Abstract
BACKGROUND:
Daily application of oral chlorhexidine gluconate (CHX) following intubation to reduce the risk of ventilator-associated pneumonia (VAP) is now the standard of care in many ICUs. This randomized clinical trial evaluated the benefit of adding a preintubation CHX dose to the known benefit of postintubation CHX to reduce the risk of early-onset VAP. A secondary aim was to test the effect of a preintubation oral application of CHX on early endotracheal tube (ETT) colonization.
METHODS:
Subjects (N = 314) were recruited from two teaching hospitals and were randomly assigned to oral application of 5 mL CHX 0.12% solution before intubation (intervention group, n = 157), or to a control group (n = 157) who received no CHX before intubation. All subjects received CHX bid after intubation. Groups were compared using a repeated-measures model with Clinical Pulmonary Infection Score (CPIS) as the response variable. In a planned subset of subjects, ETTs were cultured at extubation.
RESULTS:
Application of a preintubation dose of CHX did not provide benefit over the intervention period beyond that afforded by daily oral CHX following intubation. ETT colonization at extubation was < 20% in both groups (no statistically significant difference). Mean CPIS remained below 6 (VAP threshold score) in both groups.
CONCLUSIONS:
Although it is feasible to deliver CHX prior to intubation (including emergent or urgent intubation), the results suggest that preintubation CHX may be inconsequential when the ventilator bundle, including daily oral CHX, is in place. During the preintubation period, providers should focus their attention on other critical activities.
TRIAL REGISTRY:
ClinicalTrials.gov; No.: NCT00893763; URL: www.clinicaltrials.gov
Hospital-acquired infections in critically ill adults are associated with increased morbidity, mortality, and cost. Reduction of ventilator-associated pneumonia (VAP) has been a target for quality improvement. Over the past decade, multiple interventions have been tested to reduce the risk of VAP. The Institute for Healthcare Improvement (IHI)1 has published guidelines for the care of patients who are mechanically ventilated; the ventilator bundle incorporates interventions with evidence of effectiveness in reducing VAP risk, including 30° to 45° elevation of the head of the bed to reduce the risk of aspiration, and daily oral care with an antiseptic agent such as chlorhexidine gluconate (CHX). Many institutions have reported drastic reductions in VAP rates following implementation of a ventilator bundle.2 However, VAP has not been eliminated, and additional interventions to further prevent risk would be beneficial.
CHX is a broad-spectrum antibacterial agent that is widely used as an oral rinse in outpatient dental care for the prevention and treatment of oral diseases such as gingivitis and periodontitis. Evidence supporting the inclusion of CHX in the daily oral care of patients who are mechanically ventilated to reduce the risk of VAP was reported in our initial investigations3 and was confirmed by others in subsequent clinical trials and meta-analyses.4,5 We also found that early application of a single dose of CHX (immediately after intubation) reduced early-onset VAP.6 A Cochrane review concluded that chlorhexidine, either as a mouthwash or as a gel, is associated with a 40% reduction in the odds of developing VAP in critically ill adults.7 However, studies have failed to show that oral CHX is associated with a difference in patient outcomes such as mortality, duration of mechanical ventilation, or duration of ICU stay.4,7 Daily oral CHX was added to IHI guidelines for the care of the patient who is mechanically ventilated in 2010 and is now the standard of care in many ICUs. In contrast, a recent meta-analysis by Klompas et al8 indicated that although patients undergoing cardiac surgery derived benefit from CHX, other types of critically ill patients did not; it is interesting to note that CHX is generally begun prior to intubation in patients undergoing elective cardiac surgery, but is begun following intubation in other critically ill patients.
During intubation, the endotracheal tube (ETT) must pass through the microbially rich environment of the oropharynx. In other clinical procedures in which a tube is inserted (for example, a urinary or IV catheter), decontamination procedures are performed at the insertion site to reduce the risk of subsequent colonization or infection. Endotracheal (ET) intubation generally proceeds without any preparation of the mouth other than suctioning of secretions and removal of dentures; loose teeth may be incidentally removed. Earlier studies in subjects undergoing elective cardiac surgery demonstrated a reduction in infections (including respiratory and surgical) in subjects who used CHX as an oral rinse before hospital admission,9‐11 but preintubation administration of CHX, including emergent or urgent intubations, has not been well studied in other patient populations. It is plausible that during urgent or emergent ET intubation, microbes in the pharynx may be dragged into the trachea and lower respiratory tract, potentially predisposing to pneumonia. Preintubation chlorhexidine, but not postintubation chlorhexidine, could potentially mitigate this. We reasoned that reducing the number of microorganisms in the mouth before intubation by application of CHX, added to continual microbial suppression by CHX applied after intubation, would reduce the risk of early-onset VAP in critically ill adults.
This project focused on evaluating the benefit of adding a preintubation CHX dose to the known benefit of postintubation CHX to reduce the risk of VAP. The primary aim was to test the effect of a preintubation oral application of CHX on the development of VAP in a variety of critically ill adults who were mechanically ventilated. Because colonization of the ETT provides a habitat for microorganisms and may contribute to the development of VAP, a secondary aim was to test the effect of a preintubation oral application of CHX on early ET colonization.
Materials and Methods
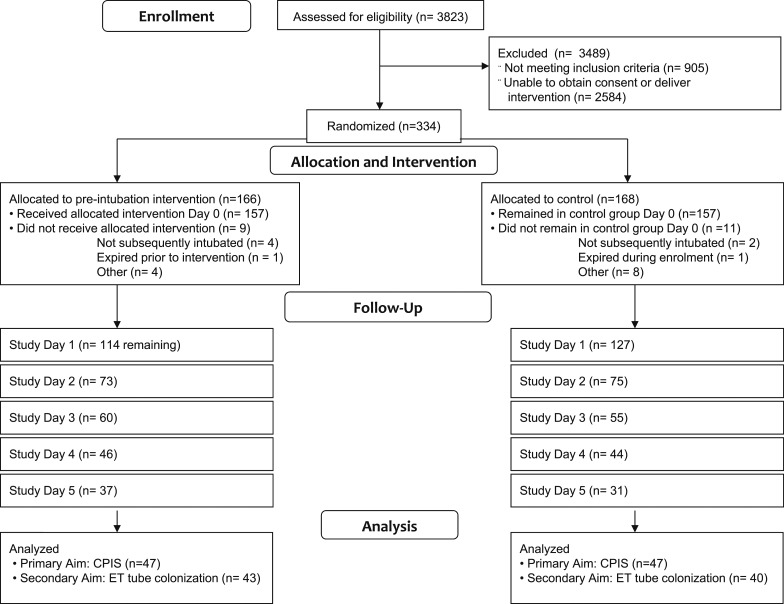
Subjects (N = 314) (Fig 1, Table 1) were enrolled from two large urban teaching medical centers in the Southeast (214 subjects at Virginia Commonwealth University Health System, an affiliate of Virginia Commonwealth University [VCU] in Richmond, Virginia, and 100 subjects at Tampa General Hospital, an affiliate of the University of South Florida [USF] in Tampa, Florida). The biostatistician investigator conducted an a priori power analysis to determine the sample size required to detect a difference in Clinical Pulmonary Infection Score (CPIS) of 1 between the two groups (intervention and control). Subjects were recruited in multiple clinical areas just prior to intubation, including critical care units, EDs, preoperative areas, procedural areas, and medical-surgical units during rapid response or code calls. Standard ETTs were selected by the clinical providers at each site. Neither site used subglottic suction tubes nor antimicrobial (eg, silver-coated) tubes. Patients with a clinical diagnosis of pneumonia at the time of intubation were excluded, because the determination of nosocomial pneumonia was confounded in subjects with preexisting pneumonia. Approval for involvement of human subjects was obtained from institutional review boards (IRBs) at both study sites (VCU IRB No. HM11519, USF IRB No. CR2_Pro00003622).
Figure 1 –
CONSORT (Consolidated Standards of Reporting Trials) 2010 flow diagram. CPIS = Clinical Pulmonary Infection Score; ET = endotracheal.
TABLE 1 ] .
Demographic and Clinical Characteristics
| Variable | Total (314) | Subjects by Collection Site | Subjects by Group | Subjects in Analysis | |||
| VCU (214) | TGH (100) | CHX (157) | Control (157) | CHX (47) | Control (47) | ||
| Age, mean (SD), y | 58.12 (16) | 55.5 (16.7) | 63.6 (12.7) | 58.1 (15.9) | 58.2 (16.2) | 59.5 (11.5) | 56.4 (16.5) |
| Ethnicity | |||||||
| Hispanic | 2.9 (9) | 3 (5) | 4 (4) | 3 (5) | 3 (4) | 2 (1) | 2 (1) |
| Non-Hispanic | 97.1 (305) | 97 (167) | 96 (96) | 97 (152) | 97 (153) | 98 (46) | 98 (46) |
| Sex | |||||||
| Male | 60.2 (189) | 59 (127) | 62 (62) | 58 (91) | 62 (98) | 55 (26) | 60 (28) |
| Female | 39.8 (125) | 41 (87) | 38 (38) | 42 (66) | 38 (59) | 45 (21) | 40 (19) |
| Race | |||||||
| White | 64.3 (202) | 51 (109) | 93 (93) | 68 (106) | 61 (96) | 64 (30) | 46 (21) |
| Black | 32.2 (101) | 46 (98) | 5 (5) | 29 (45) | 36 (56) | 34 (16) | 54 (25) |
| Other | 3.5 (11) | 4 (9) | 2 (2) | 4 (6) | 3 (5) | 2 (1) | 0 |
| Location of enrollment | |||||||
| ED | 27.7 (87) | 41 (87) | 0 | 31 (49) | 24 (38) | 17 (8) | 34 (16) |
| MICU | 9.9 (31) | 14 (30) | 1 (1) | 9 (14) | 11 (17) | 23 (11) | 13 (6) |
| SICU | 8.3 (26) | 12 (26) | 0 | 9 (14) | 8 (12) | 17 (8) | 15 (7) |
| CCU | 7.7 (24) | 11 (24) | 0 | 9 (14) | 6 (10) | 15 (7) | 2 (1) |
| CSICU | 1.9 (6) | 2 (4) | 2 (2) | 11 (2) | 3 (4) | 0 | 4 (2) |
| NSICU | 4.1 (13) | 6 (13) | 0 | 4 (6) | 4 (7) | 6 (3) | 11 (5) |
| Preop | 28.3 (89) | 1 (3) | 85 (85) | 30 (47) | 27 (42) | 13 (6) | 9 (4) |
| Other | 12.4 (39) | 13 (27) | 12 (12) | 8 (12) | 17 (27) | 9 (4) | 13 (6) |
| Reason for intubation | |||||||
| Airway control | 63.1 (198) | 47 (100) | 98 (98) | 68 (106) | 59 (92) | 51 (24) | 51 (24) |
| Hypoxemic respiratory | 10.5 (33) | 14 (31) | 2 (2) | 10 (16) | 11 (17) | 17 (8) | 11 (5) |
| Respiratory arrest | 6.7 (21) | 10 (21) | 0 | 3 (4) | 11 (17) | 2 (1) | 6 (3) |
| Ventilatory failure | 1.6 (5) | 2 (5) | 0 | 2 (3) | 1 (2) | 2 (1) | 4 (2) |
| Respiratory distress | 18.2 (57) | 27 (57) | 0 | 18 (28) | 18 (29) | 28 (13) | 28 (13) |
| APACHE score, mean (SD) | 67.1 (29.3) | 79.1 (28.4) | 48.6 (19.1) | 69.1 (28.0) | 65.4 (30.4) | 81.2 (25.2) | 73.3 (26.3) |
Data are presented as No. (%) unless indicated otherwise. APACHE = Acute Physiology and Chronic Health Evaluation; CCU = coronary care unit; CHX = chlorhexidine gluconate; CSICU = cardiac surgery ICU; MICU = medical ICU; NSICU = neuroscience ICU; Preop = preoperative area; SICU = surgical ICU; TGH = Tampa General Hospital; VCU = Virginia Commonwealth University.
The two settings differed in procedures related to informed consent. The VCU IRB approved a waiver of prospective consent and a waiver of written documentation of consent; legally authorized representatives and subjects were provided with a written study information sheet and verbal explanation of the study and were advised that they could withdraw from the study at any time. The USF IRB approved a waiver of prospective consent but required written documentation of consent (including information about voluntary withdrawal) from the subjects’ legally authorized representatives at the earliest opportunity following study enrollment. Subjects remained in the study for a maximum of 6 days; for subjects who were extubated prior to 6 days, the subject’s participation ended on the day of extubation.
Subjects were randomly assigned prior to intubation to one of two groups; the intervention group (n = 157) received oral application of 5 mL CHX 0.12% solution before intubation by swab to the oral cavity administered by study personnel, whereas the control group (n = 157) received no preintubation intervention. All subjects (both groups) received CHX bid after intubation. The bid postintubation doses of CHX were supplied as individual single application doses by the investigational pharmacies in both settings and were administered by the clinical nurse responsible for the patient’s medications. We monitored medication records for documentation of delivery. The IHI ventilator bundle was standard of care in both institutions; bundle compliance, including head-of bed elevation, was monitored for each subject. Descriptive data (demographics, clinical characteristics) and APACHE (Acute Physiology and Chronic Health Evaluation) III data were collected on enrollment.
The CPIS12 was used to evaluate the risk of early-onset VAP; we selected the CPIS because it permitted serial prospective evaluation of VAP risk in every subject, whereas BAL could not be repeated daily in subjects without clinical indication for the procedure without substantially increasing risks to human subjects. The six elements of CPIS (tracheal secretions, temperature, WBC count, oxygenation, chest radiograph, and tracheal aspirate culture) were collected every study day. Tracheal secretions and most abnormal (high or low) values for temperature, WBC count, and oxygenation (calculated by Pao2/Fio2) were obtained from the medical record by study personnel. Digital images of morning chest radiographs performed for clinical care were obtained and blinded prior to interpretation and scoring by the pulmonologist coinvestigator (C. N. S.). The scoring system originally described by Pugin et al12 was used. To facilitate scoring of changes over time, all chest radiographs for a single subject were displayed simultaneously in chronologic order and were scored in a single viewing. Tracheal aspirate cultures were obtained daily by study personnel and were analyzed by the microbiology laboratories at the data collection sites. Potentially pathogenic species were identified by semiquantitative culture, and results were reported on a 0 to 4 scale (corresponding to no, few, moderate, or many organisms). The clinical providers were blinded to study group assignment, as were the clinical laboratory personnel who performed microbial analyses and the coinvestigator who evaluated the chest radiographs.
To test the effect of the preintubation oral application of CHX on early ET colonization (secondary aim), a sample was taken by sterile culture swab from the distal end of the interior lumen of the ETTs in a planned subset of 83 subjects (43 in the CHX group and 40 in the control group) from whom the ETT could be obtained at extubation (selected separately from the analysis sample for the primary outcome variable of CPIS). We swabbed from the distal end of the interior lumen to avoid contamination of the tube with oropharyngeal organisms during extubation. Potentially pathogenic species were identified by semiquantitative culture, and results were collapsed into two categories: colonization (moderate or many organisms) or no colonization.
For the primary aim of investigating the effect of a preintubation oral application of CHX on the development of early-onset VAP, we compared groups in a single analytical model using a mixed-effects linear model with CPIS as the response variable. For this model, group (CHX, control), day, group-by-day interaction, APACHE III score, and hospital (VCU, USF) were modeled as fixed effects and subject was modeled as a random effect. We did not conduct independent analyses of subsets of subjects for each outcome day. Subjects who had complete CPIS data on admission to the study (day 0) and subsequent complete CPIS data from day 2, 3, 4, or 5 (47 in the CHX group and 47 in the control group; 438 observations) were included in the analysis in accordance with intent-to-treat analysis principles. A logistic regression analysis was performed using the binary response variable of colonization or no colonization and dependent variables for group, length of intubation, and group-by-length-of-intubation interaction. The probability of a type 1 error (α) was set to 0.05.
Results
A description of subjects by data collection site and by study group (combined sites) is presented in Table 1. Data collected at the two hospital sites were combined for final analysis because the analytical model showed no medical center fixed effects (F = 0.3935).
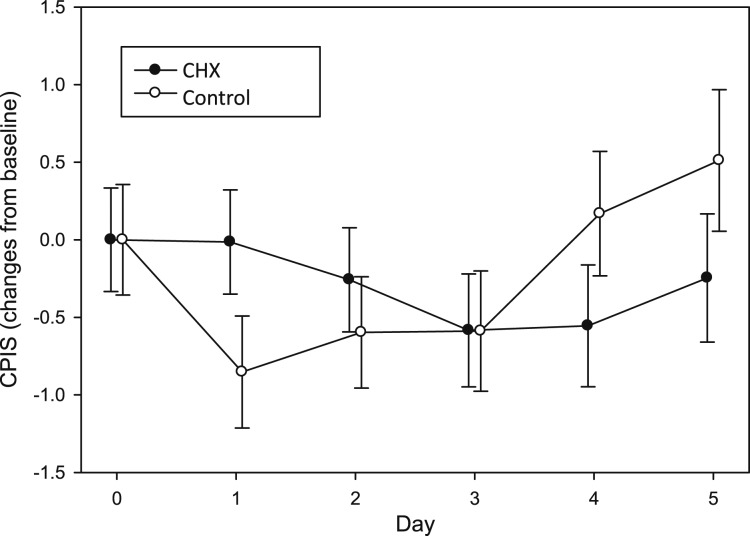
There was no statistically significant improvement in CPISs from the CHX group over the control group during the intervention period. The P values from comparing each group’s change from baseline with study days 2, 3, 4, and 5 were .4217, .9930, .1484, and .1763, respectively. Importantly, the mean CPISs from both groups remained below 6 (Table 2). Finally, the CPISs were not statistically different between the hospital sites (P = .3935), and the APACHE III score was not a statistically significant covariate (P = .1372). Figure 2 depicts changes from study admission in CPIS for each group by day, illustrating the lack of significant differences.
TABLE 2 ] .
CPISs Over Time by Group
| Day | Group | |||||
| CHX | Control | Total | ||||
| Mean ± SE | No. | Mean ± SE | No. | Mean ± SE | No. | |
| 0 | 4.53 ± 0.313 | 47 | 4.79 ± 0.339 | 47 | 4.66 ± 0.230 | 94 |
| 1 | 4.41 ± 0.276 | 46 | 3.96 ± 0.240 | 45 | 4.19 ± 0.184 | 91 |
| 2 | 4.35 ± 0.278 | 46 | 4.18 ± 0.292 | 45 | 4.26 ± 0.201 | 91 |
| 3 | 4.29 ± 0.344 | 33 | 4.18 ± 0.312 | 33 | 4.24 ± 0.231 | 67 |
| 4 | 4.46 ± 0.369 | 29 | 5.07 ± 0.441 | 29 | 4.78 ± 0.291 | 55 |
| 5 | 4.73 ± 0.417 | 22 | 5.78 ± 0.468 | 18 | 5.20 ± 0.319 | 40 |
CPIS = Clinical Pulmonary Infection Score. See Table 1 legend for expansion of other abbreviation.
Figure 2 –
Change in CPIS from baseline over time. CHX = chlorhexidine gluconate. See Figure 1 legend for expansion of other abbreviation.
The majority of ETTs in both study groups were not colonized at the time of extubation (81.4% in the CHX group and 82.5% in the control group). There was no statistically significant difference in ETT colonization between the groups (P = .8656).
Discussion
To our knowledge, the effect of preintubation application of CHX has not been reported outside of elective intubation in adult patients undergoing cardiac surgery nor has the effect of CHX on ETT colonization been reported previously. This study demonstrated that it is feasible to deliver a single dose of CHX prior to intubation in a variety of critically ill adults, including in cases of emergent or urgent intubation. However, application of a preintubation dose of CHX did not provide benefit beyond that afforded by daily oral CHX initiated following intubation.
Our results differ from those obtained in classic studies of CHX rinse administered preoperatively to adult patients undergoing elective cardiac surgery that found reductions in respiratory and other infections.9‐11 However, the classic studies were conducted prior to the widespread implementation of bundled strategies to reduce VAP, including head-of-bed elevation and daily CHX, with a resultant decrease in reported cases of VAP over time.
In the classic studies,9‐11 as well as in our previous work,3,6 the control groups received no CHX post-operatively, whereas the experimental groups continued to receive CHX postoperatively. These prior studies demonstrated the value of CHX begun prior to admission and continued throughout the hospital stay, but the contribution of the preintubation dose of CHX to the reduction in nosocomial infections was not determined. Because the ventilator bundle is now standard of care, we did not withhold daily CHX from control subjects. All subjects in our study, including control subjects, received daily oral CHX and head-of-bed elevation, and these subjects had substantially lower mean CPISs over the first 5 days of intubation than did the subjects in our previously published work,3 which was conducted prior to ventilator bundle implementation. The contribution of preintubation CHX may be inconsequential when the ventilator bundle, including daily oral CHX, is in place.
The 0.12% concentration of CHX is the only oral topical concentration with US Food and Drug Administration approval in the United States (where our study was conducted), and it is the only commercially available oral solution in the United States. Although higher concentrations of CHX have been used in some studies outside the United States, to our knowledge, there have been no head-to-head comparisons of different concentrations of CHX to assess clinical superiority or relative safety in critically ill patients. The 0.12% CHX concentration has been used in many postintubation studies conducted in the United States and other countries and is a component of commercially available postintubation oral care kits in the United States.
We were surprised by the low frequency of ETT colonization at the time of extubation in both the preintubation CHX group and the group who received CHX beginning 12 h after intubation. This could be related to the ongoing trend toward shorter durations of intubation, or to ongoing oral microbial suppression from daily CHX. Although the ETT used did not have subglottic secretion removal capability, limited aspiration of secretions is an alternative explanation for limited colonization.
The trend toward shorter length of intubation and shorter length of stay is a positive development in critical care, but it resulted in unavoidable attrition over the course of the study. A majority of subjects were extubated prior to the full 5-day intervention period.
Diagnostic certainty in the determination of VAP has been an ongoing problem for critical care research,13,14 and there is still no gold standard. We chose the CPIS to quantify the risk of development of VAP because we could obtain reproducible prospective serial data with minimal risk to subjects and without the bias introduced by a clinical provider’s suspicions or diagnosis of VAP. Recent changes in the Centers for Disease Control and Prevention surveillance definitions for ventilator-associated complications (including VAP)15 add further complexity to selecting appropriate outcome measures for future research in this area.
Conclusions
We conclude that preintubation application of CHX did not provide additional benefit in reducing the risk of development of early-onset VAP when compared with daily administration of CHX begun after intubation. Preintubation application of CHX also did not provide additional benefit in reducing ETT colonization when compared with postintubation CHX.
The results of the current project suggest that it is not essential to deliver the first dose of CHX prior to intubation to reduce the risk of early-onset VAP. Importantly, omitting the preintubation CHX application simplifies the preintubation procedures and enables providers to focus their attention on other critical preintubation activities. Initiation of CHX as early as possible following intubation, and implementation of the ventilator bundle, are important considerations in the suppression of VAP.
Acknowledgments
Author contributions: C. L. M. was the principal investigator and is the guarantor of this study; she takes full responsibility for the integrity of the submission as a whole, from inception to published article, including the data and analysis. C. L. M., M. J. G. (VCU site principal investigator following C. L. M.’s relocation to Tampa, FL), C. N. S., and R. K. E. contributed to the study conception and design; C. L. M. and P. C. contributed to the acquisition of data; C. L. M., M. J. G., C. N. S., R. K. E., D. M. (medical monitor for the Tampa General Hospital site), and R. K.-E. contributed to the analysis and interpretation of the data; C. N. S. (medical monitor for the VCU site) contributed to the scoring of all chest radiographs from both sites; D. M. and R. K.-E. contributed to the support of enrollment and liaison with medical staff at Tampa General Hospital; R. K. E. (study biostatistician) contributed to the analytical strategies, randomization of subjects at both sites, and a priori power analysis; P. C. contributed to the clinical interpretation of the data; and C. L. M. contributed to the coordination of manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank the National Institute of Nursing Research for their financial support. We are grateful to our clinical partners at Virginia Commonwealth University Medical Center and Tampa General Hospital (both Magnet Hospitals); without the enthusiastic support of nurses, physicians, research pharmacists, laboratory personnel, and administration at both sites, this work could not have been conducted. We also acknowledge the following individuals: data manager, Sally Russell, MS, MA; Data Safety and Monitoring Board members, Jessie Ketchum, PhD, Catherine Grossman, MD, and Audrey Roberson, MS, RN; Mari Miranda, BA, for assistance in manuscript preparation; and the outstanding research assistants in all phases of the project.
ABBREVIATIONS
- APACHE
Acute Physiology and Chronic Health Evaluation
- CHX
chlorhexidine gluconate
- CPIS
Clinical Pulmonary Infection Score
- ET
endotracheal
- ETT
endotracheal tube
- IHI
Institute for Healthcare Improvement
- IRB
institutional review board
- USF
University of South Florida
- VAP
ventilator-associated pneumonia
- VCU
Virginia Commonwealth University
Footnotes
FUNDING/SUPPORT: This study was supported by the National Institutes of Health National Institute of Nursing Research [Grant R01 NR07652].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.How-to guide. Prevent ventilator-associated pneumonia. Institute for Healthcare Improvement website. http://www.ihi.org/resources/Pages/Tools/HowtoGuidePreventVAP.aspx. Published 2012. Accessed October 13, 2014.
- 2.Ventilator bundle. Institute for Healthcare Improvement website. http://www.ihi.org/Engage/Memberships/MentorHospitalRegistry/Pages/VentilatorBundle.aspx. Published 2014. Accessed February 27, 2014.
- 3.Munro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing, and preventing ventilator-associated pneumonia in critically ill adults. Am J Crit Care. 2009;18(5):428-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvestri L, Weir I, Gregori D, et al. Effectiveness of oral chlorhexidine on nosocomial pneumonia, causative micro-organisms and mortality in critically ill patients: a systematic review and meta-analysis. Minerva Anestesiol. 2014;80(7):805-820. [PubMed] [Google Scholar]
- 5.Zhang TT, Tang SS, Fu LJ. The effectiveness of different concentrations of chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. J Clin Nurs. 2014;23(11-12):1461-1475. [DOI] [PubMed] [Google Scholar]
- 6.Grap MJ, Munro CL, Hamilton VA, Elswick RKJ, Jr, Sessler CN, Ward KR. Early, single chlorhexidine application reduces ventilator-associated pneumonia in trauma patients. Heart Lung. 2011;40(5):e115-e122. [DOI] [PubMed] [Google Scholar]
- 7.Shi Z, Xie H, Wang P, et al. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2013;8:CD008367. [DOI] [PubMed] [Google Scholar]
- 8.Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern Med. 2014;174(5):751-761. [DOI] [PubMed] [Google Scholar]
- 9.DeRiso AJ, II, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109(6):1556-1561. [DOI] [PubMed] [Google Scholar]
- 10.Houston S, Hougland P, Anderson JJ, LaRocco M, Kennedy V, Gentry LO. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. Am J Crit Care. 2002;11(6):567-570. [PubMed] [Google Scholar]
- 11.Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA. 2006;296(20):2460-2466. [DOI] [PubMed] [Google Scholar]
- 12.Pugin J, Auckenthaler R, Lew DP, Suter PM. Oropharyngeal decontamination decreases incidence of ventilator-associated pneumonia. A randomized, placebo-controlled, double-blind clinical trial. JAMA. 1991;265(20):2704-2710. [PubMed] [Google Scholar]
- 13.Grgurich PE, Hudcova J, Lei Y, Sarwar A, Craven DE. Diagnosis of ventilator-associated pneumonia: controversies and working toward a gold standard. Curr Opin Infect Dis. 2013;26(2):140-150. [DOI] [PubMed] [Google Scholar]
- 14.Klompas M. Interobserver variability in ventilator-associated pneumonia surveillance. Am J Infect Control. 2010;38(3):237-239. [DOI] [PubMed] [Google Scholar]
- 15.Surveillance for ventilator-associated events. Centers for Disease Control and Prevention website. http://www.cdc.gov/nhsn/acute-care-hospital/vae/. Accessed February 27, 2014.