Steinman, 1984), precluding the use of retrovirally transfected T cells. Our approach circumvented the toxicity and nonspecific effects of pharmacological Notch inhibitors. Moreover, the use of unmanipulated T cells with endogenous Notch expression ensured that Notch signaling remained ligand dependent, did not exceed physiological limits, and was sensitive to positive- and negative-feedback mechanisms. We tested the ability of DLL4-bearing APCs to drive CD4+T-cell-dependent immune responses in vivo by using adoptive transfer and tumor eradication protocols. To dissect the mechanisms underlying in vivo phenotypes, we devised an in vitro system with an Ag-presenting cell line so that the source of NotchL, MHC, and co-stimulatory molecules were defined and amenable to manipulation. This system allowed us to characterize priming of a synchronized population of T cells with or without Notch signaling.
We demonstrate that DLL4 is a physiologically relevant regulator of CD4+ T cell priming in vivo and in vitro. In concert with TCR+CD28 signaling, DLL4-induced Notch signaling allowed more T cells to respond at lower doses of Ag, which increased the magnitude of primary responses. Higher amounts of nutrient receptor expression and increased biomass indicated that Notch signaling also improved responses on a per-cell basis. DLL4-deficient APCs were less potent stimulators of CD4+ T cell metabolism, proliferation, and cytokine secretion, particularly when the concentration of Ag was low. The co-stimulatory effects of DLL4 were predicated on PI3K signaling downstream of TCR+CD28. In the absence of Ag, CD28 engagement, or PI3K activity, DLL4 had minimal effect on any of the parameters measured. Thus DLL4:Notch acted as co-stimulators of CD80:CD28-mediated co-stimulation in Ag-specific CD4+ T cells.
RESULTS
The NotchL DLL4 Is Expressed on APCs
Naive CD4+ T cells uniformly expressed Notch1 protein on their surface (Figures 1A and 1B). After stimulation Notch1 was upregulated in an Ag dose-dependent manner and Notch2 was induced. This was also true for mice with a diverse T cell repertoire and for all of the TCRαβ transgenic mice analyzed (data not shown).
Figure 1. Notch Ligand DLL4 Is Expressed on APCs and Is Functionally Important for CD4+ T Cell Activation In Vivo and In Vitro.
(A) 5cc7T cells incubated overnight with WTCD11c+ splenocytes, with or without 2.5 nM PCC, and analyzed for Notch1 and Notch2.
(B) 5cc7T cells incubated overnight with WTCD11c+ splenocytes and increasing [PCC] and analyzed for Notch1.
(C) WT splenocytes, enriched for MHCII+ cells and analyzed for expression of DLL4 (heavy black line); unstained control overlaid for comparison (dotted line). DLL4+ and DLL4 cells gated and compared for expression of the indicated proteins.
(D) Top: Marilyn T cells analyzed for signs of activation 3 days after transfer to Dll4flox Itgax-cre− (WT) or Itgax-cre+ (Dll4−/−) male mice that express H-Y Ag (solid lines). Female littermates lacking H-Y (dotted lines) served as negative controls. Bottom: each symbol represents one mouse, analyzed individually. Mean ± SEM, for FSC *p ≤ 0.05, CD98 *p ≤ 0.03, CD71 *p ≤ 0.001.
(E) Marilyn T cells analyzed for FSC after overnight stimulation by WT or Dll4−/− CD11c+ splenocytes presenting 5 nM Dby peptide, in vitro. Mean ± SEM, *p ≤ 0.05.
(F) Marilyn T cells incubated overnight with WT or Dll4−/− CD11c+ splenocytes presenting 5 nM Dby peptide analyzed for CD69 induction and IL-2 secretion. Mean ± SEM from ≥ 3 determinations, CD69 *p ≤ 0.0001, IL-2 *p = 0.0001. See also Figure S1.
It has been reported that APCs express mRNA coding for NotchL (Cheng et al., 2010). We examined MHCII+-enriched splenocytes and found that DLL4 was the only NotchL detected at the protein level (Figure S1A available online). DLL4+ cells consistently accounted for ~2% of total MHCII+ cells (Figure 1C). Cells within the DLL4+ population were predominantly large, CD19−CD11c+MHCII+CD54+CD80+CD11b+ cells, consistent with the phenotype of myeloid dendritic cells (DCs). DLL4 was not uniformly expressed by these APCs (Figures S1A and S1B); rather, the frequency of DLL4+ DCs varied from 20% to 60% of total CD19−CD11c+. The presence of DLL4 on a subset of DCs was provocative and led us to ask what effect DLL4 had on CD4+ T cell activation.
DLL4 on DCs Enhances CD4+ T Cell Activation In Vivo
To ascertain whether DLL4 expression on CD11c+ cells was functionally relevant in vivo, we generated animals in which DLL4 was conditionally deleted in DCs (Dll4−/−). Itgax-cre-mediated deletion of DLL4 was efficient and had no effect on T cell development, the frequency of DCs, or the expression of other NotchL or T cell stimulatory molecules on DCs (Figures S1B – S1D and data not shown). TCR transgenic Marilyn T cells that recognize a peptide derived from the male H-Y Ag presented by MHCII I-Ab were transferred to WT or Dll4−/− recipients. After 3 days donor cells were recovered and examined for evidence of activation (Figure 1D). Marilyn T cells transferred to recipients that did not express H-Y (WT or Dll4−/−) remained naive (FSCloCD98loCD71−). When transferred to recipients that expressed H-Y, Marilyn T cells became activated in both WT and Dll4−/− hosts. However, in Ag-bearing recipients whose CD11c+ APCs lacked DLL4, Marilyn T cells were smaller (FSC) and expressed significantly lower amounts of activation markers such as CD98 and CD71, indicative of suboptimal activation. These data indicate that DLL4 on CD11c+ DCs improved the quality of the CD4+ T cell response on a per-cell basis.
NotchL on APCs Enhances T Cell Activation In Vitro
To determine whether DLL4 influenced the frequency of cells that reached the threshold for activation, we looked at induction of an early activation marker. Within 24 hr after transfer, CD69 was uniformly high on Marilyn T cells transferred to WT or Dll4−/− Ag-bearing male recipients (data not shown). Such a rapid and homogenous response indicated that the CD69 response of Marilyn T cells had reached a plateau in response to the amount of endogenously expressed Ag. To better control the concentration of Ag, we primed Marilyn T cells in vitro by using freshly isolated WT or Dll4−/− DCs to present Dby peptide. As was the case in vivo, T cells stimulated by Dll4−/− DCs were smaller than T cells stimulated by WT DCs (Figure 1E). Once the amount of Ag was titrated down, it became clear that DLL4 increased the frequency of T cells that became CD69+ (Figure 1F).
An essential helper function performed by activated CD4+ T cells is IL-2 production. To quantify the frequency of cells secreting IL-2, we used capture assays (Figure 1F). As expected, IL-2+ cells were a subset of the activated CD69+CD4+ T cell population. Significantly fewer T cells secreted IL-2 when stimulated with Dll4−/− than with WT APCs. Thus DLL4 increased the frequency of cells that became activated and acquired effector function, as measured by CD69 induction and cytokine secretion, respectively. Taken altogether, the data show that DLL4 expression on DCs enhanced the frequency of CD4+ T cells that responded and the quality of the response on a per-cell basis, in vivo and in vitro.
The CD11c+ DC population was heterogeneous in DLL4 expression. To compare the stimulatory capacity of APCs that differed only in the expression of a NotchL, we sought a cell line that could substitute for the DLL4+ splenocytes. P13.9 cells were originally created by engineering L cells to express MHCII, CD54, and CD80. This APC line has been used to demonstrate that CD80:CD28 interactions enhance CD69 and IL-2 induction by CD4+ T cells stimulated by peptide Ag (Naramura et al., 1998; Itoh and Germain, 1997). The MHCII+CD80+CD54+phenotype of P13.9 cells matched the phenotype of the DLL4+ APCs we identified in spleen but lacked endogenous NotchL. We transfected P13.9 cells with DLL4 or empty vector and established stable cell lines with equivalent expression of MHC, CD54, GFP, and CD80.
When used to present pigeon cytochrome c (PCC) peptide in the context of I-Ek to 5cc7 TCR transgenic T cells, DLL4-P13.9 cells were comparable to splenic DCs, and both of these cell types were more potent stimulators than control P13.9 cells (Figure 2A). CD69 induction was Ag dependent; DLL4 alone did not induce CD69 expression. The frequency of CD69+ T cells was significantly higher with DLL4-P13.9 than control P13.9 APCs across a range of Ag doses (Figure 2B). The ability of DLL4 to reduce the threshold of Ag necessary for a naive CD4+ T cell to respond is demonstrated in that the frequency of CD69+ T cells reached a plateau by 10 nM PCC in the presence of DLL4; whereas in its absence, the frequency of CD69+ cells still increased significantly as PCC was increased from 100 nM to 1,000 nM (p = 0.006).
Figure 2. An In Vitro System Accurately Recapitulates In Vivo Observations.
(A) 5cc7 T cells stimulated overnight with 1 nM PCC presented by CD11c+ DCs or control or DLL4-P13.9 analyzed for CD69.
(B) 5cc7 T cells stimulated overnight with PCC presented by control or DLL4-P13.9 analyzed for CD69. Mean ± SEM from ≥ 3 determinations for each dose of Ag; control versus DLL4: 0.1 nM *p = 0.044, 1 nM *p ≤ 0.0001, 10 nM *p ≤ 0.0001, 100 nM *p ≤ 0.0001, 1,000 nM *p ≤ 0.0001.
(C) T cells cultured for 3 days with control orDLL4-P13.9 presenting PCC analyzed after a 24hr pulse with 3H thymidine. Mean± SEM from ≥ 3 determinations for each dose of PCC; control versus DLL4: 0.01 nM *p = 0.04, 0.03 nM *p = 0.0001, 0.10 nM *p ≤ 0.005, 0.30 nM *p ≤ 0.0001, 1 nM *p = 0.0002, 3 nM *p ≤ 0.0001, 10 nM *p ≤ 0.0001, 30 nM *p ≤ 0.0001, 100 nM *p ≤ 0.0005, 300 nM *p ≤ 0.0001, 1,000 nM *p ≤ 0.0001.
(D) 5cc7 T cells were stimulated as in (A). Live CD4+ cells were analyzed for increased biomass. n > 10.
(E) 5cc7 T cells cultured for 3 days with control or DLL4-P13.9 and 1 nM PCC harvested and analyzed for CFSE dilution. n = 2.
(F) 5cc7 T cells counted after 3 days in culture with control or DLL4-P13.9 and PCC. Mean ± SEM of ≥ 4 determinations; control versus DLL4: 1 nM *p ≤ 0.02, 10 nM *p ≤ 0.03, 1,000 nM n.s. See also Figure S2.
T cell proliferation, as assessed by 3H uptake and CFSE dilution, was impaired when APCs lacked DLL4 (Figures 2C and 2E). This was largely because in the absence of a NotchL, fewer cells were recruited into the proliferating pool (Figures 2D, 2E, and S2A). As was the case with Marilyn T cells responding to Dby, DLL4 increased the frequency of 5cc7 T cells that committed to proliferation, as measured by increased biomass at day 1 across a range of PCC concentrations (Figure 2D). On day 3, the frequency of cells that remained undivided decreased by 80% from 4.7% to 0.8% (Figures 2E and S2A), which reflects a 3-fold increase in the frequency of input cells that remained quiescent in the absence of DLL4.
Notch signaling has been reported to influence T cell survival (Helbig et al., 2012; Jehn et al., 1999). In the presence of NotchL, the number of cells recovered after 3 days of culture was significantly higher when T cells were stimulated with low-, but not with high-, dose Ag (Figure 2F). The decreased cellularity in DLL4-P13.9 cultures with high-dose PCC was probably due to increased activation-induced cell death (Lenardo, 1991), increased cytokine secretion (Figure S2B), and nutrient depletion. In any case, we saw no evidence that DLL4 provided protection from cell death.
DLL4 Facilitates T Cell Expansion by Relieving Metabolic Constraints
Proliferation requires increased nutrient uptake (MacIver et al., 2013). Because the expression of nutrient transporters is low on quiescent T cells, naive T cells have a cell-intrinsic limitation on the amount of nutrients that they can acquire. In thymocytes, several nutrient transporters are Notch responsive, including CD71 and Glut1 (Ciofani and Zúñga-Pflücker, 2005; Kelly et al., 2007). When mature T cells were primed with low-dose peptide, DLL4 increased the frequency of T cells with high CD71 and Glut1 (Figures 3A). In addition to an increase in the frequency of responders, T cells activated by DLL4+ APCs expressed more of these transporters on a per-cell basis than T cells stimulated in the absence of NotchL.
Figure 3. DLL4 Augments Metabolic Parameters in Activated CD4+ T Cells.
(A) Rbpjflox Cd4-cre− (WT) or -cre+ (Rbpj−/−) 5cc7 T cells stimulated overnight with 4 nM PCC presented by control or DLL4-P13.9 and analyzed for activation markers.
(B and C) WT 5cc7 T cells stimulated with 3 nM PCC presented by control or DLL4-P13.9 analyzed for induction of activation markers, and uptake of fluorescently labeled analogs of transferrin (B) or glucose (C). Data are representative of ≥ 3 determinations. Data from multiple experiments with WTT cells were compiled and subjected to statistical analysis; see Figure 5.
DLL4 increased expression of nutrient receptors on Marilyn T cells stimulated by DCs in vivo and on 5cc7 T cells stimulated by DLL4-P13.9 APCs in vitro. In vitro we could quantify the functional impact that the changes in receptor expression had on nutrient uptake. Fluorescently labeled analogs of transferrin (Tf) or glucose were added to the media of T cells cultured with PCC and control or DLL4-P13.9 APCs (Figures 3B and 3C). Only activated CD69+CD71+ T cells took up Tf. Despite a high concentration of Tf in the culture media, resting (CD71−) cells did not have the capacity to take in Tf. DLL4 on APCs increased the frequency of T cells that became activated and able to endocytose Tf. Moreover, the amount of Tf acquired by a cell was directly correlated with its Tf receptor expression, such that the mean fluorescence intensity (MFI) of Tf was higher for T cells primed in the presence of NotchL. Thus on a per-cell basis, T cells stimulated in the presence of DLL4 were able to acquire more Tf:iron complexes than CD71+ T cells deprived of Notch signaling. These data are consistent with previous studies illustrating that CD71 is a rate-limiting factor for Tf transport in T cells (Bayer et al., 1998; Brekelmans et al., 1994a). DLL4+ APCs also induced more T cells to increase Glut1 expression and glucose consumption, although the increase in MFI of glucose taken up on a per-cell basis between control and DLL4 cultures did not reach statistical significance. Nevertheless, the data support the hypothesis that one mechanism by which DLL4-induced Notch signaling facilitates T cell activation and expansion is to relieve cell-intrinsic metabolic constraints that are imposed by the paucity of nutrient transporters on naive T cells.
Rbpj−/− T cells can bind to NotchL and undergo cleavage of Notch receptors but cannot modify transcription of target genes. The frequency of activated, blasting Rbpj−/− T cells was the same regardless of whether or not APCs expressed DLL4 and was equivalent to control T cells stimulated by DLL4− APCs (Figure 3A). These data are consistent with a previous report in which Rbpj−/− and WT T cells proliferated equivalently when stimulated by anti-CD3+CD28. However, when APCs were added as a source of NotchL, only the proliferation of WT cells was enhanced (Tanigaki et al., 2004). The fact that Rbpj−/−T cells were refractory to DLL4 also indicates that the effects of DLL4 on priming were largely the result of canonical Notch signaling, rather than increased adhesion or noncanonical Notch signaling.
DLL4 Increases IL-2
We used capture assays to quantify the frequency of cells secreting IL-2 in response to pMHC presented by our APC lines (Figures 4A and 4B). More T cells secreted IL-2 in response to DLL4+ than to control APCs, consistent with what we observed when using WT and Dll4−/− DCs to stimulate Marilyn T cells. As was the case with CD69 induction, IL-2 expression was RBP J dependent, peptide dependent, and dose responsive, and the frequency of IL-2+ cells reached aplateauata lower concentration of Ag when T cells were stimulated in the presence of DLL4. The data from IL-2 capture assays were corroborated by measuring total IL-2 in culture supernatants (Figure 4C). These data show that DLL4 reduced the threshold of Ag necessary to induce IL-2 across a range of Ag doses, but was best appreciated when pMHC was limiting.
Figure 4. DLL4 on APCs Increases IL-2 Production.
(A) 5cc7 T cells cultured overnight with control or DLL4-P13.9 and PCC and analyzed for CD69 and IL-2 secretion.
(B) RbpjfloxCd4-Cre− or -Cre+ 5cc7 T cells stimulated overnight with 2.5 nM PCC presented by control or DLL4-P13.9 analyzed for IL-2 secretion. Mean ± SEM of ≥ 4 determinations. Control *p ≤ 0.03, Rbpj−/− n.s.
(C) Concentration of IL-2 in 48 hr supernatants of 5cc7T cells cultured with control or DLL4-P13.9. Mean ± SEM of ≥ 3 determinations; 1 nM *p ≤ 0.05; 10 nM *p ≤ 0.04; 1,000 nM n.s.
(D) 5cc7 Il2-GFP T cells cultured overnight with control or DLL4-P13.9 and 1 nM PCC analyzed for CD69 and GFP. Mean ± SEM of ≥ 5 determinations, *p ≤ 0.002.
(E) 5cc7 Il2-GFP T cells cultured for up to 4 days with control or DLL4-P13.9 and 2.5 nM PCC analyzed for GFP. Open symbols on day 1 are 0 nM PCC. Mean ± SEM, day 1 *p ≤ 0.0001, day 2 *p ≤ 0.03, days 3, 4 n.s. See also Figure S3.
To determine whether Notch signaling influenced the kinetics of an IL-2 response, Il2 gene expression in T cells of mice with an Il2-green fluorescent protein reporter (Il2-GFP) was analyzed (Figures 4D, 4E, and S3). In the absence of DLL4, not only was the magnitude of the response diminished, but the peak of the Il2-GFP response was delayed. Collectively, the data demonstrate that Notch signaling accelerates the kinetics of the response, increases the magnitude of the response by increasing the frequency of IL-2 expressing cells, and, ultimately, produces more IL-2.
Notch Works in Concert with CD28-Mediated Co-Stimulation
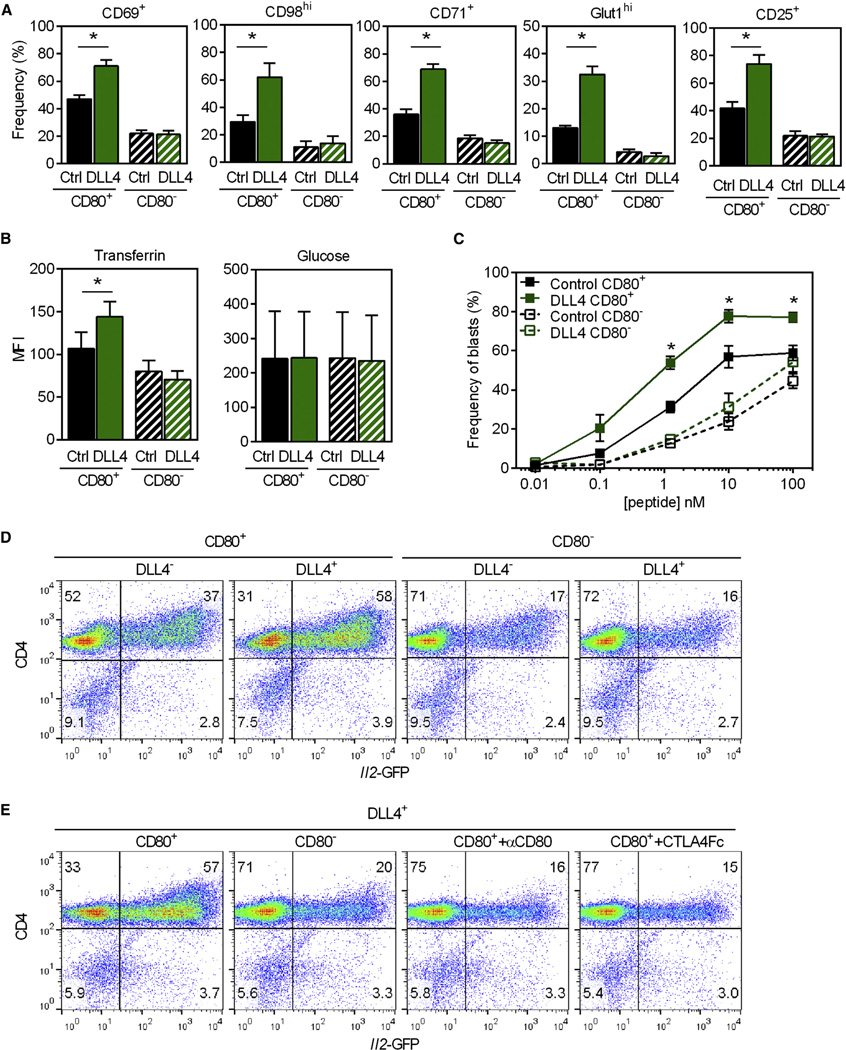
The co-stimulatory effects of DLL4 on primary T cell responses were reminiscent of the effects typically associated with the CD28 ligands, CD80 and CD86, also known as “signal 2” (Frauwirth et al., 2002; Jacobs et al., 2008). To isolate the individual consequences of Notch and CD28 signaling, additional APC lines were created. Fibroblasts expressing equivalent amounts of CD54 and MHCII, without CD80, were transfected with DLL4 or control vector and used to present peptide to T cells in side-by-side comparisons with CD80-bearing APCs (Figure S4). As established in Figures 2, 3, and 4, APCs that expressed both CD80 and DLL4 were potent stimulators of naive CD4+ T cells. As expected, CD80+ APCs were more efficient than CD80− APCs in their ability to stimulate nutrient transporter expression and function (Figures 5A, 5B, and S4), IL-2Rα expression (Figures 5A and S4), blastogenesis (Figures 5C and Figure S4), and Il2-GFP expression (Figure 5D). Surprisingly, APCs that expressed only DLL4, and not CD80, were poor inducers of T cell activation. Similar results were obtained when CD80:CD28 interactions were abrogated by addition of blocking anti-CD80 antibody (mAb) or CTLA4-Fc (Figure 5E). Thus, the co-stimulatory effects of DLL4 were predicated on the presence of pMHC and CD80, suggesting that the major effect of Notch signaling was to potentiate Ag-specific signaling downstream of CD28.
Figure 5. DLL4:Notch Work in Concert with CD80:CD28 Signaling.
(A) 5cc7T cells cultured with 1 nM PCC and control or DLL4-P13.9, with or without CD80, analyzed for activation markers. Mean ± SEM of ≥ 3 determinations; CD80+ control versus DLL4: CD69 *p ≤ 0.0001, CD98 *p ≤ 0.02, CD71 *p ≤ 0.002, Glut1 *p ≤ 0.001, CD25 *p ≤ 0.001. CD80− control versus DLL4: n.s.
(B) 5cc7 T cells were stimulated as in (A) and the amount of fluorescently labeled Tf or glucose compared as in Figures 3B and 3C. Mean ± SEM of ≥ 3 determinations. Tf: CD80+ control versus DLL4 0.01 nM *p ≤ 0.008, CD80− control versus DLL4 n.s. Glucose: CD80+ control versus DLL4 n.s. p = 0.17, CD80− control versus DLL4 n.s. p = 0.76.
(C) T cells cultured overnight with control or DLL4-P13.9, with or without CD80, and increasing doses of PCC, analyzed for increased biomass. Data are the frequency of blasting cells (FSChi) mean ± SEM from ≥ 3 experiments at each dose. Control versus DLL4:1 nM *p ≤ 0.001,10 nM *p ≤ 0.008,100 nM *p ≤ 0.002.
(D) 5cc7 Il2-GFP T cells, cultured with 10 nM PCC and control or DLL4-P13.9, with or without CD80 analyzed for GFP.
(E) 5cc7 T cells were stimulated as in (D) and where indicated αCD80 mAb or CTLA4-Fc was added. Data are representative of ≥ 3 determinations. See also Figure S4.
Notch Augments the PI3K Pathway Downstream of CD80:CD28
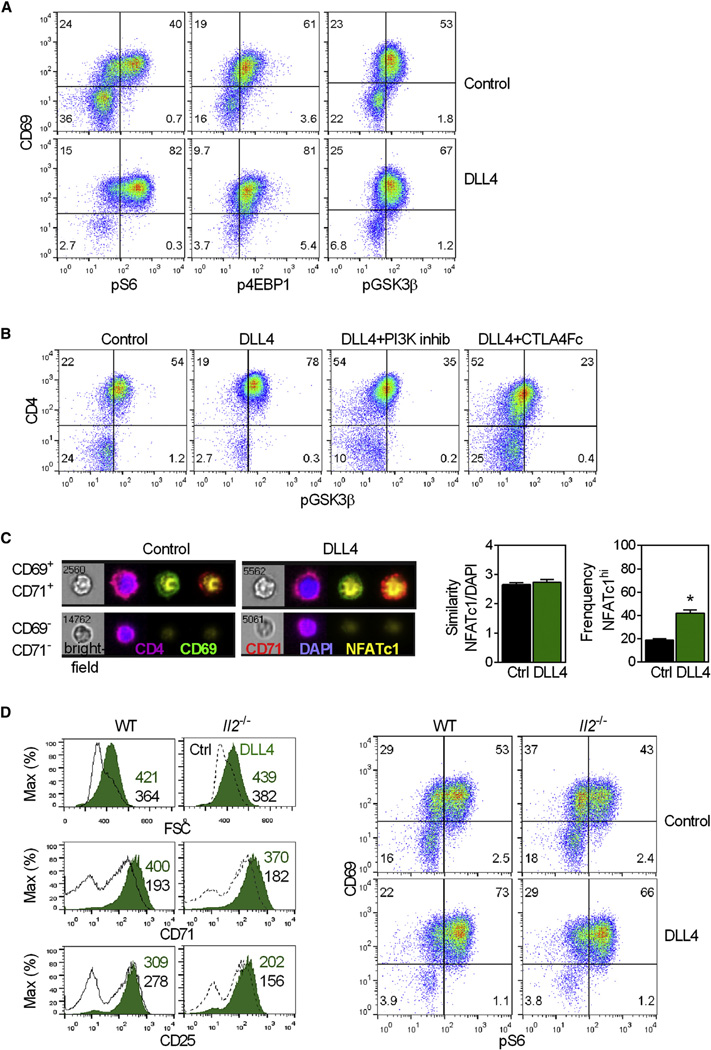
One signaling cascade activated downstream of CD28 involves PI3K and mTOR (Riha and Rudd, 2010). We found that T cells primed in the absence of NotchL showed evidence of decreased PI3K activity, such as decreased biomass and less of the PI3K-regulated proteins CD71 and Glut1. To formally demonstrate that PI3K activity was linked to Notch signaling, we confirmed that phosphorylation of downstream targets of mTOR were decreased when T cells were stimulated in the absence of a NotchL. The frequency of cells with activated ribosomal protein S6 (pS6), phospho-inactivated 4 elongation factor binding protein 1 (p4EBP1), and phospho-inactivated glycogen synthase kinase-3 beta (pGSK3b) was diminished when APCs lacked DLL4 (Figures 6A, 6B, and S5), indicating that Notch signaling potentiates signaling downstream of PI3K. The increased phosphorylation of proteins that occurred when DLL4 was present was dependent on CD28, PI3K, and pyruvate dehydrogenase kinase-1 (PDK1), as shown by the fact that the co-stimulatory effects of DLL4 were negated by treatment with pharmacological inhibitors of these kinases or when CD80:CD28 interactions were sterically hindered (Figures 6B, S5, and data not shown).
Figure 6. DLL4:Notch Increase PI3K–Dependent Phosphorylation of Proteins Downstream of CD80:CD28.
(A and B) 5cc7T cells cultured overnight with 2.5–5 nM PCC and CD80+ control or DLL4-P13.9 analyzed for phosphorylation of the proteins indicated. Where indicated, inhibitors were added.
(C) 5cc7 T cells cultured overnight with CD80+ control or DLL4-P13.9 presenting 3 nM PCC analyzed for induction of NFATc1, CD69, and CD71. The images shown are live, diploid, CD4+ cells. Numbers in the upper left corner of the bright field images are the unique object identifier assigned to each cell by Inspire (Amnis). Graphs are the mean ±SEM. Similarity between NFATc1 and DAPI control versus DLL4 n.s. Frequency of NFATc1bright control versus DLL4*p ≤ 0.002.
(D) WT or Il2−/− 5cc7 T cells cultured overnight with control or DLL4-P13.9 APCs and 2.5 nM PCC analyzed for signs of activation and PI3K signaling. Data are representative of ≥3 experiments. See also Figures S5 and S6.
Sustained signaling via TCR+CD28 leads to the synthesis and nuclear translocation of the transcription factor NFATc1 (Nurieva et al., 2007). Phospho-inactivation of GSK3β is a prerequisite for IL-2 expression because it prolongs the nuclear retention of transcription factors, including NFAT (Ohteki et al., 2000). We examined the induction and cellular localization of NFATc1 in individual T cells (Figures 6C and S6). NFATc1 was difficult to detect in resting (CD69−CD71−) T cells but was highly expressed in activated (CD69+CD71+) cells. NFATc1 in activated cells was largely confined to the nucleus, regardless of whether cells became activated in the presence or absence of DLL4. However, the frequency of cells that became NFATc1 bright was significantly higher in the presence of DLL4, consistent with the observed increases in the frequency of cells with pGSK3b and secreting IL-2.
We considered two explanations for how DLL4 on APCs could lead to increased PI3K activity in T cells. One possibility was that Notch signaling potentiates PI3K signaling downstream of CD28. A second possibility was that increased PI3K signaling was secondary to the increased IL-2 because IL-2R signaling also activates PI3K (Liao et al., 2013). To determine whether DLL4 increased PI3K signaling upstream of IL-2 secretion and/or downstream of IL-2R signaling, we used control or DLL4-APCs to stimulate IL-2-deficient (Il2−/−) T cells. The magnitude of the DLL4-associated increases in biomass, CD69, CD71, CD98, Glut1, pS6, and p4EBP1 were the same in the presence and absence of IL-2 (Figures 6D, S5, and data not shown). These findings indicated that during the priming phase, DLL4-mediated increases in PI3K signaling were a consequence of Notch re-enforcing signals downstream of CD28 and occurred independently of DLL4’s ability to increase the availability of IL-2.
DLL4 on DCs Is Essential for an Effective Anti-Tumor Response
Our data suggest that Notch signals can enhance CD4+ T cell priming, which could critically alter the course of an immune response. To test this hypothesis, we used a model in which eradication of a tumor is dependent on cross-presentation of tumor Ag to CD4+ T cells by host MHCII+ APCs (Perez-Diez et al., 2007). In the absence of T cells, tumor cells injected into WT or Dll4−/− recipients grew at a similar rate and resulted in 100% mortality in less than 5 weeks (Figure 7A). Adoptively transferred Marilyn T cells responded to Ag derived from the tumor cells and constrained tumor growth in WT mice. The anti-tumor effect of Marilyn T cells was impaired in Dll4−/− mice. Tumors grew larger, as compared to WT littermates, and this led to a significant increase in mortality.
Figure 7. DLL4 on DC Is Essential for a Protective CD4+ T-Cell-Dependent Anti-Tumor Response.
Tumor cells were injected intoCd3ε−/−Dll4floxItgax-cre− (WT) or -cre+ (Dll4−/−) animals and some mice received Marilyn T cells 2 days later.
(A) Tumor volumes (top) plotted until the day that the first mouse in the group was lost (bottom). Data are representative of ≥2 experiments, each with ≥3 mice per experimental group. Log-rank (Mantel-Cox) tests of survival control versus Dll4−/−*p ≤ 0.004.
(B) Tumors analyzed for the presence of Marilyn T cells (CD4+TCRVp6+).
(C) Tumor-infiltrating T cells analyzed for signs of activation. Data are representative of ≥ 2 pairs of mice.
The inability of T cells to control tumor growth in the absence of DLL4+ APCs could not be attributed to a failure of T cells to survive in Dll4−/− mice, because CD4+ T cells were present in the tumor draining lymph node (LN) and the tumor itself (Figure 7B and data not shown). This suggested to us that the impaired ability of mice with Dll4−/− DCs to eradicate tumors was due to qualitative differences in T cells stimulated in the presence or absence of DLL4 on APCs. T cells isolated from Dll4−/− mice tended to have less CD98 than T cells isolated from WT mice (Figure 7C), consistent with diminished PI3K activity in the absence of DLL4-induced Notch signaling. This occurred in spite of the fact that Dll4−/− mice tended to have larger tumors, and thus increased Ag burden. This phenotype was similar to the diminution in CD98 that was observed on T cells transferred into Dll4−/− or WT male mice (Figure 1D). Thus, DLL4 on APCs was essential for efficient activation and function of CD4+ T cells during an anti-tumor response.
DISCUSSION
The data presented here provide compelling evidence that interactions between DLL4 on APCs and Notch on naive T cells are a critical factor in determining the magnitude and quality of primary immune responses. We have shown that DLL4 improved CD4+ T cell priming in vivo and in vitro. On a population level, the frequency of CD4+ T cells that became activated and secreted IL-2 in response to a given concentration of Ag was greater when T cells were primed in the presence of DLL4. On a per-cell basis, Notch signaling was associated with elevated expression of nutrient receptors, improved nutrient uptake, greater biomass, and increased proliferation. DLL4-mediated enhancement of T cell activation was pMHC dependent, Ag dose responsive, and predicated on the co-engagement of other co-stimulatory molecules expressed on APCs. Collectively, these data indicate that DLL4-induced Notch signaling is a mechanism by which APCs can fine tune the Ag sensitivity of CD4+ T cell responses.
DLL4 facilitated T cell priming as measured by numerous indicators of T cell activation, including CD69, CD71, CD98, Glut1, FSC, and proliferation. When a single parameter was followed over a series of peptide concentrations, it was quite clear that Notch facilitated priming across a broad range of Ag doses. For certain parameters, the effects of DLL4 were difficult to appreciate if T cells received a strong stimulus because once a parameter reached its plateau, the effects of Notch were obscured. For example, Marilyn T cells transferred into recipients that ubiquitously express H-Y Ag became uniformly CD69+ in WT or Dll4−/− mice. However, when Ag was titrated down, it became clear that fewer T cells became CD69+ if stimulated in the absence of a NotchL.
The dose-dependent nature of Notch effects might be one reason that the role of Notch in T cell priming has not been widely appreciated. For example, Adler et al. (2003) concluded that Notch signaling had no effect on T cell activation, based on CD69 induction. However, only one dose of Ag was assayed, and that dose of peptide was high enough to induce nearly 100% of cells to become CD69+. Similarly, two groups reported that Notch signaling does not enhance T cell proliferation (Ong et al., 2008; Helbig et al., 2012). In both cases a very strong TCR stimulus was provided, and therefore a likely explanation for their results is that proliferation had already reached its plateau. These observations are analogous to the finding that with high doses of TCR stimulation, anti-CD28 co-stimulation cannot further increase Glut1 expression or the frequency of dividing cells (Wells et al., 1997; Teh and Teh, 1997; Jacobs et al., 2008).
Most notably, our data illustrate that the source of NotchL is critically important in vivo. DLL4 is expressed by many cell types. However, deletion of DLL4 specifically on CD11c+ APCs impaired CD4+ T-cell-dependent immune responses to endogenously expressed protein or exogenously added peptide Ag. It will be interesting to see how targeted deletion of NotchL on different subsets of APCs, such as CD103+ DCs in the mucosa or B220+ plasmacytoid DCs, impacts T cell responses including infection and tolerance.
Nutrient transporters are low on resting T cells and are among the earliest proteins upregulated in response to PI3K signaling downstream of TCR+CD28 (Cotner et al., 1983; Edinger, 2007; Jacobs et al., 2008). We found that phosphorylation of proteins downstream of PI3K was enhanced when T cells were stimulated by DLL4+ APCs, and this was associated with increased induction of nutrient receptors. Conversely, when T cells were activated in the absence of DLL4, we found that induction of CD71, endocytosis of Tf:iron complexes, IL-2 secretion, and proliferation were diminished. Because proliferation and cytokine secretion are constrained in cells that cannot take up adequate iron (Bayer et al., 1998), our data might explain why mice with iron deficiency are less susceptible to Notch1-induced leukemia (Khwaja et al., 2010).
The effects of Notch signaling that we describe during the priming phase of CD4+ T cell responses bear similarities to the cooperative relationship between preTCR and Notch during β-selection in thymocytes. During both processes, signaling through Ag receptor and Notch allows selective expansion of cells by upregulating nutrient receptors in a PI3K- and PDK1-dependent manner (Cotner et al., 1983; Kelly et al., 2007; Brekelmans et al., 1994b). In mature CD4+ T cells, we found that the trophic effects of DLL4 boosted metabolism only in T cells responding to pMHC+CD80. With a diverse TCR repertoire, this mechanism would allow Ag-specific T cells to preferentially expand. It might also help to maintain peripheral tolerance, because NotchL are ubiquitously expressed and might otherwise facilitate expansion of T cells that see Ag in the absence of signal 2.
We observed that Rbpj−/− T cells were refractory to the presence of DLL4 on APCs, suggesting that DLL4-induced changes during the priming phase are the consequence of canonical Notch signaling. For example, DLL4 did not increase nutrient transporter expression or FSCs of Rbpj−/− T cells. Complementary data show that inhibition of Notch in T-ALL cells by dominant-negative mastermind-like-1 or a γ-secretase inhibitor decreased cell size (Chan et al., 2007). The promoters of the Notch-responsive genes that we monitored lack consensus RBP J binding sites, but the possibility that some of these genes have RBP J binding sites in distal enhancers cannot be excluded (Wang et al., 2014). Nevertheless, we favor the interpretation that the Notch-responsive proteins that we measured were indirectly regulated by Notch signaling.
By regulating the activity of GSK3β, Notch signaling can influence the activity of >40 proteins that are targets of GSK3β-me-diated regulation including NFAT, AP-1, and NF-κB transcription factors (McCubrey et al., 2014; Pollizzi and Powell, 2014). The Il2 promoter contains binding sites for numerous transcription factors, all of which must be bound for optimal transcription of Il2 mRNA (Rothenberg and Ward, 1996). Inactivation of GSK3P prolongs the nuclear residency of transcription factors at the Il2 promoter and increases Il2 gene expression (Ohteki et al., 2000). Previous studies have shown that Notch signaling increases the nuclear retention of NF-κB and the total amount of IL-2(Shin et al., 2006; Dongre et al., 2014). We have demonstrated here that pGSK3β, NFATc1 induction and nuclear translocation, Il2 gene expression, and IL-2 secretion are all increased when T cells are primed in the presence of NotchL. Thus, Notch signaling coordinately regulates the expression, activity, and cellular localization of multiple proteins that control Il2 gene expression.
Notch has been described as instructive for the differentiation of Th1, Th2, Th17, follicular Th, and Treg cells (Amsen et al., 2007; Minter et al., 2005; Samon et al., 2008; Mukherjee et al., 2009; Auderset et al., 2013; Keerthivasan et al., 2011). Although it is formally possible that Notch specifies commitment to all of these lineages, our data offer a simpler explanation. Because these CD4+ T cell effector cell lineages are each dependent on IL-2 (Liao et al., 2013; MacIver et al., 2013), decreased IL-2 production in the absence of Notch would explain most of the earlier findings. We propose that Notch promotes the generation of CD4+ T cell effector lineages not by specifying lineage choice or inducing expression of master regulators, but by facilitating T cell activation, metabolic reprogramming, and IL-2 secretion during the first few hours after Ag encounter. This hypothesis does not exclude the possibility that at later time points Notch signaling reinforces or amplifies the expression of lineage-specific genes in fully differentiated T cells.
The findings presented here suggest that the therapeutic potential of harnessing Notch is not limited to a particular Th-cell-biased pathology, but will be broadly applicable. DLL4- blocking mAbs suppress disease in models of multiple sclerosis, graft-versus-host disease, and transplantation (Takeichi et al., 2010; Tran et al., 2013; Ishida et al., 2011). Based on our findings that CD4+ T cell responses were impaired in the absence of DLL4 on APCs, we posit that the immune-suppressive effects of DLL4 mAb in these models were due to less-efficient T cell priming. Our results suggest that strategies targeting DLL4 on CD11c+ cells (Kontermann, 2012) would offer powerful remedies for autoimmune disease without the adverse effects of systemic blockade of DLL4 (Pellegrinet et al., 2011; Yan et al., 2010). Our observation that DLL4 confers a growth advantage to Ag-specific T cells also suggests that DLL4-induced Notch signaling could be used to optimize immunotherapy. Clinical trials evaluating the efficacy of ex vivo expanded autologous T cells to treat malignancies report that a major limitation is the time required to grow enough T cells to transfer back into the patient (Weber et al., 2011). Our data suggest that DLL4 could facilitate expansion of tumor-specific T cells and decrease the time required to generate sufficient T cells for immunotherapy.
In conclusion, we show that DLL4 is a mechanism by which APCs can fine tune adaptive immune responses, by influencing the sensitivity, magnitude, and quality of the initial CD4+ T cell response. The findings presented here provide insight as to how a generic signaling pathway like Notch can work in concert with Ag- and APC-specific signals to enhance T cell priming. The highly context-dependent nature of Notch signaling is ideal for tailoring immune responses to specific situations. The outcome of Notch signaling in T cells is likely to vary depending on the identity of the APCs, co-engagement of other co-stimulatory or inhibitory molecules, cytokine milieu, and other environmental factors. Coupling in vivo observations with mechanistic data acquired in willfully reductionist in vitro systems could provide innovative approaches for manipulating Notch signaling to treat autoimmunity, infection, and other pathologies.
EXPERIMENTAL PROCEDURES
Mice
Mice were maintained under specific-pathogen-free conditions. All experiments were performed in compliance with NIAID Animal Care and Use Committee (ACUC) protocols and regulations. A detailed list of mice is provided in the Supplemental Experimental Procedures.
Cell Isolation
Spleens, LNs, and tumors were harvested in M199 (Invitrogen) supplemented with fetal calf serum (FCS) (Gemini Bio-Products), L-glutamine (Quality Biological), and antibiotic-antimycotic (Invitrogen). Single-cell suspensions were made by passing cells through 100 µm nylon mesh. Splenocytes were enriched for MHCII+ or CD11c+ cells via magnetic beads and an AutoMACS (Miltenyi). T cells were not previously activated. No exogenous adjuvants were added.
Adoptive Transfers
0.5–2.5 × 106 Marilyn LN cells, which are specific for an H-Y-derived peptide presented by MHCII I-Ab, were injected intravenously into T-cell-replete H-2b male and female recipients. 3 days later, cells isolated from spleen and LN were analyzed for the presence of donor cells.
Tumor Challenge
105 MB49 carcinoma cells (originally isolated from an H-2b male mouse) were injected subcutaneously on the right flank of H-2b Cd3ε−/− female recipients. 2 days after tumor challenge, 106 Marilyn T cells were injected intraperitoneally. Tumors were measured with calipers every other day, and tumor volumes were calculated as length × width × height ÷ 2. Mice were euthanized when they became distressed or if tumor volume exceeded 1,000 mm3.
Antibodies and Fluorescently Labeled Probes
A detailed list of antibodies and fluorescently labeled probes is provided in the Supplemental Experimental Procedures.
Antigen-Presenting Cell Lines
L cells with constitutive expression of CD54 and MHCII I-Ek with (P13.9) or without CD54 (EKAM) (Lucas and Germain, 2000) were provided by R. Germain (NIAID, NIH). Cells were maintained in complete DMEM (cDMEM) (DMEM [Invitrogen] supplemented with FCS, L-glutamine, sodium pyruvate [Invitrogen], antibiotic-antimycotic, and gentamycin [Invitrogen]). MIGR1-empty vector and MIGR1-mDLL4 constructs were nucleofected into P13.9 or EKAM cells with an Amaxa Nucleofector (Amaxa Biosystems). Flow cytometric sorting was used to select stable clones with comparable retrovirus integration (GFP), MHCII, CD54, and CD80. Surface expression of DLL4 was confirmed by staining with anti-DLL4 antibody.
In Vitro Stimulation
T cells and APCs were incubated overnight at a ratio of 10:1 in complete RPMI (cRPMI)(RPMI1640 [Invitrogen] supplemented with FCS, L-glutamine, sodium pyruvate, non-essential amino acids (Invitrogen), antibiotic-antimycotic, and 2-ME [Invitrogen]). Dby and Pigeon cytochrome c peptides were purchased from Anaspec. A pharmacological inhibitor of PI3K (Ly294002) was purchased from Cell Signaling.
Flow Cytometry
For surface staining, cells were resuspended in HBSS+BSA+NaN3 (Quality Biological) with properly diluted antibodies. For intracellular staining, cells were fixed with 4% PFA in PBS (Ted Pella) for 30 min and then permeabilized with 0.5% Triton X-100 (Sigma) in PBS with 0.1% IgG-free BSA (Jackson Immunoresearch). IL-2 secretion was detected with a cytokine secretion assay (Miltenyi). Live cells were gated based on exclusion of 7AAD (eBioscience) or Live Dead Fixable dyes (Invitrogen). Data collected on a FACS Calibur or FACS Canto II (BD) were analyzed in FlowJo (Treestar). For NFATc1 localization, data were collected with an ImagestreamX Mark II imaging flow cytometer (Amnis/EMD Millipore). Data were analyzed with IDEAS (Amnis/EMD Millipore). Images were taken at 603 magnification.
Proliferation
For 3H incorporation, APCs were incubated for 2 hr with mitomycin C (10 mg/ml; Calbiochem) in cDMEM and then rinsed with 1× PBS. T cells and APCs were resuspended in cRPMI at a ratio of 10:1 with PCC peptide. After 48 hr, culture supernatants were removed and replaced with cRPMI supplemented with 1 µCi [3H] thymidine. After an additional 24 hr, cultures were harvested and the amount of [3H]thymidine incorporated was determined with a scintillation counter.
For CFSE dilution, T cells were incubated with 1 µM carboxy-fluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) in PBS+0.5% FCS, washed with FCS, and then T cells and APCs were re-suspended in cRPMI at a ratio of 10:1 with PCC peptide. After 3 days, cultures were harvested and the amount of CFSE dilution was determined.
IL-2 ELISA
APCs were incubated for 2 hr with mitomycin C in cDMEM and rinsed with 1 × PBS. T cells and APCs were incubated in cRPMI at a ratio of 10:1 with PCC peptide. Supernatants were collected after 48 hr and analyzed by Quanti-body Array (RayBiotech). The limit of detection for IL-2 was 30 pg/ml.
Statistics
Data are presented as the mean ± SEM. Two-tailed unpaired Student’s t tests and log-rank (Mantel-Cox) tests of survival curves were performed with Prism6 (GraphPad). A p value of ≤ 0.05 was considered significant and marked with an asterisk (*); a p value >0.05 was considered non-significant (n.s.). Similarity scores, calculated by IDEAS, are the log transformed Pearson’s Correlation Coefficient of pixel intensity for NFATc1 and DAPI.
Supplementary Material
Highlights.
Notch enhances the magnitude, kinetics, and quality of primary immune responses
Ligand-induced Notch signaling increases the Ag sensitivity of naive CD4+ T cells
In CD4+ T cells, Notch signaling potentiates PI3K signaling downstream of TCR+CD28
DLL4 on APCs is physiologically important for CD4+-mediated T cell responses in vivo
ACKNOWLEDGMENTS
We thank N. Singh, R. Varma, E. Shevach, F. Ramsdell, L. Lefrançois, and P. Schwartzberg for their valuable suggestions regarding experimental design, interpretation of the data, and/or writing of the manuscript; R. Germain, T. Honjo, and S. Habu for critical reagents; T. Moyer and C. Eigsti of the NIAID Flow Cytometry Core Facility for sorting; and S. Friend and R. DeMarco of Amnis/EMD Millipore and D. Stephany of the NIAID Flow Cytometry Core Facility for assistance with the Imagestream analyses. This research was supported by the Intramural Research Program of the NIAID, NIH.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures, one table, and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2014.12.027.
REFERENCES
- Adler SH, Chiffoleau E, Xu L, Dalton NM, Burg JM, Wells AD, Wolfe MS, Turka LA, Pear WS. Notch signaling augments T cell responsiveness by enhancing CD25 expression. J. Immunol. 2003;171:2896–2903. doi: 10.4049/jimmunol.171.6.2896. [DOI] [PubMed] [Google Scholar]
- Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, Busslinger M, McCright B, Gridley T, Flavell RA. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auderset F, Schuster S, Fasnacht N, Coutaz M, Charmoy M, Koch U, Favre S, Wilson A, Trottein F, Alexander J, et al. Notch signaling regulates follicular helper T cell differentiation. J. Immunol. 2013;191:2344–2350. doi: 10.4049/jimmunol.1300643. [DOI] [PubMed] [Google Scholar]
- Bayer AL, Baliga P, Woodward JE. Transferrin receptor in T cell activation and transplantation. J. Leukoc. Biol. 1998;64:19–24. doi: 10.1002/jlb.64.1.19. [DOI] [PubMed] [Google Scholar]
- Brekelmans P, van Soest P, Leenen PJM, van Ewijk W. Inhibition of proliferation and differentiation during early T cell development by anti-transferrin receptor antibody. Eur. J. Immunol. 1994a;24:2896–2902. doi: 10.1002/eji.1830241147. [DOI] [PubMed] [Google Scholar]
- Brekelmans P, van Soest P, Voerman J, Platenburg PP, Leenen PJM, van Ewijk W. Transferrin receptor expression as a marker of immature cycling thymocytes in the mouse. Cell. Immunol. 1994b;159:331–339. doi: 10.1006/cimm.1994.1319. [DOI] [PubMed] [Google Scholar]
- Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Zhou J, Gabrilovich D. Regulation of dendritic cell differentiation and function by Notch and Wnt pathways. Immunol. Rev. 2010;234:105–119. doi: 10.1111/j.0105-2896.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- Ciofani M, Zúñiga-Pflücker JC. Notch promotes survival of pre-T cells at the β-selection checkpoint by regulating cellular metabolism. Nat. Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- Cotner T, Williams JM, Christenson L, Shapiro HM, Strom TB, Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J. Exp. Med. 1983;157:461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongre A, Surampudi L, Lawlor RG, Fauq AH, Miele L, Golde TE, Minter LM, Osborne BA. Non-canonical Notch signaling drives activation and differentiation of peripheral CD4+ T cells. Front. Immunol. 2014;5:54. doi: 10.3389/fimmu.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL. Controlling cell growth and survival through regulated nutrient transporter expression. Biochem. J. 2007;406:1–12. doi: 10.1042/BJ20070490. [DOI] [PubMed] [Google Scholar]
- Fischer A, Gessler M. Delta-Notch—and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 2007;35:4583–4596. doi: 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Helbig C, Gentek R, Backer RA, de Souza Y, Derks IA, Eldering E, Wagner K, Jankovic D, Gridley T, Moerland PD, et al. Notch controls the magnitude of T helper cell responses by promoting cellular longevity. Proc. Natl. Acad. Sci. USA. 2012;109:9041–9046. doi: 10.1073/pnas.1206044109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Steinman RM. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J. Exp. Med. 1984;160:1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida W, Fukuda K, Sakamoto S, Koyama N, Koyanagi A, Yagita H, Fukushima A. Regulation of experimental autoimmune uveoretini-tis by anti-delta-like ligand 4 monoclonal antibody. Invest. Ophthalmol. Vis. Sci. 2011;52:8224–8230. doi: 10.1167/iovs.11-7756. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Germain RN. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogeneity for individual cytokine responses of CD4+ T cells. J. Exp. Med. 1997;186:757–766. doi: 10.1084/jem.186.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn BM, Bielke W, Pear WS, Osborne BA. Cutting edge: protective effects of notch-1 on TCR-induced apoptosis. J. Immunol. 1999;162:635–638. [PubMed] [Google Scholar]
- Jelley-Gibbs DM, Strutt TM, McKinstry KK, Swain SL. Influencing the fates of CD4 T cells on the path to memory: lessons from influenza. Immunol. Cell Biol. 2008;86:343–352. doi: 10.1038/icb.2008.13. [DOI] [PubMed] [Google Scholar]
- Keerthivasan S, Suleiman R, Lawlor R, Roderick J, Bates T, Minter L, Anguita J, Juncadella I, Nickoloff BJ, Le Poole IC, et al. Notch signaling regulates mouse and human Th17 differentiation. J. Immunol. 2011;187:692–701. doi: 10.4049/jimmunol.1003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AP, Finlay DK, Hinton HJ, Clarke RG, Fiorini E, Radtke F, Cantrell DA. Notch-induced T cell development requires phosphoinositide-dependent kinase 1. EMBO J. 2007;26:3441–3450. doi: 10.1038/sj.emboj.7601761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja SS, Liu H, Tong C, Jin F, Pear WS, van Deursen J, Bram RJ. HIV-1 Rev-binding protein accelerates cellular uptake of iron to drive Notch-induced T cell leukemogenesis in mice. J. Clin. Invest. 2010;120:2537–2548. doi: 10.1172/JCI41277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4:182–197. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, Iezzi G, Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- Lenardo MJ. Interleukin-2 programs mouse alpha beta Tlymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas B, Germain RN. Opening a window on thymic positive selection: developmental changes in the influence of cosignaling by integrins and CD28 on selection events induced by TCR engagement. J. Immunol. 2000;165:1889–1895. doi: 10.4049/jimmunol.165.4.1889. [DOI] [PubMed] [Google Scholar]
- MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, Montalto G, D’Assoro AB, Libra M, Nicoletti F, et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014;5:2881–2911. doi: 10.18632/oncotarget.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter LM, Osborne BA. Canonical and non-canonical notch signaling in CD4+ T cells. In: Radtke F, editor. Notch Regulation of the Immune System. Berlin Heidelberg: Springer; 2012. pp. 99–114. [Google Scholar]
- Minter LM, Turley DM, Das P, Shin HM, Joshi I, Lawlor RG, Cho OH, Palaga T, Gottipati S, Telfer JC, et al. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat. Immunol. 2005;6:680–688. [PubMed] [Google Scholar]
- Mukherjee S, Schaller MA, Neupane R, Kunkel SL, Lukacs NW. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J. Immunol. 2009;182:7381–7388. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramura M, Hu RJ, Gu H. Mice with a fluorescent marker for interleukin 2 gene activation. Immunity. 1998;9:209–216. doi: 10.1016/s1074-7613(00)80603-2. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chuvpilo S, Wieder ED, Elkon KB, Locksley R, Serfling E, Dong C. A costimulation-initiated signaling pathway regulates NFATc1 transcription in T lymphocytes. J. Immunol. 2007;179:1096–1103. doi: 10.4049/jimmunol.179.2.1096. [DOI] [PubMed] [Google Scholar]
- O’Donnell KA, Yu D, Zeller KI, Kim J-W, Racke F, Thomas-Tikhonenko A, Dang CV. Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Mol. Cell. Biol. 2006;26:2373–2386. doi: 10.1128/MCB.26.6.2373-2386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T, Parsons M, Zakarian A, Jones RG, Nguyen LT, Woodgett JR, Ohashi PS. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J. Exp. Med. 2000;192:99–104. doi: 10.1084/jem.192.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CT, Sedy JR, Murphy KM, Kopan R. Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PLoS ONE. 2008;3:e2823. doi: 10.1371/journal.pone.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, Ciofani M, Caparros E, Buteau J, Brown K, Perkins SL, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat. Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240. e1–e7. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat. Rev. Immunol. 2014;14:435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat. Rev. Immunol. 2013;13:427–437. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- Riha P, Rudd CE. CD28 co-signaling in the adaptive immune response. Self Nonself. 2010;1:231–240. doi: 10.4161/self.1.3.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg EV, Ward SB. A dynamic assembly of diverse transcription factors integrates activation and cell-type information for interleukin 2 gene regulation. Proc. Natl. Acad. Sci. USA. 1996;93:9358–9365. doi: 10.1073/pnas.93.18.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samon JB, Champhekar A, Minter LM, Telfer JC, Miele L, Fauq A, Das P, Golde TE, Osborne BA. Notch1 and TGFbeta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood. 2008;112:1813–1821. doi: 10.1182/blood-2008-03-144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE, Sonenshein GE, Osborne BA. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J. 2006;25:129–138. doi: 10.1038/sj.emboj.7600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi N, Yanagisawa S, Kaneyama T, Yagita H, Jin YH, Kim BS, Koh CS. Ameliorating effects of anti-Dll4 mAb on Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Int. Immunol. 2010;22:729–738. doi: 10.1093/intimm/dxq059. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Tsuji M, Yamamoto N, Han H, Tsukada J, Inoue H, Kubo M, Honjo T. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- Teh HS, Teh SJ. High concentrations of antigenic ligand activate and do not tolerize naive CD4 T cells in the absence of CD28/B7 costimulation. Cell. Immunol. 1997;179:74–83. doi: 10.1006/cimm.1997.1137. [DOI] [PubMed] [Google Scholar]
- Tran IT, Sandy AR, Carulli AJ, Ebens C, Chung J, Shan GT, Radojcic V, Friedman A, Gridley T, Shelton A, et al. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J. Clin. Invest. 2013;123:1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zang C, Taing L, Arnett KL, Wong YJ, Pear WS, Blacklow SC, Liu XS, Aster JC. NOTCH1-RBPJ complexes drive target gene expression through dynamic interactions with superenhancers. Proc. Natl. Acad. Sci. USA. 2014;111:705–710. doi: 10.1073/pnas.1315023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J, Atkins M, Hwu P, Radvanyi L, Sznol M, Yee C Immunotherapy Task Force of the NCI Investigational Drug Steering Committee. White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy. Clin. Cancer Res. 2011;17:1664–1673. doi: 10.1158/1078-0432.CCR-10-2272. [DOI] [PubMed] [Google Scholar]
- Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J. Clin. Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, Ridgway JB, Niessen K, Plowman GD. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:E6–E7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.