Abstract
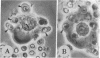
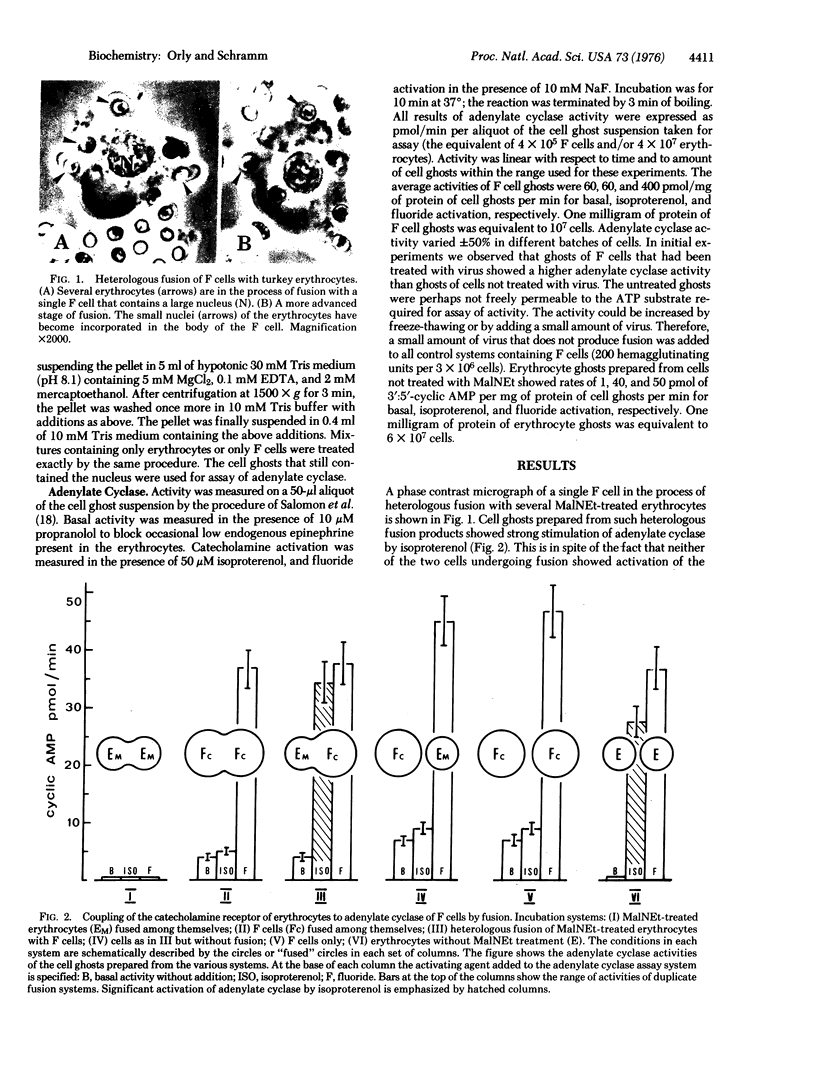
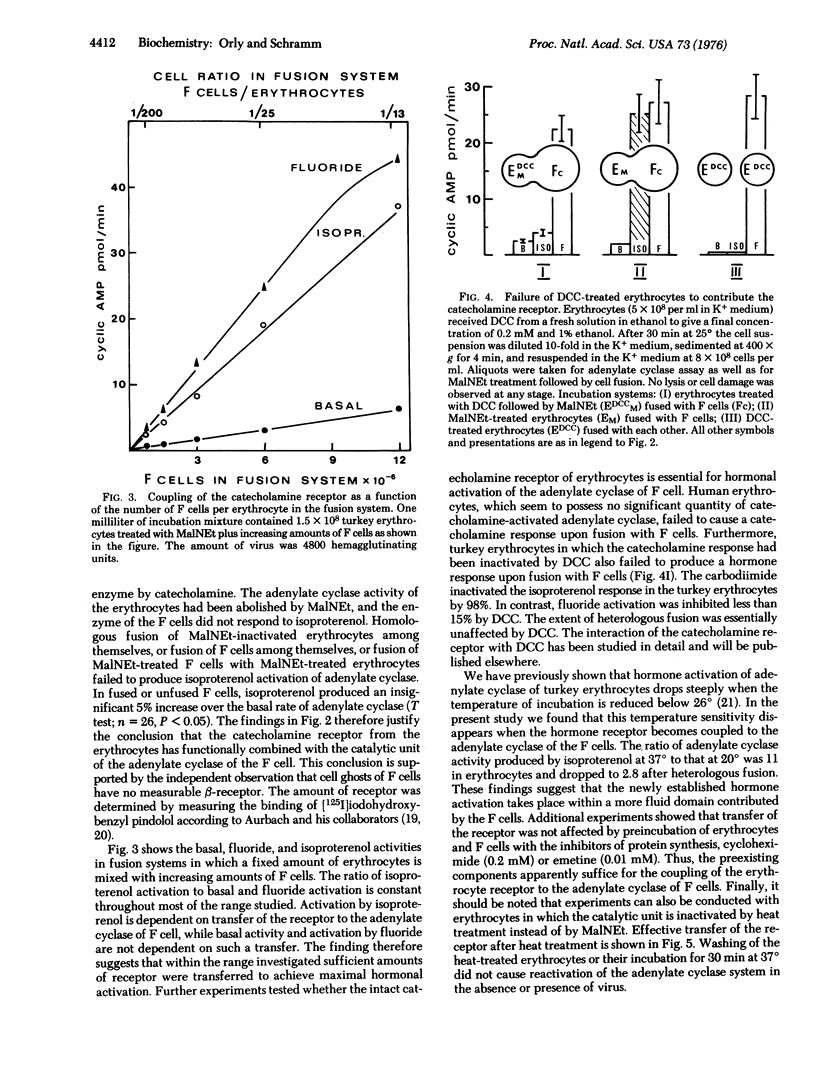
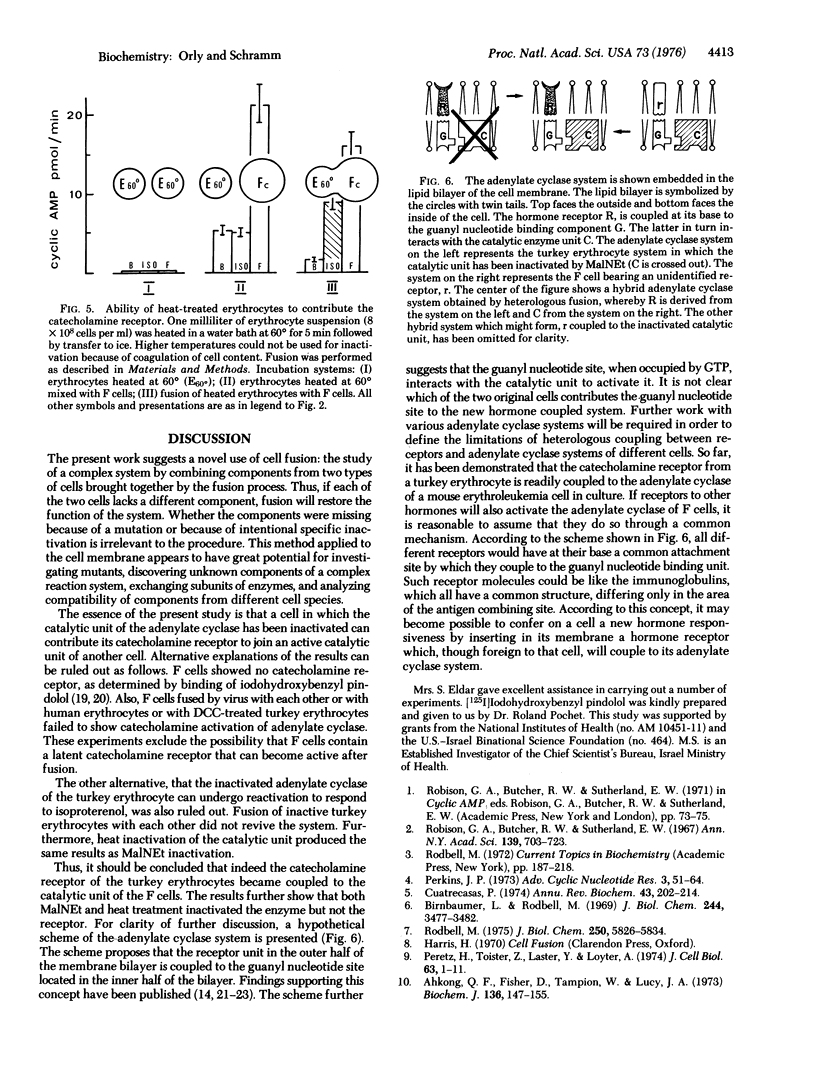
The experiments test the hypothesis that beta-adrenergic receptor is an independent unit that can be transferred from one adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4-6-1-1[ system to another. Turkey erythrocytes in which the catalytic activity of adenylate cyclase had been inactivated by N-ethylmaleimide or by heat contributed the beta-adrenergic receptor. Friend erythroleukemia cells (F cells) that possessed no measurable beta-adrenergic receptor contributed the adenylate cyclase. The erythrocytes in which the enzyme had been inactivated were fused with the F cells by Sendai virus. The cell ghosts of the fused preparation demonstrated adenylate cyclase activity which was strikingly enhanced by isoproterenol. Controls of fusion of F cells with each other or with human erythrocytes failed to show a response to isoproterenol. It was therefore concluded that the beta-adrenergic receptor of the turkey erythrocytes must have become functionally coupled to the adenylate cyclase of the mouse F cells. Activation by isoproterenol was demonstrable within a few minutes after fusion, and inhibitors of protein synthesis had no effect. Thus, coupling must have occurred between the preexisting components. The findings suggest that it may be possible in the future to confer on cells that possess an adenylate cyclase system new hormonal responses by inserting a receptor into their cell membrane. It is proposed that the procedure of massive heterologous cell fusion, as used in the present study, can be used to analyze the function of other cell membrane components.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. The fusion of erythrocytes by fatty acids, esters, retinol and alpha-tocopherol. Biochem J. 1973 Sep;136(1):147–155. doi: 10.1042/bj1360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurbach G. D., Fedak S. A., Woodard C. J., Palmer J. S., Hauser D., Troxler F. Beta-adrenergic receptor: stereospecific interaction of iodinated beta-blocking agent with high affinity site. Science. 1974 Dec 27;186(4170):1223–1224. doi: 10.1126/science.186.4170.1223. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Rodbell M. Adenyl cyclase in fat cells. II. Hormone receptors. J Biol Chem. 1969 Jul 10;244(13):3477–3482. [PubMed] [Google Scholar]
- Brown E. M., Fedak S. A., Woodard C. J., Aurbach G. D. Beta-Adrenergic receptor interactions. Direct comparison of receptor interaction and biological activity. J Biol Chem. 1976 Mar 10;251(5):1239–1246. [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Gilman A. G., Minna J. D. Expression of genes for metabolism of cyclic adenosine 3':5'-monophosphate in somatic cells. I. Responses to catecholamines in parental and hybrid cells. J Biol Chem. 1973 Oct 10;248(19):6610–6617. [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. I. Microscopic observation of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:98–107. doi: 10.1016/0014-4827(62)90205-7. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orly J., Schramm M. Fatty acids as modulators of membrane functions: catecholamine-activated adenylate cyclase of the turkey erythrocyte. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3433–3437. doi: 10.1073/pnas.72.9.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oye I., Sutherland E. W. The effect of epinephrine and other agents on adenyl cyclase in the cell membrane of avian erythrocytes. Biochim Biophys Acta. 1966 Oct 31;127(2):347–354. doi: 10.1016/0304-4165(66)90389-8. [DOI] [PubMed] [Google Scholar]
- Peretz H., Toister Z., Laster Y., Loyter A. Fusion of intact human erythrocytes and erythrocyte ghosts. J Cell Biol. 1974 Oct;63(1):1–11. doi: 10.1083/jcb.63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci. 1967 Feb 10;139(3):703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- Rodbell M. On the mechanism of activation of fat cell adenylate cyclase by guanine nucleotides. An explanation for the biphasic inhibitory and stimulatory effects of the nucleotides and the role of hormones. J Biol Chem. 1975 Aug 10;250(15):5826–5834. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schramm M., Naim E. Adenyl cyclase of rat parotid gland. Activation by fluoride and norepinephrine. J Biol Chem. 1970 Jun;245(12):3225–3231. [PubMed] [Google Scholar]
- Schramm M., Rodbell M. A persistent active state of the adenylate cyclase system produced by the combined actions of isoproterenol and guanylyl imidodiphosphate in frog erythrocyte membranes. J Biol Chem. 1975 Mar 25;250(6):2232–2237. [PubMed] [Google Scholar]