Abstract
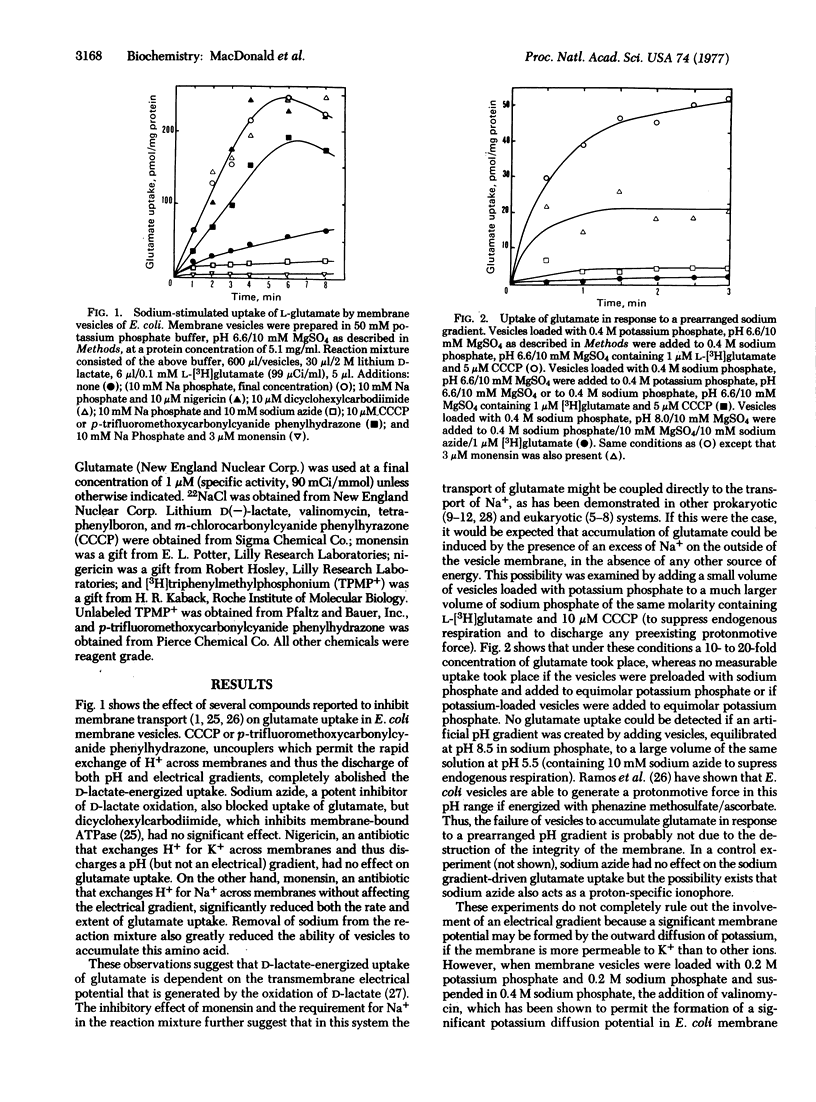
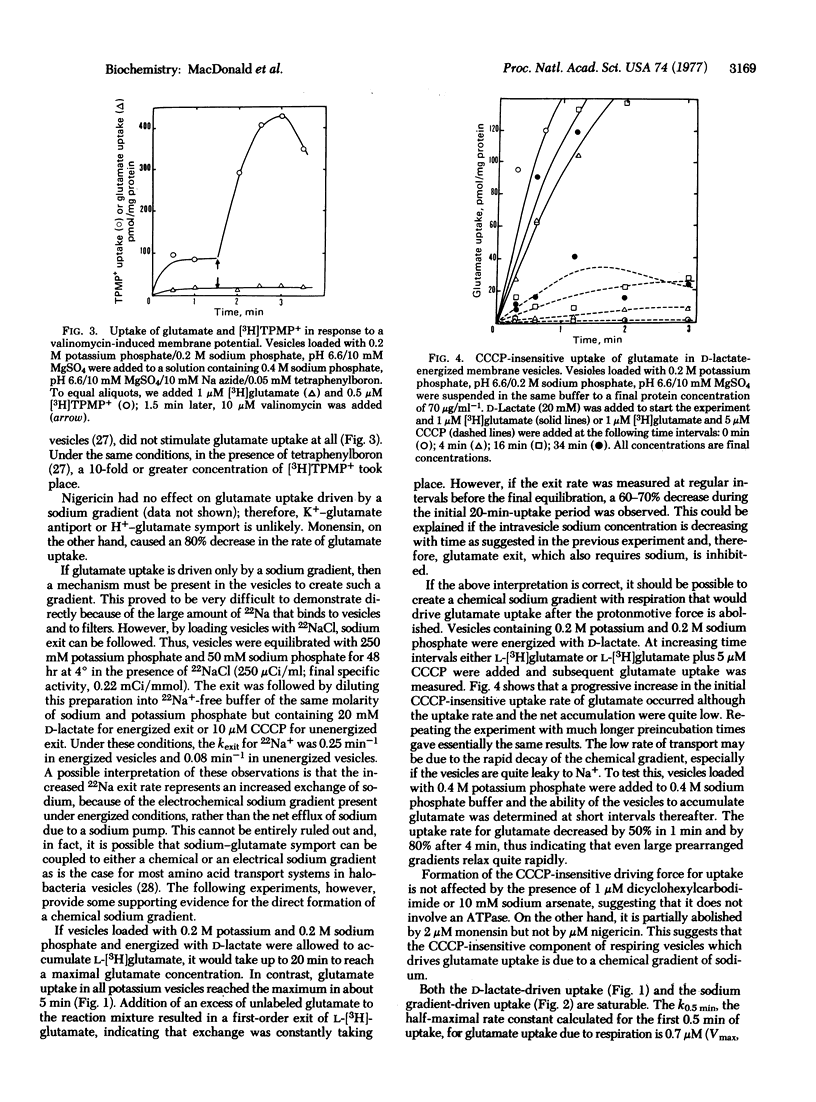
Membrane vesicles prepared from Escherichia coli B/r grown on glutamate as a sole source of carbon and energy require sodium for glutamate accumulation when energized by D-lactate oxidation. Glutamate uptake can also be driven by a prearranged sodium gradient (out to in) in the absence of an energy source or a protonmotive force. Sodium ions are exchanged rapidly in respiring vesicles and the sodium gradient may be large enough under certain conditions to drive glutamate uptake after the protonmotive force is abolished with m-chlorocarbonylcyanide phenylhydrazone. Glutamate uptake due to a prearranged sodium gradient or lactate oxidation is inhibited by monensin but not by nigericin. Transport does not occur in response to valinomycin-induced membrane potential. We interpret these results to indicate that glutamate transport is obligately coupled to sodium transport and can only occur when there is a net flux of sodium ions. This flux is driven by a chemical gradient of sodium that is created by the protonmotive force generated by respiration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. C., Sacktor B. Energetics of the Na+-dependent transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1975 Nov 25;250(22):8674–8680. [PubMed] [Google Scholar]
- Emilio M. G., Menano H. P. The excretion of hydrogen ion by the isolated amphibian skin: effects of antidiuretic hormone and amiloride. Biochim Biophys Acta. 1975 Mar 25;382(3):344–352. doi: 10.1016/0005-2736(75)90276-x. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol. 1972;8(1):45–62. doi: 10.1007/BF01868094. [DOI] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Asano A., Brodie A. F. Respiration dependent transport of proline by electron transport particles from mycobacterium phlei. Biochem Biophys Res Commun. 1971 Jul 16;44(2):368–374. doi: 10.1016/0006-291x(71)90609-7. [DOI] [PubMed] [Google Scholar]
- Hopfer U. Sugar and amino acid transport in animal cells. Horiz Biochem Biophys. 1976;2:106–133. [PubMed] [Google Scholar]
- KJELDGAARD N. O. The kinetics of ribonucleic acid- and protein formation in Salmonella typhimurium during the transition between different states of balance growth. Biochim Biophys Acta. 1961 Apr 29;49:64–76. doi: 10.1016/0006-3002(61)90870-8. [DOI] [PubMed] [Google Scholar]
- Kahane S., Marcus M., Barash H., Halpern Y. S. Sodium-dependent glutamate transport in membrane vesicles of Escherichia coli K-12. FEBS Lett. 1975 Aug 15;56(2):235–239. doi: 10.1016/0014-5793(75)81099-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanyi J. K., MacDonald R. E. Existence of electrogenic hydrogen ion/sodium ion antiport in Halobacterium halobium cell envelope vesicles. Biochemistry. 1976 Oct 19;15(21):4608–4614. doi: 10.1021/bi00666a010. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Renthal R., MacDonald R. E. Light-induced glutamate transport in Halobacterium halobium envelope vesicles. II. Evidence that the driving force is a light-dependent sodium gradient. Biochemistry. 1976 Apr 20;15(8):1603–1610. doi: 10.1021/bi00653a002. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Yearwood-Drayton V., MacDonald R. E. Light-induced glutamate transport in Halobacterium halobium envelope vesicles. I. Kinetics of the light-dependent and the sodium-gradient-dependent uptake. Biochemistry. 1976 Apr 20;15(8):1595–1603. doi: 10.1021/bi00653a001. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi J. K. Light-activated amino acid transport in Halobacterium halobium envelope vesicles. Fed Proc. 1977 May;36(6):1828–1832. [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi L. K. Light-induced leucine transport in Halobacterium halobium envelope vesicles: a chemiosmotic system. Biochemistry. 1975 Jul;14(13):2882–2889. doi: 10.1021/bi00684a014. [DOI] [PubMed] [Google Scholar]
- Miner K. M., Frank L. Sodium-stimulated glutamate transport in osmotically shocked cells and membrane vesicles of Escherichia coli. J Bacteriol. 1974 Mar;117(3):1093–1098. doi: 10.1128/jb.117.3.1093-1098.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976 Mar 15;154(3):597–604. [PMC free article] [PubMed] [Google Scholar]
- Murer H., Sigrist-Nelson K., Hopfer U. On the mechanism of sugar and amino acid interaction in intestinal transport. J Biol Chem. 1975 Sep 25;250(18):7392–7396. [PubMed] [Google Scholar]
- Niven D. F., Hamilton W. A. Mechanisms of energy coupling to the transport of amino acids by Staphylococcus aureus. Eur J Biochem. 1974 May 15;44(2):517–522. doi: 10.1111/j.1432-1033.1974.tb03510.x. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Stock J., Roseman S. A sodium-dependent sugar co-transport system in bacteria. Biochem Biophys Res Commun. 1971 Jul 2;44(1):132–138. doi: 10.1016/s0006-291x(71)80168-7. [DOI] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Na+ and K+ gradients and alpha-aminoisobutyric acid transport in a marine pseudomonad. J Biol Chem. 1973 Oct 25;248(20):7106–7111. [PubMed] [Google Scholar]
- Tokuda H., Kaback H. R. Sodium-dependent methyl 1-thio-beta-D-galactopyranoside transport in membrane vesicles isolated from Salmonella typhimurium. Biochemistry. 1977 May 17;16(10):2130–2136. doi: 10.1021/bi00629a013. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Proton/sodium ion antiport in Escherichia coli. Biochem J. 1974 Oct;144(1):87–90. doi: 10.1042/bj1440087. [DOI] [PMC free article] [PubMed] [Google Scholar]


