Abstract
BACKGROUND:
Surgical and medical treatments for emphysema may affect both quality and quantity of life. The purpose of this article is to report outcomes from the National Emphysema Treatment Trial (NETT) using an index that combines quality and quantity of life.
METHODS:
This was a prospective randomized clinical trial. Following pulmonary rehabilitation, 1,218 patients with severe emphysema were randomly assigned to maximal medical therapy or to lung volume reduction surgery (LVRS). A generic quality-of-life measure, known as the Quality of Well-being Scale (QWB), was administered at baseline and again at 6, 12, 24, 36, 48, 60, and 72 months following treatment assignment.
RESULTS:
At baseline, QWB scores were comparable for the Medical and LVRS groups. For both groups, scores significantly improved following the rehabilitation program. The QWB scores before death for patients in the LVRS group improved up to the year 2 visit, whereas scores for the Medical group dropped significantly following the baseline visit. Imputing zeros (0) for death, QWB scores decreased significantly for both groups. With or without scoring death as 0, the LVRS group achieved better outcomes, and the significant differences were maintained until the sixth year. Over 6 years of follow-up, LVRS produced an average of 0.30 quality-adjusted life years (QALYs), or the equivalent of about 3.6 months of well life.
CONCLUSIONS:
Compared with maximal medical therapy alone, patients undergoing maximal medical therapy plus LVRS experienced improved health-related quality of life and gained more QALYs.
TRIAL REGISTRY:
ClinicalTrials.gov; No.: NCT00000606; URL: www.clinicaltrials.gov
As a result of chronic airflow obstruction, patients with advanced emphysema experience significant limitations in daily activities and reduced quality of life. Evidence supports the value of medical management and pulmonary rehabilitation for these patients.1,2 Despite important advances in medical management, the promise of medical therapy is limited. Lung volume reduction surgery (LVRS) may offer the potential to extend the life expectancy and enhance quality of life for selected patients who have completed pulmonary rehabilitation.3
The National Emphysema Treatment Trial (NETT) compared medical and surgical approaches with the management of severe emphysema using a wide range of outcome assessments. Although mortality outcomes have been reported previously,4 less is known about the effects of LVRS treatment on quality of life because most studies have significant loss to follow-up. Studying survivors only is subject to several biases, including bias against treatments that keep the sickest patients alive for inclusion in assessment.
A variety of other articles reporting outcomes from the NETT have been published. However, the trial suffered from significant loss to follow-up. For example, quality-of-life (QoL) data were available for only about 22% of the planned participants at the time of the 6-year follow-up. Loss to follow-up causes significant problems because those who are unable to complete quality-of-life questionnaires are not a random subgroup of the population. Often, they are the patients who either died or were too sick to complete the questionnaire. The purpose of this article is to report outcomes from the NETT using an index that combines life duration with quality of life. Because there was selective loss to follow-up, we used imputation methods to estimate quality-of-life scores for those too sick to attend follow-up evaluations.
Materials and Methods
Subjects
The subjects were 746 male and 472 female volunteers with an average age of 64 years at baseline. Participants were studied at one of 17 approved NETT sites. The inclusion criteria were (1) radiographic evidence of bilateral emphysema, (2) severe airflow obstruction and hyperinflation, and (3) completion of pulmonary rehabilitation. Exclusion criteria were (1) a broad range of medical conditions that place patients at risk for perioperative morbidity and/or mortality, (2) emphysema believed to be unsuitable for LVRS, and (3) medical conditions or other circumstances that make it likely that the patient would be unable to complete the trial. The exclusion criteria relating to cardiologic issues were based upon the work of Goldman and colleagues.5 All participants provided written informed consent to participate, and the study protocol was approved by the institutional review boards of each of the 17 participating centers. The Coordinating Center at the Johns Hopkins Bloomberg School of Public Health provided oversight over all participating institutions and did not accept data from any center without an active approval (Johns Hopkins Institutional Review Board, approved protocol number H34.02.01.29.A1). A more detailed description of the NETT methodology is available elsewhere.6
Following baseline evaluation, all participants completed comprehensive pulmonary rehabilitation. A second assessment was completed after rehabilitation and prior to randomization.
Treatment Groups
Following extensive screening and completion of pulmonary rehabilitation for eligible subjects, patients were randomly assigned to LVRS or to continued maximal medical therapy. LVRS was achieved using a median sternotomy approach (406 patients) or by means of video-assisted surgery (174 patients). Among patients randomized to LVRS, 21 declined surgical intervention and seven subsequently were deemed unsuitable for surgical intervention by the surgeon or anesthesiologist at some point after randomization. Sixty-nine of the 580 patients who underwent LVRS were in a subgroup identified by the Data and Safety Monitoring Board as being at high risk of mortality after LVRS with little chance of functional benefit; enrollment of this patient subgroup in the NETT was terminated in May 2001. Outcomes for the high-risk subgroup were reported in 2001.6 Maximal medical therapy included state-of-the-art medical management of advanced emphysema.
QoL Data Collection
Data collection for NETT subjects began in October 1997. NETT randomizations began in January 1998 and ended in July 2002. The QoL assessments were collected at all visits. The final QoL forms were collected by mail between December 31, 2003, and June 30, 2004. Based on date of randomization, the follow-up time for QoL data collection of NETT patients varied from 2.0 to 6.5 years. As a result, not all QoL measures were assessed for patients in the later phases of the trial (due to right censoring). NETT created time windows for follow-up visits and mapped the forms into them. In this article, we include analyses of the QoL measures from pre-rehabilitation, post-rehabilitation (or baseline before randomization), and follow-up visits 0.5, 1, 2, 3, 4, 5, and 6 years after randomization.
QoL Measurement: Self-Administered Quality of Well-being Scale
This article concentrates on outcomes measured by the Self-Administered Quality of Well-being Scale (QWB). This 73-item instrument combines preference-weighted values for symptoms and functioning. Among the items, 11 ask about chronic symptoms, seven focus on use of health aides, 25 concern acute physical symptoms, and 14 ask about mental health symptoms. The remaining 16 items concentrate on mobility, physical activity, and social activity. Combinations of symptoms and functioning are weighted on a continuum of wellness by independent observers. The preference weights were obtained from ratings by 856 people from the general population. These judges rated the desirability of health conditions to place each on the continuum between death (0) and optimum health (1.0). Symptoms are assessed by questions that ask about the presence or absence of different symptoms complexes.
One of the advantages of the QWB is the comprehensiveness of the 57-item symptom/problem complex list. Patients are asked about symptoms relevant to all physiologic systems. The detailed symptom inquiries allow the capture of unexpected as well as expected benefits and side effects. Functioning is assessed by a series of questions designed to record functional limitations over the previous 3 days, within three separate domains (mobility, physical activity, and social activity). The preference-weighted four domain scores are combined into a total score that provides a numerical point-in-time expression of well-being that ranges from zero (0) for death to one (1.0) for asymptomatic optimum functioning.
The QWB has been used in numerous clinical trials and studies to evaluate medical and surgical therapies in conditions such as diabetes,7 COPD,8 HIV,9,10 cystic fibrosis,11,12 diabetes mellitus,7 atrial fibrillation,13 lung transplantation,14 arthritis,15,16 end-stage renal disease,17 cancer,18 depression,19,20 and several other conditions.21 Further, the method has been used for health resource allocation modeling and has served as the basis for an innovative experiment on rationing of health care by the state of Oregon.22,23 Studies have also demonstrated that the QWB is responsive to clinical change derived from surgery14 or medical conditions such as rheumatoid arthritis,24 AIDS,10 and cystic fibrosis.11 The QWB was developed more recently. It has been shown to be highly correlated with the interviewer-administered version.25 The full questionnaire is available at: https://hoap.ucsd.edu/qwb-info/EnglishQWB-SA_2.pdf.
Missing Data:
The NETT follow-up evaluations suffered from substantial missing data for many of the measures, including the QoL assessments. Among the concerns was that missing data were likely to be systematically related to outcomes including death and poor health.
There were three major reasons for missing QoL data in NETT: (1) later entry, (2) censoring due to death, and (3) failure to complete the questionnaire. Our evaluations addressed these problems as follows: (1) Later entry: NETT enrolled patients for 4.5 years, (January 1998 to July 2002) and the QoL data collection ended 2 years later on June 30, 2004. Therefore, subjects who entered the study later in the enrollment period could not complete all 6 years of QoL assessments. This type of missing data due to later randomization does not complicate our analysis because it affects both groups equally. (2) Censoring because of death: Some participants were censored by death, and these events are nonrandom and, possibly, a function of treatment. Death is an outcome that can be scored as 0 in the QWB system. For deceased participants, we included 0s for the QWB scores after the death date up to the maximum follow-up visit prior to June 30, 2004. (3) Failure to complete questionnaire: A missed or incomplete visit form included eight possible reasons: (a) patient was ill, (b) patient was temporarily away from area, (c) patient refused to return, (d) patient permanently moved from the area, (e) unable to contact patient, (f) patient’s physical condition precluded attending visit, (g) patient’s mental condition precluded attending visit, and (h) other (specify). When part of the visit was completed, the form(s) that were not completed were checked and the reason for the missed form(s) was assessed by a four-category question: (a) patient was ill, (b) patient refused procedure, (c) procedure forgotten, and (d) other (specify). In both situations, steps or attempts were made to avoid missing the visit or to complete form(s). Since this missing mechanism might be related to our outcome of interest, to be conservative, we imputed these missed questionnaire data by randomly selecting from the lower quartile of the observed records by treatment group at each follow-up visit.
Mortality Ascertainment:
The NETT coordinating center engaged in continuing surveillance for mortality. Deaths were confirmed, and a date of death was acquired.
Statistical Methods
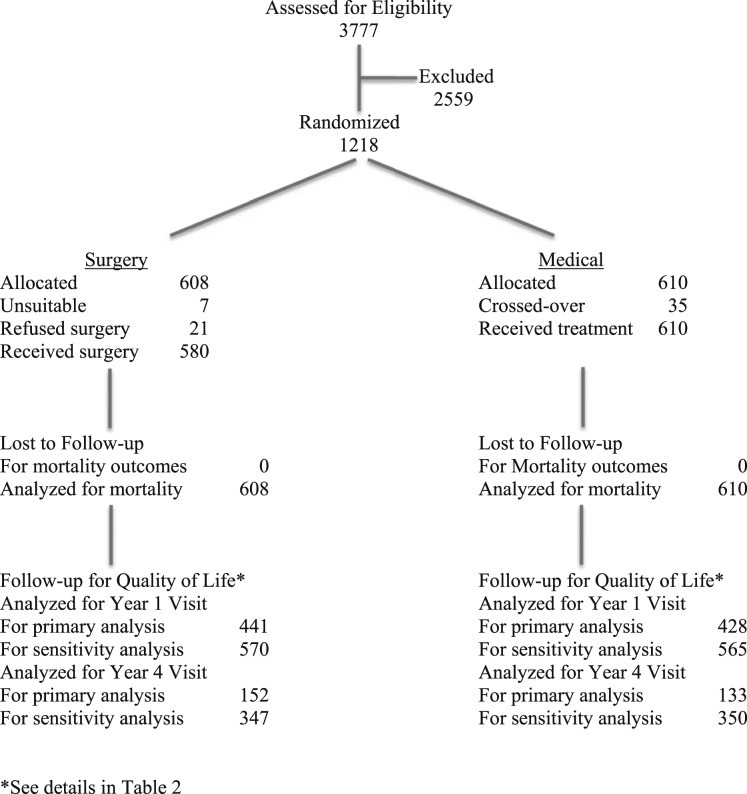
A CONSORT diagram for the study flow is shown in Figure 1. All analyses were performed using R software (www.r-project.org). Group assignment was based on intention to treat. We estimated the numbers of the planned assessments based on randomization dates. The frequencies of the observed, censored by death, and missed questionnaire records were tabulated and compared with the planned assessments by visit and treatment group. The distributions of the QWB scores were displayed via box plots.
Figure 1 –
Summary of participant allocation using standardized CONSORT diagram.
Our primary analysis was based on available data without any imputation (Table 1). We analyzed these longitudinal data using a random intercept model with treatment, visit, and the interaction term of treatment and visit as fixed effects. The nominal visit was treated as a categorical predictor in the model using missing at random assumption. The average QWB score, change from baseline score, treatment effect, corresponding 95% CIs, and P values at each visit were estimated and tabulated for both groups. The unadjusted group means and standard errors were also included in Table 2 for comparison. We displayed the estimated response profiles for both groups in a figure by plotting the estimated average QWB scores and the 95% CIs. In addition to the primary analysis, we imputed the censored by death and missing questionnaires records via the previously described method and performed a sensitivity analysis with and without imputed data (Table 3).
TABLE 1 ] .
Frequencies and Percentages of Background and Longitudinal Characteristics at Baseline
| Characteristics | Therapy (n = 610) | Surgery (n = 608) | Total (N = 1,218) |
| Ethnicity | |||
| White | 575 (94.3) | 581 (95.6) | 1,156 (94.9) |
| Black | 23 (3.8) | 19 (3.1) | 42 (3.5) |
| Other | 12 (2.0) | 8 (1.3) | 20 (1.6) |
| Sex | |||
| Male | 391 (64.1) | 355 (58.4) | 746 (61.3) |
| Female | 219 (35.9) | 253 (41.6) | 472 (38.8) |
| Distribution of emphysema on CT scan | |||
| Predominantly non-upper lobe | 204 (33.5) | 223 (36.7) | 427 (35.1) |
| Predominantly upper lobe | 405 (66.5) | 385 (63.3) | 790 (64.9) |
| Homogeneous | 274 (44.9) | 278 (45.7) | 552 (45.3) |
| Heterogeneous | 336 (55.1) | 330 (54.3) | 666 (54.7) |
| Variables measured in scaled units, mean (SD) | |||
| Age at randomization, y | 67.22 (5.89) | 67.04 (6.342) | 67.13 (6.12) |
| Perfusion ratio | 0.28 (0.22) | 0.298 (0.211) | 0.29 (0.22) |
| Maximal workload, W | 39.23 (22.10) | 38.533 (21.18) | 38.88 (21.64) |
| Distance walked in 6 min, fta | 1,196.87 (311.20) | 1,197.75 (312.52) | 1,197.31 (311.73) |
| FEV1 after bronchodilator use, % predicted | 26.60 (7.05) | 26.77 (7.33) | 26.68 (7.19) |
| Total lung capacity after bronchodilator use, % predicted | 128.47 (14.96) | 128.07 (15.23) | 128.27 (15.09) |
| Residual volume after bronchodilator use, % predicted | 223.65 (49.353) | 221.01 (49.99) | 222.33 (49.67) |
| Carbon monoxide diffusion capacity, % predicted | 28.39 (9.76) | 28.22 (9.57) | 28.31 (9.66) |
| Pao2, mm Hg | 64.21 (10.15) | 64.43 (10.44) | 64.32 (10.29) |
| Paco2, mm Hg | 43.07 (5.78) | 43.31 (5.86) | 43.19 (5.82) |
| Total score on St. George’s Respiratory Questionnaire | 53.65 (12.67) | 52.35 (12.66) | 53.00 (12.68) |
| Average daily QWB score | 0.56 (0.11) | 0.57 (0.11) | 0.57 (0.11) |
| Total UCSD Shortness of Breath score | 63.53 (18.55) | 61.80 (18.10) | 62.67 (18.34) |
QWB = Quality of Well-being Scale; UCSD = University of California, San Diego.
To convert values from feet to meters, divide by 3.28.
TABLE 2 ] .
Unadjusted and Linear Mixed Model Estimated Mean QWB Average Scores (Available Case Cohort)
| Visit Year | Treatment Group | Unadjusted Mean (SE) | Linear Mixed Model Estimates | |||||
| Raw Score | Treatment Effect | Change From Baseline | ||||||
| Mean (SE) | 95% CI | Mean (SE) | P Value | Mean (SE) | P Value | |||
| −0.25 | LVRS | 0.546 (0.005) | 0.545 (0.005) | 0.53, 0.55 | 0.02 (0.007) | .023 | −0.03 (0.005) | < .001 |
| Medical | 0.528 (0.0048) | 0.529 (0.0052) | 0.52, 0.54 | … | … | −0.04 (0.005) | < .001 | |
| Baseline | LVRS | 0.578 (0.0047) | 0.58 (0.005) | 0.57, 0.59 | 0.01 (0.007) | .065 | … | … |
| Medical | 0.564 (0.0046) | 0.56 (0.005) | 0.55, 0.57 | … | … | … | … | |
| 0.5 | LVRS | 0.587 (0.006) | 0.59 (0.005) | 0.58, 0.59 | 0.05 (0.008) | < .001 | 0.01 (0.006) | .079 |
| Medical | 0.54 (0.005) | 0.53 (0.005) | 0.52, 0.54 | … | … | −0.03 (0.006) | < .001 | |
| 1 | LVRS | 0.59 (0.006) | 0.58 (0.005) | 0.57, 0.59 | 0.06 (0.008) | < .001 | 0.005 (0.006) | .353 |
| Medical | 0.53 (0.006) | 0.52 (0.005) | 0.51, 0.53 | … | … | −0.04 (0.06) | < .001 | |
| 2 | LVRS | 0.58 (0.0066) | 0.57(0.005) | 0.56, 0.58 | 0.06 (0.008) | < .001 | −0.01 (0.01) | .15 |
| Medical | 0.52 (0.006) | 0.51 (0.006) | 0.50, 0.52 | … | … | −0.05 (0.006) | < .001 | |
| 3 | LVRS | 0.56 (0.008) | 0.55 (0.006) | 0.53, 0.56 | 0.04 (0.01) | < .001 | −0.03 (0.01) | < .001 |
| Medical | 0.52 (0.007) | 0.50 (0.0068) | 0.49, 0.52 | … | … | −0.06 (0.01) | < .001 | |
| 4 | LVRS | 0.54 (0.013) | 0.52 (0.008) | 0.51, 0.54 | 0.03 (0.012) | .02 | −0.05(0.01) | < .001 |
| Medical | 0.51 (0.012) | 0.50 (0.009) | 0.48, 0.51 | … | … | −0.069 (0.01) | < .001 | |
| 5 | LVRS | 0.56 (0.013) | 0.54 (0.009) | 0.52, 0.55 | 0.06 (0.014) | < .001 | −0.04 (0.01) | < .001 |
| Medical | 0.50 (0.016) | 0.47 (0.011) | 0.45, 0.50 | … | … | −0.09 (0.01) | < .001 | |
| 6 | LVRS | 0.52 (0.029) | 0.48 (0.019) | 0.44, 0.51 | 0.02 (0.020) | .564 | −0.10 (0.02) | < .001 |
| Medical | 0.54 (0.031) | 0.46 (0.019) | 0.42, 0.50 | … | … | −0.104 (0.02) | < .001 | |
LVRS = lung volume reduction surgery. See Table 1 legend for expansion of other abbreviation.
TABLE 3 ] .
Unadjusted and Linear Mixed Model Estimated Mean QWB Average Scores (With Data Imputations for Missed Visits and Deaths)
| Visit Year | Treatment Group | Unadjusted Mean (SE) | Linear Mixed Model Estimates | |||||
| Raw Score | Treatment Effect | Change From Baseline | ||||||
| Mean (SE) | 95% CI | Mean (SE) | P Value | Mean (SE) | P Value | |||
| −0.25 | LVRS | 0.546 (0.0051) | 0.546 (0.0084) | 0.5292, 0.5623 | 0.02 (0.012) | .14 | −0.032 (0.009) | < .001 |
| Medical | 0.52 (0.004) | 0.528 (0.0084) | 0.5115, 0.5446 | … | … | −0.036 (0.009) | < .001 | |
| Baseline | LVRS | 0.57 (0.004) | 0.578 (0.0084) | 0.5612, 0.5943 | 0.01 (0.012) | .26 | … | … |
| Medical | 0.56 (0.004) | 0.56 (0.008) | 0.548, 0.581 | … | … | … | … | |
| 0.5 | LVRS | 0.51 (0.009) | 0.55 (0.008) | 0.4985, 0.5316 | 0.03 (0.012) | .03 | −0.06 (0.009) | < .001 |
| Medical | 0.489 (0.006) | 0.48 (0.008) | 0.4726, 0.5057 | … | … | −0.07 (0.009) | < .001 | |
| 1 | LVRS | 0.54 (0.008) | 0.51 (0.009) | 0.499, 0.534 | 0.05 (0.013) | < .001 | −0.06 (0.009) | < .001 |
| Medical | 0.48 (0.007) | 0.47 (0.008) | 0.453, 0.488 | … | … | −0.09 (0.009) | < .001 | |
| 2 | LVRS | 0.44 (0.011) | 0.44 (0.008) | 0.427, 0.461 | 0.05 (0.012) | 0 | −0.13 (0.009) | < .001 |
| Medical | 0.39 (0.009) | 0.39 (0.008) | 0.374, 0.408 | … | … | −0.17 (0.009) | < .001 | |
| 3 | LVRS | 0.36 (0.012) | 0.37 (0.009) | 0.353, 0.388 | 0.050 (0.013) | 0 | −0.20 (0.009) | < .001 |
| Medical | 0.32 (0.011) | 0.32 (0.009) | 0.304, 0.339 | … | … | −0.24 (0.009) | < .001 | |
| 4 | LVRS | 0.24 (0.015) | 0.27 (0.010) | 0.256, 0.296 | 0.05 (0.014) | < .001 | −0.30 (0.010) | < .001 |
| Medical | 0.20 (0.014) | 0.22 (0.010) | 0.205, 0.245 | … | … | −0.34 (0.010) | < .001 | |
| 5 | LVRS | 0.26 (0.018) | 0.27 (0.011) | 0.255, 0.301 | 0.09 (0.016) | < .001 | −0.30 (0.011) | < .001 |
| Medical | 0.17 (0.016) | 0.19 (0.011) | 0.168, 0.214 | … | … | −0.37 (0.012) | < .001 | |
| 6 | LVRS | 0.15 (0.026) | 0.18 (0.018) | 0.151, 0.222 | 0.02 (0.025) | .31 | −0.39 (0.018) | < .001 |
| Medical | 0.14 (0.026) | 0.16 (0.017) | 0.126, 0.196 | … | … | −0.40 (0.018) | < .001 | |
Results
At the point of randomization, those assigned to medical therapy or surgery were comparable in terms of ethnicity, sex, and distribution of emphysema on CT scan. Further, the groups were equivalent on a variety of pulmonary function, exercise tolerance, blood gas, and QoL measures (Table 1).
Table 4 summarizes the numbers of observed and missing records at each visit by treatment group. The estimated planned assessments are listed in the last column. One hundred fourteen out of 608 patients in the LVRS group and 122 out of 610 in the Medical group had entered the study early enough to have a year 6 follow-up visit. All other patients enrolled later did not have the opportunity to complete the year 6 follow-up visit when QoL data collection ended on June 30, 2004. In reality, not all measures would be included in the nominal time windows. Starting with the year 4 visit, the censored by death records outnumbered the observed records (eg, 39.8% vs 32.9% for the LVRS and 44.4% vs 28.5% for the Medical group. There were more censored-by-death records in the LVRS group than in the Medical group up to the year 3 visit. Also, there were more missing questionnaires in the Medical group, 0% to 13.8% vs 0% to 6.2% for LVRS. Due to late entry, the censored-by-death numbers are not cumulative after the year 4 visit.
TABLE 4 ] .
Number of Observed and Missing Data Within Each Treatment Group at Each Time Point
| Visit Year | Treatment Group | Observed | Censored by Death | Missed Visits | Total | Planned |
| −0.25 | LVRS | 607 (99.8) | 0 (0) | 0 (0) | 607 (99.8) | 608 |
| Medical | 608 (99.7) | 0 (0) | 0 (0) | 608 (99.7) | 610 | |
| Baseline | LVRS | 608 (100) | 0 (0) | 0 (0) | 608 (100) | 608 |
| Medical | 610 (100) | 0 (0) | 0 (0) | 610 (100) | 610 | |
| 0.5 | LVRS | 507 (83.4) | 65 (10.7) | 34 (5.6) | 606 (99.7) | 608 |
| Medical | 496 (81.3) | 29 (4.8) | 81 (13.3) | 606 (99.3) | 610 | |
| 1 | LVRS | 441 (72.5) | 93 (15.3) | 36 (5.9) | 570 (93.8) | 608 |
| Medical | 428 (70.2) | 53 (8.7) | 84 (13.8) | 565 (92.6) | 610 | |
| 2 | LVRS | 426 (70.1) | 125 (20.6) | 30 (4.9) | 581 (95.6) | 608 |
| Medical | 373 (61.1) | 120 (19.7) | 72 (11.8) | 565 (92.6) | 610 | |
| 3 | LVRS | 308 (54.5) | 164 (29) | 35 (6.2) | 507 (89.7) | 565 |
| Medical | 271 (47.7) | 181 (31.9) | 38 (6.7) | 490 (86.3) | 568 | |
| 4 | LVRS | 152 (32.9) | 184 (39.8) | 11 (2.4) | 347 (75.1) | 462 |
| Medical | 133 (28.5) | 207 (44.4) | 10 (2.1) | 350 (75.1) | 466 | |
| 5 | LVRS | 121 (39.7) | 136 (44.6) | 1 (0.3) | 258 (84.6) | 305 |
| Medical | 82 (26.9) | 159 (52.1) | 3 (1) | 244 (80) | 305 | |
| 6 | LVRS | 27 (23.7) | 63 (55.3) | 0 (0) | 90 (78.9) | 114 |
| Medical | 26 (21.3) | 69 (56.6) | 0 (0) | 95 (77.9) | 122 |
Data are presented as No. (%) or No. See Table 2 legend for expansion of abbreviation.
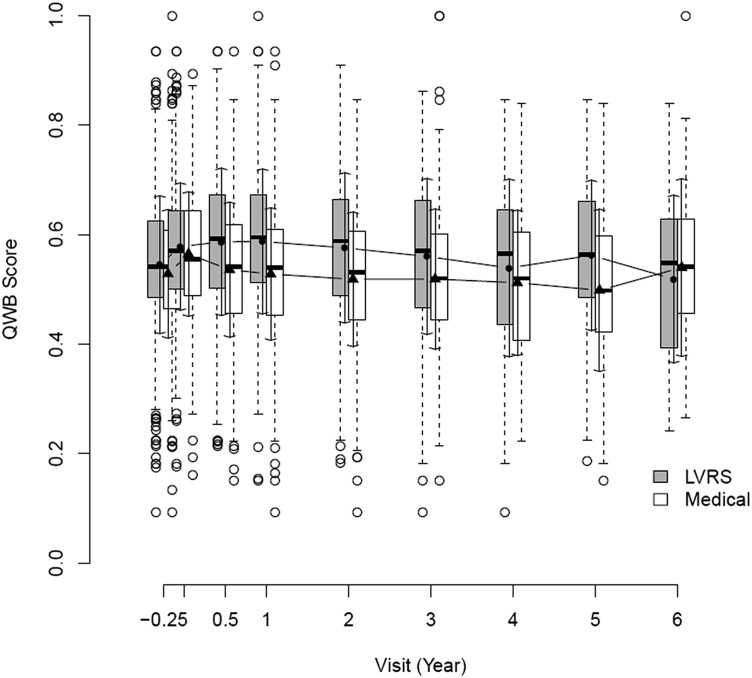
The distributions of the QWB scores for the observed records are depicted in Figure 2. The shaded boxes are for the LVRS group, and the open ones for the medical group. The filled circles are the group means for LVRS group and the triangles for the Medical group. The SDs are indicated by arrows. Although most of the scores fall between 0.4 and 0.7, there were some extreme values. The patients in the LVRS group had higher QWB scores before death than the Medical group.
Figure 2 –
Box and whiskers display for QWB (self-administered version) outcomes at each follow-up. The center of each box is the group median. The lower boundary of the box is the 25th percentile, and the upper boundary of the box is the 75th percentile. Extensions to each box show the lowest and highest values, excluding outlier cases. Cases outside the expected range (outliers) are shown as circles. Outliers are defined as beyond 1.5 times the interquartile range. LVRS = lung volume reduction surgery; QWB = Quality of Well-being Scale.
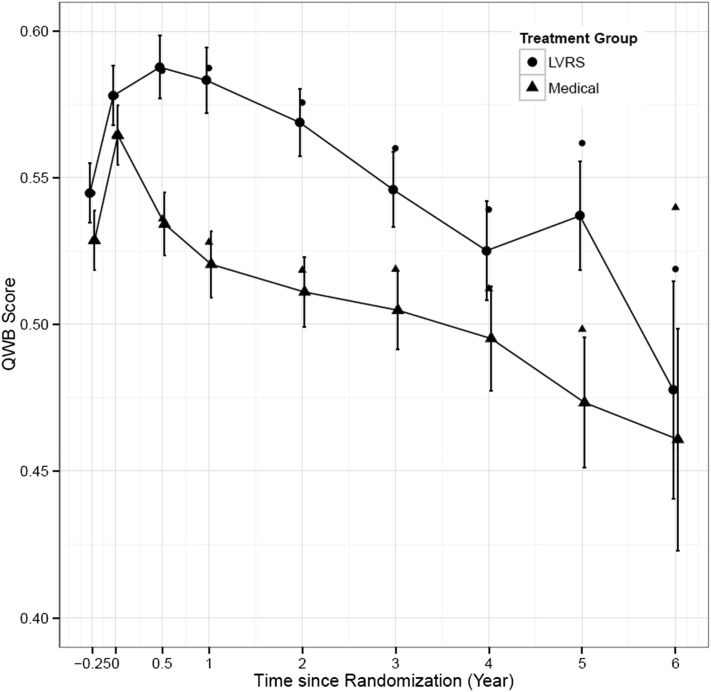
Linear mixed model-based analysis results derived from the available observed data are summarized in Table 2 and displayed in Figure 3. The unadjusted group means and SEs are also included. Both descriptive and model-based results indicate that patients in the LVRS group had higher QoL scores before death. The two groups differed at the pre-rehabilitation visit, with a mean score difference of 0.0170 (P = .023). Following the rehabilitation program, there were significant gains for both groups, about 6% increase for Surgery (0.032/0.546) and about 7% for the Medical group (0.036/0.528). Although LVRS group patients on average had a 0.013 higher QWB score than the Medical group, the difference was not statistically significant (P = .065) prior to randomization. After treatment assignment, scores for the LVRS group improved up to the year 2 visit, whereas scores for the Medical group continued to drop. The patients in the LVRS group consistently achieved better QoL outcomes before death, and the differences between the two groups were significant through the fifth year of evaluation but were nonsignificant for the sixth year.
Figure 3 –
Average QWB observed scores and 95% CIs by assessment period. QWB scores for surgery and medical treatments did not differ prior to randomization. Both groups improved following rehabilitation, but differences between groups were nonsignificant. Following surgery, the groups diverged, and differences between them remained statistically significant for all follow-ups with the exception of years 4 and 6. See Figure 2 legend for expansion of abbreviations.
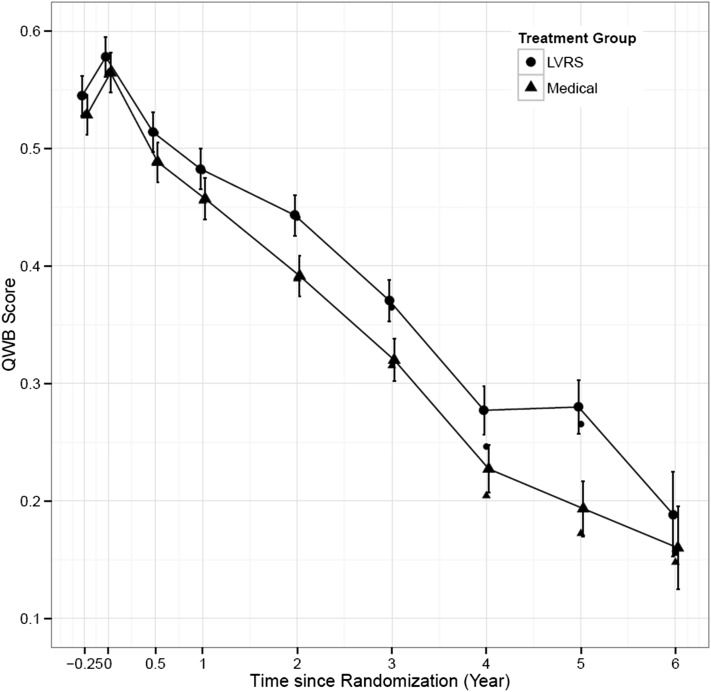
Sensitivity analysis results that include missing data imputation are summarized in Table 3 and Figure 4. Using these adjusted values, QWB scores were always higher in the surgery group than the medical group, as shown in Table 3, column 6 (treatment effect mean). These differences included the very first assessment that was taken prior to rehabilitation, although the difference 0.018 was not statistically significant (P = .138). QWB scores for both groups significantly increased after the 3-month rehabilitation program (see Table 3 change from baseline mean [SE], columns 8 and 9, rows 1 and 2). The baseline difference following rehabilitation was not statistically significant (P = .266, column 8, row 3). After treatment assignment, both groups declined significantly, but the difference from baseline for the surgery group tended to be smaller than for the medical group (Table 2, column 8). Using the imputed modeled data, the same pattern appeared, and differences between the surgery and medical arms were statistically significant for all follow-up years (P < .001).
Figure 4 –
Average QWB scores based on imputation and 95% CIs by assessment period. QWB scores for surgery and medical treatments did not differ prior to randomization. Both groups improved following rehabilitation, but differences between groups were nonsignificant. Following surgery, the groups diverged, and differences between them remained statistically significant for all follow-up periods. See Figure 2 legend for expansion of abbreviations.
To estimate quality-adjusted life years (QALYs), survival was weighted by QWB scores. For patients who were deceased, a QWB score of 0 was imputed. For surviving participants, the QWB score was used to adjust survival time. For example, for each 1 year a participant with a QWB score of 0.60 survived, they would be credited with about 7.2 QALYs (0.6 × 12 months = 7.2 months). With this adjustment for quality of life, the average quality-adjusted life expectancy over 6 years for the LVRS group was 2.07 years, and 1.77 years for the Medical group. Thus, LVRS produced an average of 0.30 QALYs or about 3.6 quality-adjusted months of life. In other words, being in the surgery group was associated with living an estimated 3.6 months of well life longer than being in the medical group. Both the primary and the sensitivity analyses indicated that patients in the surgery group achieved higher QoL outcomes in comparison with those assigned to the medical group.
Discussion
COPD is an important public health problem because it affects both the length and quality of life. The NETT was particularly important in establishing the effects of LVRS surgery on outcomes for this group. The NETT offered a unique opportunity to evaluate quality of life in a prospective randomized controlled trial. Quality of life is a meaningful outcome for patients with emphysema. Evidence from the NETT suggests that quality of life is correlated with other outcomes, including 6-min walk distance and health-care use.26 In addition, the QWB includes mental health symptoms and has been shown to be responsive to changes in psychologic well-being.27 The QoL measures were responsive to the effects of pulmonary rehabilitation and to the surgical intervention.
Patients randomly assigned to LVRS achieved better outcomes on the QWB, with and without imputation for missing data. Combining QWB with mortality outcomes to express the benefits of LVRS in terms of QALYs allowed the use of a metric that could be used for cost-effectiveness analysis.28 Other evidence from the NETT suggests that quality of life and measures of function are independent predictors of exacerbations of emphysema.29 The QWB was also responsive to improvement following the rehabilitation phase of the NETT. Consistent with our findings, other analyses have suggested that those who had not previously attended pulmonary rehabilitation improved more on these measures than patients with previous experience in rehabilitation.30 The analyses offered in this report update previous reports by including many of the participants who had been excluded from previous reports because of loss to follow-up. We also update previous reports by including more deaths in the analysis.
Outcomes for life expectancy and for some QoL measures have been reported in previous publications.4 However, combining morbidity and mortality into a single index is challenging and requires a specialized index such as the QWB. Previous reports have also been challenged because the NETT had selective loss to follow-up. A significant number of participants had missing data and the reasons for missing data were nonrandom. One of the most important reasons for missing a follow-up is that the participant was too sick to come to the clinic for testing. Using the missed visit questionnaire from the NETT, we were able to confirm that the missed visit was for health reasons in many cases. This information allowed a reasonable imputation for level of wellness. Further, participants who missed a follow-up because of death were scored 0 on the QWB scale. Using both of these estimation methods allowed us to recover a significant amount of information on individuals who were not assessed at their follow-up visits.
In concert with previous NETT articles, this work suggests that LVRS produces a significant benefit independently for quality of life and mortality. Our results suggest that, independent of the imputation for death, there is a significant advantage of LVRS for QoL outcomes. Adding quality and quantity of life together allows a better overall estimate of treatment benefit. Our results are different from previous reports from the NETT trial because we included more years of follow-up and because we were able to make greater use of imputation for missing data. Using the imputation increased the power of the analysis and suggested that the treatment effect was larger. But, the imputation did not change the basic conclusion that QoL outcomes are better for surgical patients. Ramsey and colleagues31 reported that LVRS was associated with 0.28 additional QALYs in comparison with our estimate of 0.30. Thus, the imputations we applied were associated with an estimate slightly more favorable to surgery. Although we use longer follow-up and include more participants in the analysis, the analysis suggests that the Ramsey estimates were quite reasonable.
There are several important limitations with our analyses. Patients willing to volunteer for a surgical trial are not likely to be representative of all people who suffer from emphysema. Further, the study was conducted in highly specialized centers, and there is some evidence that the surgical treatment was more effective in some centers than in others. As the study progressed, the number of participating patients systematically declined, and we have no assurance that the loss to follow-up was random. We also recognize that quality of life is a broad concept with many components. Although the QWB includes psychologic symptoms, the core items emphasize physical symptoms and limitations of activities. As a result, the QoL focus may be somewhat narrower than found in other studies of patients with emphysema. Another potential concern is that QWB scores for the surgery group were slightly higher at the first study visit, prior to rehabilitation (by about 0.036 units on the 0.0 to 1.0 scale). Following rehabilitation and prior to randomization, the difference in QWB scores between the two groups declined to a nonsignificant difference (0.014). Even though the NETT was a randomized trial, it is possible that an unmeasured variable caused the baseline difference and could have influenced later QWB differences observed during the years of follow-up. In summary, our analyses of data from the NETT provide additional evidence that LVRS enhances longevity and quality of life for patients with emphysema.
Acknowledgments
Author contributions: R. M. K. is guarantor of the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis. R. M. K. contributed as a member of the NETT investigators group, co-chaired the cost-effectiveness committee for the study, and was involved in the writing, analysis, and all revisions of this manuscript; Q. S. contributed to the data analysis and drafting and revision of the manuscript; and A. L. R. contributed as a member of the NETT steering committee, was the principal investigator for the San Diego site, and participated in the development and revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors participated in the design, execution, and reporting of the study.
ABBREVIATIONS
- LVRS
lung volume reduction surgery
- NETT
National Emphysema Treatment Trial
- QALY
quality-adjusted life year
- QoL
quality of life
- QWB
Quality of Well-being Scale
Footnotes
Dr Kaplan is currently at the Agency for Healthcare Research and Quality (Rockville, MD).
FUNDING/SUPPORT: This study was supported by contracts with the National Heart, Lung, and Blood Institute [Grants N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01-HR76119], the Center for Medicare and Medicaid Services (formerly the Health Care Financing Administration), and the Agency for Healthcare Research and Quality.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Edelman NH, Kaplan RM, Buist AS, et al. Chronic obstructive pulmonary disease. Task Force on Research and Education for the Prevention and Control of Respiratory Diseases. Chest. 1992;102(3_supplement):243S-256S. [DOI] [PubMed] [Google Scholar]
- 2.Johannessen A, Nilsen RM, Storebø M, Gulsvik A, Eagan T, Bakke P. Comparison of 2011 and 2007 Global Initiative for Chronic Obstructive Lung Disease guidelines for predicting mortality and hospitalization. Am J Respir Crit Care Med. 2013;188(1):51-59. [DOI] [PubMed] [Google Scholar]
- 3.Fishman A, Martinez F, Naunheim K, et al. ; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059-2073. [DOI] [PubMed] [Google Scholar]
- 4.Criner GJ, Cordova F, Sternberg AL, Martinez FJ. The National Emphysema Treatment Trial (NETT) part II: lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med. 2011;184(8):881-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297(16):845-850. [DOI] [PubMed] [Google Scholar]
- 6.The National Emphysema Treatment Trial Research Group. Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest. 1999;116(6):1750-1761. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan RM, Hartwell SL, Wilson DK, Wallace JP. Effects of diet and exercise interventions on control and quality of life in non-insulin-dependent diabetes mellitus. J Gen Intern Med. 1987;2(4):220-228. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis. 1984;37(2):85-95. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan RM, Patterson TL, Kerner DN, Atkinson JH, Heaton RK, Grant I; HNRC Group. HIV Neural Behavioral Research Center. The Quality of Well-Being scale in asymptomatic HIV-infected patients. Qual Life Res. 1997;6(6):507-514. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan RM, Anderson JP, Patterson TL, et al. ; HNRC Group. HIV Neurobehavioral Research Center. Validity of the Quality of Well-Being Scale for persons with human immunodeficiency virus infection. Psychosom Med. 1995;57(2):138-147. [DOI] [PubMed] [Google Scholar]
- 11.Orenstein DM, Pattishall EN, Nixon PA, Ross EA, Kaplan RM. Quality of well-being before and after antibiotic treatment of pulmonary exacerbation in patients with cystic fibrosis. Chest. 1990;98(5):1081-1084. [DOI] [PubMed] [Google Scholar]
- 12.Orenstein DM, Kaplan RM. Measuring the quality of well-being in cystic fibrosis and lung transplantation. The importance of the area under the curve. Chest. 1991;100(4):1016-1018. [DOI] [PubMed] [Google Scholar]
- 13.Ganiats TG, Palinkas LA, Kaplan RM. Comparison of Quality of Well-Being scale and Functional Status Index in patients with atrial fibrillation. Med Care. 1992;30(10):958-964. [DOI] [PubMed] [Google Scholar]
- 14.Squier HC, Ries AL, Kaplan RM, et al. Quality of well-being predicts survival in lung transplantation candidates. Am J Respir Crit Care Med. 1995;152(6 pt 1):2032-2036. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan RM, Schmidt SM, Cronan TA. Quality of well being in patients with fibromyalgia. J Rheumatol. 2000;27(3):785-789. [PubMed] [Google Scholar]
- 16.Kaplan RM, Alcaraz JE, Anderson JP, Weisman M. Quality-adjusted life years lost to arthritis: effects of gender, race, and social class. Arthritis Care Res. 1996;9(6):473-482. [DOI] [PubMed] [Google Scholar]
- 17.Rocco MV, Gassman JJ, Wang SR, Kaplan RM. Cross-sectional study of quality of life and symptoms in chronic renal disease patients: the Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1997;29(6):888-896. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan RM. Quality of life assessment for cost/utility studies in cancer. Cancer Treat Rev. 1993;19(suppl A):85-96. [DOI] [PubMed] [Google Scholar]
- 19.Pyne JM, Patterson TL, Kaplan RM, et al. Preliminary longitudinal assessment of quality of life in patients with major depression. Psychopharmacol Bull. 1997;33(1):23-29. [PubMed] [Google Scholar]
- 20.Pyne JM, Patterson TL, Kaplan RM, Gillin JC, Koch WL, Grant I. Assessment of the quality of life of patients with major depression. Psychiatr Serv. 1997;48(2):224-230. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan RM, Ganiats TG, Sieber WJ, Anderson JP. The Quality of Well-Being Scale: critical similarities and differences with SF-36. Int J Qual Health Care. 1998;10(6):509-520. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan RM. California Policy Seminar. Allocating Health Resources in California: Learning From the Oregon Experiment. Berkeley, CA: California Policy Seminar; 1993. [Google Scholar]
- 23.Kaplan RM. Value judgment in the Oregon Medicaid experiment. Med Care. 1994;32(10):975-988. [DOI] [PubMed] [Google Scholar]
- 24.Bombardier C, Ware J, Russell IJ, Larson M, Chalmers A, Read JL. Auranofin therapy and quality of life in patients with rheumatoid arthritis. Results of a multicenter trial. Am J Med. 1986;81(4):565-578. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan RM, Ganiats TG, Sieber WJ, Anderson JP. The Quality of Well-Being Scale: critical similarities and differences with SF-36. Int J Qual Health Care. 1998;10(6):509-520. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan RM, Ries AL, Reilly J, Mohsenifar Z; for the National Emphysema Treatment Trial Research Group. Measurement of health-related quality of life in the national emphysema treatment trial. Chest. 2004;126(3):781-789. [DOI] [PubMed] [Google Scholar]
- 27.Sarkin AJ, Groessl EJ, Carlson JA, et al. Development and validation of a mental health subscale from the Quality of Well-Being Self-Administered. Qual Life Res. 2013;22(7):1685-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsey SD, Shroyer AL, Sullivan SD, Wood DE. Updated evaluation of the cost-effectiveness of lung volume reduction surgery. Chest. 2007;131(3):823-832. [DOI] [PubMed] [Google Scholar]
- 29.Fan VS, Ramsey SD, Make BJ, Martinez FJ. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD. 2007;4(1):29-39. [DOI] [PubMed] [Google Scholar]
- 30.Ries AL, Make BJ, Lee SM, et al. ; for the National Emphysema Treatment Trial Research Group. The effects of pulmonary rehabilitation in the national emphysema treatment trial. Chest. 2005;128(6):3799-3809. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey SD, Berry K, Etzioni R, Kaplan RM, Sullivan SD, Wood DE; National Emphysema Treatment Trial Research Group. Cost effectiveness of lung-volume-reduction surgery for patients with severe emphysema. N Engl J Med. 2003;348(21):2092-2102. [DOI] [PubMed] [Google Scholar]