Abstract
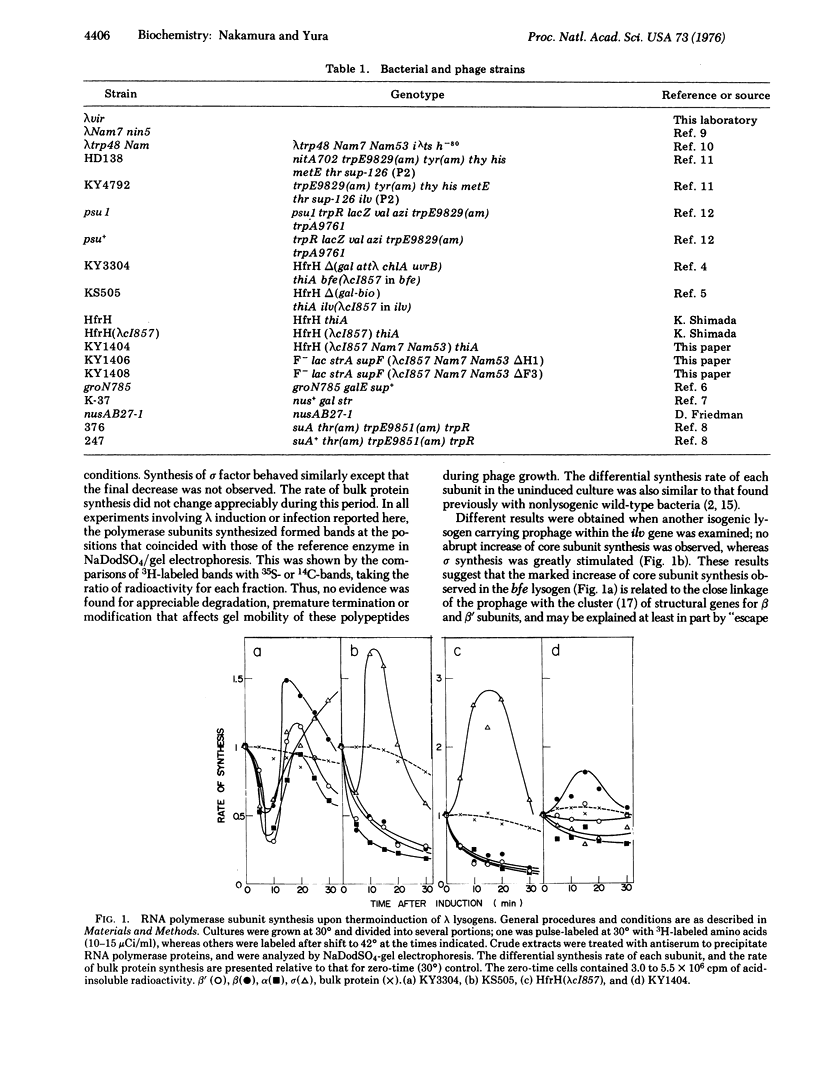
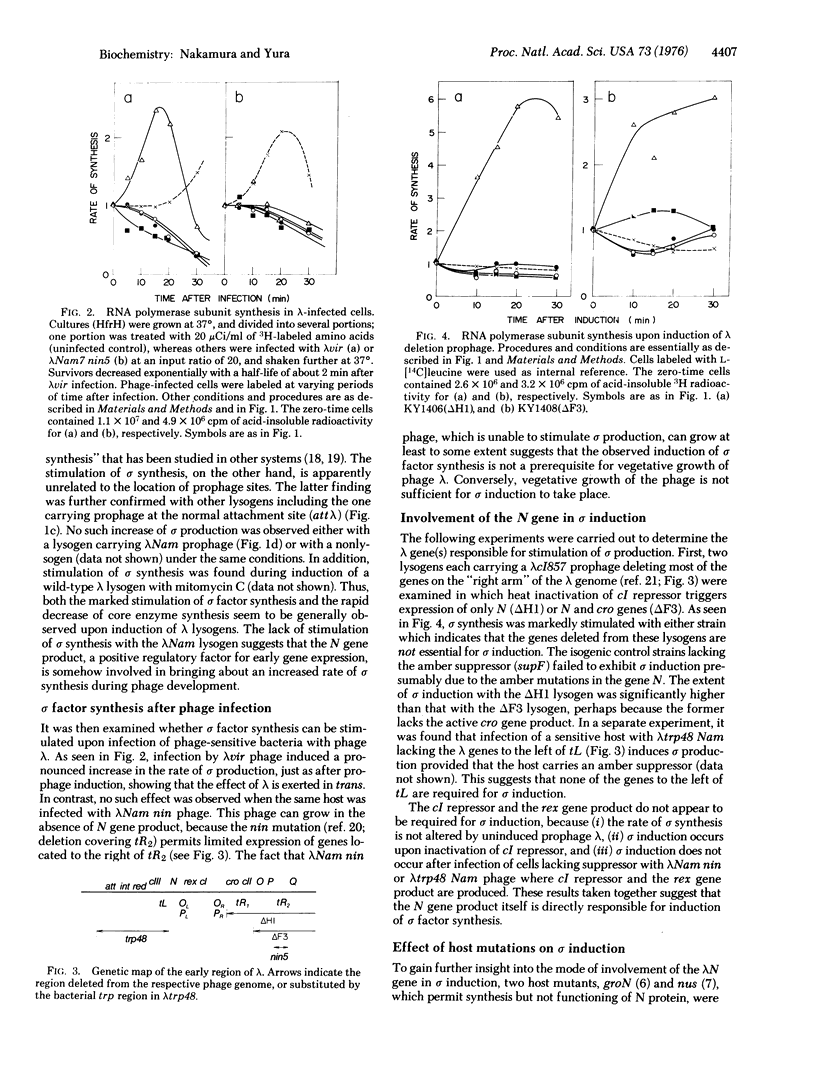
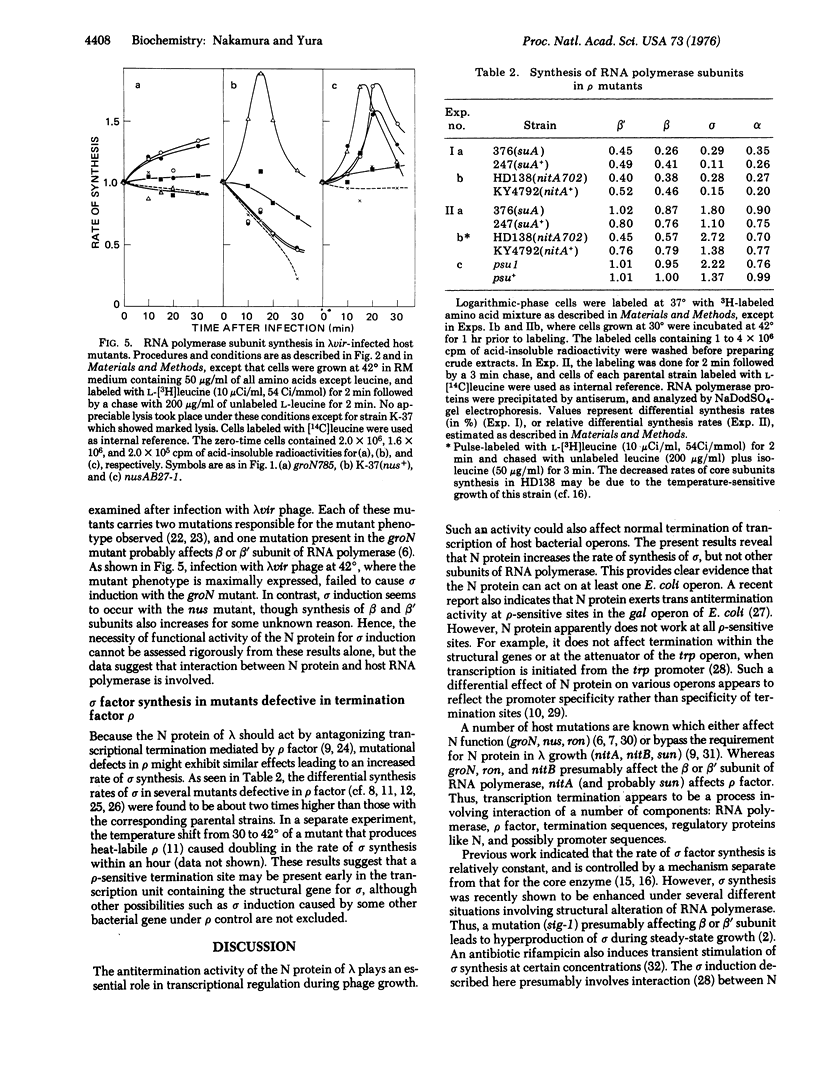
Thermoinduction of cells of E. coli carrying prophage lambdacI857 within the bfe gene brings about not only "escape synthesis" of core subunits of the DNA-dependent RNA polymerase (RNA nucleotidyltransferase, nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2-7-7-6), but also a striking stimulation of sigma factor synthesis. The latter phenomenon, termed sigma induction, is generally observed after lambda phage infection or prophage induction. A series of experiments with various bacterial and phage strains led us to conclude that the N gene product of lambda is directly involved to the sigma induction. These and other results obtained with mutants defective in transcription termination factor rho suggest the involvement of a rho-sensitive site in the control of sigma gene expression in E. coli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTTIN G., JACOB F., MONOD J. [Constituent synthesis of galactokinase following the development of lambda bacteriophages in Escherichia coli K 12]. C R Hebd Seances Acad Sci. 1960 Mar 28;250:2471–2473. [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Brunel F., Davison J. Bacterial mutants able to partly suppress the effect of N mutations in bacteriophage lambda. Mol Gen Genet. 1975;136(2):167–180. doi: 10.1007/BF00272037. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. Separation and characterization of the subunits of ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6168–6176. [PubMed] [Google Scholar]
- Castellazzi M., Brachet P., Eisen H. Isolation and characterization of deletions in bacteriophage lambda residing as prophage in E. coli K 12. Mol Gen Genet. 1972;117(3):211–218. doi: 10.1007/BF00271648. [DOI] [PubMed] [Google Scholar]
- Court D., Sato K. Studies of novel transducing variants of lambda: dispensability of genes N and Q. Virology. 1969 Oct;39(2):348–352. doi: 10.1016/0042-6822(69)90060-9. [DOI] [PubMed] [Google Scholar]
- Errington L., Glass R. E., Hayward R. S., Scaife J. G. Structure and orientation of an RNA polymerase operon in Escherichia coli. Nature. 1974 Jun 7;249(457):519–522. doi: 10.1038/249519a0. [DOI] [PubMed] [Google Scholar]
- Franklin N. C. Altered reading of genetic signals fused to the N operon of bacteriophage lambda: genetic evidence for modification of polymerase by the protein product of the N gene. J Mol Biol. 1974 Oct 15;89(1):33–48. doi: 10.1016/0022-2836(74)90161-2. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Baumann M., Baron L. S. Cooperative effects of bacterial mutations affecting lambda N gene expression. I. Isolation and characterization of a nusB mutant. Virology. 1976 Aug;73(1):119–127. doi: 10.1016/0042-6822(76)90066-0. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Ponce-Campos R. Differential effect of phage regulator functions on transcription from various promoters: evidence that the P22 gene 24 and the lambda gene N products distinguish three classes of promoters. J Mol Biol. 1975 Nov 5;98(3):537–549. doi: 10.1016/s0022-2836(75)80085-4. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Bacterial mutants in which the gene N function of bacteriophage lambda is blocked have an altered RNA polymerase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2977–2981. doi: 10.1073/pnas.68.12.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P., Reichardt K. M., Eisen H. Cro mutants of phage 434. Virology. 1975 Nov;68(1):35–40. doi: 10.1016/0042-6822(75)90145-2. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Pironio M. Relationship between the N function of bacteriophage lambda and host RNA polymerase. J Mol Biol. 1972 Mar 28;65(2):259–272. doi: 10.1016/0022-2836(72)90281-1. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T., Yura T., Yamagishi H. Genetic and physical studies of lambda transducing bacteriophage carrying the beta subunit gene of the Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1975 Jun;122(3):1247–1256. doi: 10.1128/jb.122.3.1247-1256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoko H., Imai M. Isolation and genetic characterization of the nitA mutants of Escherichia coli affecting the termination factor rho. Mol Gen Genet. 1976 Jan 16;143(2):211–221. doi: 10.1007/BF00266924. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Ito K., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. I. Control of RNA polymerase content at various growth rates. Mol Gen Genet. 1974;133(1):1–23. doi: 10.1007/BF00268673. [DOI] [PubMed] [Google Scholar]
- Kasai T. Regulation of the expression of the histidine operon in Salmonella typhimurium. Nature. 1974 Jun 7;249(457):523–527. doi: 10.1038/249523a0. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors increase expression of the wild-type tryptophan operon of Escherichia coli. J Mol Biol. 1976 May 15;103(2):395–409. doi: 10.1016/0022-2836(76)90319-3. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D., Morse D. E. Loss of dispensable endonuclease activity in relief of polarity by suA. Nat New Biol. 1971 Jun 16;231(24):214–217. doi: 10.1038/newbio231214a0. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Effects of rifampicin on synthesis and functional activity of DNA-dependent RNA polymerase in Escherichia coli. Mol Gen Genet. 1976 Jun 15;145(3):227–237. doi: 10.1007/BF00325817. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Evidence for a positive regulation of RNA polymerase synthesis in Escherichia coli. J Mol Biol. 1975 Oct 5;97(4):621–642. doi: 10.1016/s0022-2836(75)80063-5. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Hyperproduction of the sigma subunit of RNA polymerase in a mutant of Escherichia coli. Mol Gen Genet. 1975 Nov 24;141(2):97–111. doi: 10.1007/BF00267677. [DOI] [PubMed] [Google Scholar]
- Petit-Koskas E., Contesse G. Stimulation in trans of synthesis of E. coli gal operon enzymes by lambdoid phages during low catabolite repression. Mol Gen Genet. 1976 Jan 16;143(2):203–209. doi: 10.1007/BF00266923. [DOI] [PubMed] [Google Scholar]
- Ratner D. Evidence that mutations in the suA polarity suppressing gene directly affect termination factor rho. Nature. 1976 Jan 15;259(5539):151–153. doi: 10.1038/259151a0. [DOI] [PubMed] [Google Scholar]
- Richardson J. P., Grimley C., Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci U S A. 1975 May;72(5):1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. II. Mutations induced by bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1973 Oct 25;80(2):297–314. doi: 10.1016/0022-2836(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A., Burgessrr Cyclic re-use of the RNA polymerase sigma factor. Nature. 1969 May 10;222(5193):537–540. doi: 10.1038/222537a0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H. REGULATION BY COLIPHAGE LAMBDA OF THE EXPRESSION OF THE CAPACITY TO SYNTHESIZE A SEQUENCE OF HOST ENZYMES. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1626–1645. doi: 10.1073/pnas.46.12.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]


